INTRODUCTION
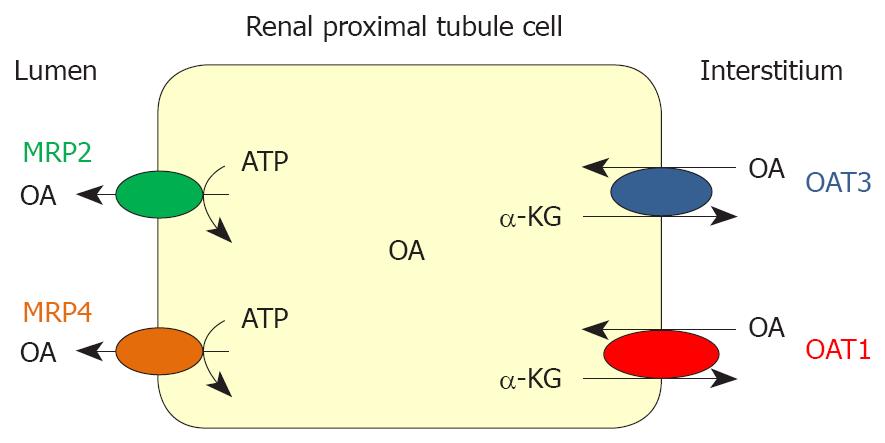
Figure 1 Schematic presentation of a renal proximal tubule cell, showing Organic Anion Transporters 1/3 (OAT1/3) and Multidrug Resistance Proteins 2/4 (MRP2/4).
OA: organic anion; α-KG: α-ketoglutarate.
Pharmacotherapeutic efficacy and toxicity are governed by pharmacodynamic and pharmacokinetic factors. The ability of a drug to reach its site of action is dependent on absorption, distribution, metabolism and excretion, all of which are intimately related to transport mechanisms in barrier epithelia. The majority of drugs and their metabolites have a positive or negative charge in the body, rendering them somewhat polar and hydrophilic. This polarity limits passive permeation across lipophilic barrier membranes. Thus the disposition of most drugs is highly dependent on specialized transporters embedded in these barrier epithelia. Transporters mediate selective permeation of charged molecules through barrier membranes and are thought to play a pivotal role in detoxification and drug disposition[1,2]. Membrane transporters have been classified into the solute carrier (SLC) and the ATP-binding cassette (ABC) transporter families. The SLC22 family comprises organic cation transporters (OCTs), zwitterion/cation transporters (OCTNs), and organic anion transporters (OATs). These transporters contain 12 predicted alpha-helical transmembrane domains (TMDs) and one large extracellular loop between TMDs 1 and 2. Transporters of the SLC22 family function in different ways: (1) as uniporters that mediate facilitated diffusion in either direction (OCTs), (2) as anion exchangers (OAT1, OAT3 and URAT1), and (3) as Na(+)/l-carnitine cotransporters (OCTN2)[1-5].
OATs play an essential role in the elimination of numerous endogenous and exogenous organic anions from the body. Numerous compounds, such as drugs, environmental substances, plant and animal toxins, and metabolites of both foreign and endogenous origins, are classified as organic anions. As for drugs of pharmacological interest, it is possible to mention β-lactam antibiotics, diuretics, nonsteroidal anti-inflammatory drugs, and several antiviral drugs that are organic anions[1-5].
Two organic anion transporters, OAT1 (Slc22a6) and OAT3 (Slc22a8), are expressed in the basolateral membrane of renal proximal tubule cells and have been identified as contributors to xenobiotic and endogenous organic anion secretion[3-5]. This membrane localization and function position them for an active role in determining drug exposure and efficacy. The regulation of OATs expression is not well understood. The function of OATs in renal cells under physiological and pathological conditions has not been, as yet, fully elucidated. Recent studies indicated that the expressions of OATs are affected by pathological states. Modifications in the renal expression of OAT1 and OAT3 have been described in renal diseases; for example, chronic renal failure[6], ischemic acute renal failure[7] and obstructive nephropathy[8,9] and in other diseases, such as arterial calcinosis[10,11] and extrahepatic cholestasis[12-14].
GENERAL CHARACTERISTICS OF OAT1 AND OAT3
OAT1 has been cloned from rat, mouse, flounder, human, pig, rabbit and C. elegans[1-5,15-17]. OAT1 is expressed predominantly in the kidneys and weakly in the brain. OAT1 couples organic anions entry to dicarboxylate exit[16]. This protein has been immunolocalized to the basolateral surface of the proximal tubule[18]. OAT1 mediates the transport of many compounds (dicarboxylates, nucleotides, prostaglandins, antivirals, loop and thiazide diuretics, β lactam antibiotics, and nonsteroidal anti-inflammatory drugs, including the prototypical substrate of the classical organic anions secretion pathway, para-aminohipurate (PAH)). For methotrexate, transport was demonstrated for rat OAT1, but not for human OAT1, suggesting species differences. This has been demonstrated in vitro by its heterologous expression following microinjection of OAT1 cRNA into Xenopus oocytes or transfection of OAT1 cDNA into epithelial cell lines[3-5,15-17]. Eraly et al[19] have recently generated a colony of OAT1 knockout mice, permitting elucidation of the role of OAT1 in the context of other potentially functionally redundant transporters. They found that the knockout mice manifest a large loss of organic anion transport (e.g. PAH) both ex vivo (in isolated renal slices) as well as in vivo (as indicated by loss of renal secretion). In the case of the organic anion, furosemide (FS), loss of renal secretion in knockout animals resulted in impaired diuretic responsiveness to this drug. These results indicate an important role for OAT1 in the functioning of the classical pathway. The gene for human OAT1, SLC22A6, is located on chromosome 11q12.3, being paired with the gene for OAT3. The mammalian OAT1 consist of 545-551 amino acids, and secondary structure algorithms predict 12 TMDs with the N- and C-termini located at the cytosolic side of the plasma membrane. In man, a longer splice variant with 563 amino acids and two shorter, non functional splice variants were found[1-5].
OAT3 was cloned from human, monkey, pig, rabbit, rat and mouse kidneys. The human OAT3 is mainly expressed in kidneys, and to a lesser extent in the brain. The human OAT3 gene is paired with that of OAT1 and located on chromosome 11q12.3. In rats, more message for rat OAT3 was found in liver than in kidneys and brain suggesting species differences. The mammalian OAT3 proteins consist of 536-542 amino acids, arranged in 12 TMDs. Inmunohistochemistry revealed the location of OAT3 at the basolateral membrane of human, rat and mouse renal proximal tubules[18,20]. In rats, OAT3 was also found in several other nephron segments including the thick ascending limb of Henle’s loop, distal convoluted tubule and collecting ducts. OAT3 in proximal tubules is involved in organic anion secretion, but the physiological and pharmacological roles of OAT3 in deeper nephron segments are presently not clear. Based on message abundance, OAT3 is the predominant organic anion transporter in the human kidney. Besides kidneys and liver, OAT3 was also found in human choroid plexus and in rat cerebral capillaries. Rat OAT3 contains three potential PKC phosphorylation sites and human OAT3 contains eight. OAT3 recognizes a broad spectrum of substrates, and it mediates the transport of PAH, ochratoxin A, estrone sulfate (ES), cimetidine, benzylpenicillin, cephaloridine and glutarate. Although it is evident that the selectivity of OAT3 overlaps that of OAT1, affinities for several substrates appear to permit discrimination between both transporters. For example, OAT3 displays a moderately high affinity for ES, whereas OAT1 interacts little with ES[2]. So, ES has been frequently used as a test substrate in studies for OAT3 activity. The mechanisms of OAT3 activity revealed that it can operate as an organic anion/dicarboxylate exchanger[21,22]. Consistent with an important role for OAT3-mediated uptake in kidney and choroid plexus, recent experiments with tissue from OAT3 knockout mice show reduced uptake of PAH, ES, and taurocholate in renal cortical slices and nearly complete inhibition of transport of the fluorescent organic anion fluorescein in intact choroid plexus[23]. For some drugs, there are differences in the specificity between human and rodent OAT3. For example, rat OAT3 transports ranitidine, famotidine and AZT, and human OAT3 transport little ranitidine and no famotidine and AZT[5]. Nevertheless, a systematic survey of possible species differences does not exist.
CHOLESTASIS AND RENAL DAMAGE
Kidney and liver play a major role in the elimination of numerous potentially toxic xenobiotics, including drugs, toxins, and endogenous metabolites. In some cases, the loss of one route of elimination can be compensated by another[24-26]. It must also be mentioned that impairment of liver or kidney functions can cause syndromes characterized by injury of the alternative elimination organ[24,27,28]. For example, prolonged cholestasis, characterized by retention of bile compound, may cause renal damage (reduction in renal hemodynamics, impairment of renal excretion of water and salts, and sensitization of the kidney to anoxia damage), which sometimes leads to renal failure. Moreover, obstructive jaundice predisposes the kidney to acute renal failure and oxidative stress[29-32]. Extrahepatic biliary obstruction significantly decreased the reduced and oxidated forms of glutathione, and significantly inhibited glutathione peroxidase activity in rat kidneys. Increases of thiobarbituric acid reactive substances were also observed[32]. The F2-isoprostanes formed during oxidant injury are renal vasoconstrictors acting via thromboxane (TX)-like receptors. Holt et al[30] have demonstrated that the antioxidants N-acetyl-cysteine and α-lipoid acid and a TX receptor antagonist can prevent renal dysfunction in experimental cholestasis. Endothelin, a potent renal vasoconstrictor and modulator of the tubular action of arginine vasopressin, shows an increased synthesis in kidneys from rats with extrahepatic cholestasis, which is reflected by increased urinary excretion and may reduce distal tubular water reabsorption in these rats, being also involved in the renal damage observed during this liver pathology[31]. In human beings and rats, extrahepatic cholestasis has been shown to render the kidney susceptible to a variety of nephrotoxic agents[29,30]. The pathophysiological cause of renal damage during the course of bile flow impairment is not well understood, even if several phenomena, such as increased access of various constituents into the kidney (bilirubin and bile salts), have been suggested[30-34]. Impairment of kidney function produces modifications in the renal elimination of drugs mediated by alterations in blood flow to the kidney, glomerular filtration, active tubular secretion, and passive tubular reabsorption[6-11,35]. Because ethical considerations usually preclude meaningful clinical investigation in patients with acute obstructive jaundice, most studies in this condition have been carried out in animals, usually dogs and rats[31,32,36]. Bile duct ligation in dogs and rodents serves as an experimental model of extrahepatic cholestasis[33,34].
RENAL ELIMINATION OF ORGANIC ANIONS AND EXPRESSION OF OAT1 AND OAT3 IN EXTRAHEPATIC CHOLESTASIS
Brandoni et al[12,13]. have studied the cortical renal expression of OAT1 and OAT3 in association with the pharmacokinetics and renal excretion of PAH and FS in rats with acute extrahepatic cholestasis. Male Wistar rats underwent bile duct ligation (BDL rats). All studies were carried out 21 h after surgery. The systemic and renal clearance of both PAH and FS increased in BDL rats. In kidneys from BDL rats, immunoblotting showed a significant increase in the abundance of both OAT1 and OAT3 in homogenates from renal cortex. In basolateral membranes from the kidney cortex of BDL rats, OAT1 abundance was also increased and OAT3 abundance was not modified. Immunohistochemical techniques confirmed these results. Acute obstructive jaundice is associated with an upregulation of OAT1 and OAT3, which might explain, at least in part, the increased systemic and renal elimination of PAH and FS.
In this connection, extrahepatic cholestasis is associated with the production of various cytokines and growth factors that may affect gene transcription[37]. Similarly, with OAT1 and OAT3, MRP2 upregulation in BDL rats has been described[38-40]. These authors found that bilirubin ditaurate, sulfate-conjugated bile acids, and some components of the human bile upregulate the expression of MRP2 in human renal tubular cells. MRP2 and MRP4 are two members of the multidrug resistance protein (MRP) family located at the apical membranes from the proximal tubule cells[3-5]. Both proteins mediate the apical renal transport of several anionic substances, such as PAH[3-5,41]. An up-regulation of renal MRP2 has been described at 1 d and 3 d after BDL[38-40]. On the contrary, MRP4 decreases at 3 d after BDL[42].
Three days of BDL is the period in which serum bile acids and bilirubin levels reach the peak of elevation[38,40,43]. Brandoni et al[14] have also studied OAT1 and OAT3 function and expression after 3 d of BDL. After this time, BDL rats displayed a reduction in the renal elimination of PAH. OAT1 protein expression in kidney homogenates was not modified, but it decreased in the basolateral membranes. In contrast, OAT3 abundance in both kidney cortex homogenates and in basolateral membranes increased by 3 d after the ligation. This study demonstrated the key role of OAT1 expression in the impaired elimination of PAH after 3 d of obstructive cholestasis.
OAT1, when heterologously expressed in oocytes or mammalian cells, is inhibited by more or less selective PKC activators. It was demonstrated that PKC induces human OAT1 down-regulation through carrier retrieval from the cell membrane without phosphorylation[3-5,44]. Angiotensin II[45] modulates the renal proximal tubule function via activation of PKC. Although the role of the renin-angiotensin system in the BDL model remains controversial[30], some humoral factors including angiotensin II induced by the 3 d BDL may induce the activation of PKC. Moreover, bile acids and high bilirubin levels can activate PKC[46,47]. Three days of BDL is the period in which serum bile acids and bilirubin levels reach peak elevation. So, it was postulated that the peak elevation of bile acids and bilirubin can also trigger PKC activation. This PKC activation may cause the phosphorylation of caveolin-2, which may induce internalization of caveolae with OAT1 protein anchored with caveolin, as has been recently suggested by Kwak et al[48]. This OAT1 downregulation (30%) was associated with a concomitant decrease of renal and systemic PAH clearance (40% and 30% respectively). The medium PAH plasma concentrations reached during the renal clearance infusion studies were 295 μmol/L and 376 μmol/L for the Sham and BDL rats respectively. The OAT1 mediated uptake of PAH is saturable with apparent Michaelis constants ranging from 15 μmol/L to 70 μmol/L for rat OAT1[6]. Therefore, PAH concentrations that we obtained in our “in vivo” experiments were sufficiently higher than the reported Km of rat OAT1. The diminished secreted load of PAH measured under saturating conditions was in part accounted for by the lower number of OAT1 protein units observed in renal basolateral plasma membranes after 3 d of BDL by immunoblot technique. The opposite was observed in the early phase of extrahepatic cholestasis where an increase of 30% of OAT1 abundance was associated with a similar increase in PAH clearance[12]. The differences observed in OAT1 abundance between 21 h and 72 h of BDL remain to be explained. The increased OAT1 abundance observed in the early phase of extrahepatic cholestasis suggests a transient up-regulation similar to those described for renal OCT1 in cholestatic rats[49]. Different levels of cytokines and growth factors that may affect gene transcription might be involved in this differential response[50].
On the contrary, OAT3 expression increased both in homogenates and basolateral membranes from BDL kidneys. OAT3 is found in various cells and in all parts of the nephron, whereas OAT1 is confined to proximal tubules. The human and rat OAT3 transport PAH with relatively high affinity (87 μmol/L and 65 μmol/L respectively)[3-5,20], similarly to OAT1. On the contrary, ES, cholate, and taurocholate are substrates for OAT3 and not for OAT1 using in vivo and in vitro methodologies[3-5,19,20,23]. The over-expression of OAT3 does not compensate for the down-regulation of OAT1 regarding PAH transport because in this disease the high plasma levels of bile acids compete with PAH for OAT3 transport. Moreover, bile acids regulate the expression of several genes involved in bile salt transport[51,52]. It is possible that high bile acid levels up-regulate OAT3 expression without affecting OAT1 expression; this being another example of substrate specific regulation.
As has been mentioned above, MRP2 up-regulation was observed in the kidneys from rats with BDL of 1 d and 3 d[38-40]. On the other hand, renal MRP4 was down-regulated at 3 d after biliary obstruction[42]. Therefore, the protein expression of the luminal (MRP2 and MRP4) and basolateral organic anion renal transporters (OAT1 and OAT3) are differently regulated in extrahepatic biliary obstruction, thus indicating that different roles are played by these transporters in the pathogenesis of cholestasis. Tubular secretion is a vectorial transcellular transport system consisting of basolateral entry into the epithelial cells and secretion across the brush border membranes. Defects in either of these two processes should therefore influence the tubular secretion of anionic drugs.
Therefore, the time elapsed after obstructive cholestasis has an important impact in the regulation of OAT1 and OAT3. Studies regarding the possible role of bile acids and bilirubin, which are increased in this pathology, in the regulation of OATs are currently being performed in our laboratory.
Highly accumulated anionic drugs, as observed during cholestasis, may cause general body deterioration. The molecular mechanism(s) involved in the differential renal regulation of MRP2, MRP4, OAT3 and OAT1 expression should therefore be elucidated to prevent the occurrence of drug-induced toxicity. The renal expression of OAT1 and OAT3 should be taken into account in order to improve pharmacotherapeutic efficacy and to prevent drug toxicity during the onset of this hepatic disease.
Figure 1 shows the expression pattern of the transporters OAT1, OAT3, MRP2 and MRP4 in a cell of renal proximal tubule.
Peer reviewer: Dr. Milan Jirsa, Laboratory of Experimental Medicine-building Z1, Institute for Clinical and Experimental Medicine, Videnska 1958/9, Praha 4, 14000, Czech
S- Editor Xiao LL L- Editor Lutze M E- Editor Lin YP