Published online Nov 7, 2008. doi: 10.3748/wjg.14.6312
Revised: October 30, 2008
Accepted: November 6, 2008
Published online: November 7, 2008
Neuropeptide Y (NPY) is a potent neurotransmitter for feeding. Besides NPY, orexigenic neuropeptides such as agouti-related protein (AgRP), and anorexigenic neuropeptides such as α-melatonin stimulating hormone (MSH) and cocaine-amphetamine-regulated transcript (CART) are also involved in central feeding regulation. During fasting, NPY and AgRP gene expressions are up-regulated and POMC and CART gene expressions are down-regulated in hypothalamus. Based on the network of peptidergic neurons, the former are involved in positive feeding regulation, and the latter are involved in negative feeding, which exert these feeding-regulated peptides especially in paraventricular nucleus (PVN). To clarify the compensatory mechanism of knock-out of NPY system on feeding, change in gene expressions of appetite-related neuropeptides and the feeding behavior was studied in NPY Y5-KO mice. Food intake was increased in Y5-KO mice. Fasting increased the amounts of food and water intake in the KO mice more profoundly. These data indicated the compensatory phenomenon of feeding behavior in Y5-KO mice. RT-PCR and ISH suggested that the compensation of feeding is due to change in gene expressions of AgRP, CART and POMC in hypothalamus. Thus, these findings indicated that the compensatory mechanism involves change in POMC/CART gene expression in arcuate nucleus (ARC). The POMC/CART gene expression is important for central compensatory regulation in feeding behavior.
- Citation: Higuchi H, Niki T, Shiiya T. Feeding behavior and gene expression of appetite-related neuropeptides in mice lacking for neuropeptide Y Y5 receptor subclass. World J Gastroenterol 2008; 14(41): 6312-6317
- URL: https://www.wjgnet.com/1007-9327/full/v14/i41/6312.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6312
Feeding behavior is a complicated process to regulate the body weight. The body weight is determined by balance between calorie intake mainly by feeding and energy consumption including exercise, body temperature and metabolism. Obesity derived from excess of food intake results in the metabolic syndrome, diabetes mellitus and associated cardiovascular diseases. Since hyperphagia contributes onset and progression of the metabolic syndrome and so on, central feeding regulation is an important issue to be clarified[1,2].
Feeding behavior is regulated strictly by more than 20 appetite-related neuropeptides which are expressed in feeding center in central hypothalamus[1-4]. The main regulation to control feeding are the opposing controls between the orexigenic NPY (neuropeptide Y)/AgRP (agouti-related protein) neurons and the anorexigenic POMC (proopiomelanocortin)/CART (cocaine-amphetamine-regulated transcript) neurons, which originate from the arcuate nucleus (ARC) to the paraventricular nucleus (PVN)[3-6]. The main pathway of two opposite neuron groups obtain and integrate nutritional informations, and communicate information regarding nutrient status and energy stores to the second-order neurons of feeding regulation in PVN[3,5,6]. Among these appetite-related neuropeptides, NPY is the endogenous, strongest orexigenic neuropeptide and fasting induces the most remarkable increase in NPY gene expression in hypothalamus, indicating its principal and physiological relevance in feeding regulation[3-7].
We have been studying the regulatory mechanism of NPY gene expression in hypothalamus, so as to elucidate the mechanism of feeding-related regulation of NPY gene expression[8-11]. Leptin is an anti-obesity hormone derived from adipose tissue and reduces the NPY gene expression in hypothalamus. This inhibition of NPY gene expression by leptin is shown to be due to activation of SOCS3 in the NPY neurons in arcuate nucleus[11].
In contrast the NPY receptors have been classified into at least 6 subclasses (Y1-y6). Y1, Y2, Y4, Y5, and y6 receptors have been cloned and Y1, Y2 and Y5 receptors are mainly involved in central feeding regulation in hypothalamic ARC and PVN[12-19]. NPY-induced marked induction of feeding behavior intracerebroventricular (icv) is mediated through mainly Y1 and Y5 receptors in mammalians[13,15,16]. Interestingly, although activation of Y1 and Y5 receptors is involved in NPY-induced hyperphagia, the Y1-KO (knockout) and Y5-KO mice develop the late-onset obesity with increase in food intake and adiposity, while NPY-KO mice did not change the feeding behavior or body weight[17,20]. This implies a compensatory mechanism in feeding behavior in these KO mice, which is important for understanding the long-term treatment with the current Y1 and Y5 receptor antagonists for anti-obesity drugs. In addition the compensation against the orexigenic NPY system has not been elucidated to date yet. Therefore, first we chose the Y5-KO mice to investigate the central compensatory mechanism for knockout of NPY system, since the expression of Y5 receptor is much restricted in the brain[16,18,21]. Then we characterized change in the feeding behavior and gene expressions of various appetite-related neuropeptides in the hypothalamus of the NPY Y5-KO mice.
NPY is the endogenous neuropeptide with most orexigenic potency in hypothalamus, and its injection icv or direct injection into PVN in hypothalamus produces marked increase in feeding behavior in rodents[13-16]. Fasting or central glucoprivation evoked by 2-deoxy-glucose (2DG) induces eating with simultaneous increase in orexigenic NPY and AgRP gene expressions in ARC, followed by augmented NPY peptide release[7,8]. Since this fasting-induced food intake is significantly inhibited by selective Y1 or Y5 antagonists, the induction is mediated through Y1 and Y5 receptors (apparently, mainly through Y1 receptors in mice)[13,16]. This NPY/AgRP neurons are essential for feeding in adult mice[22].
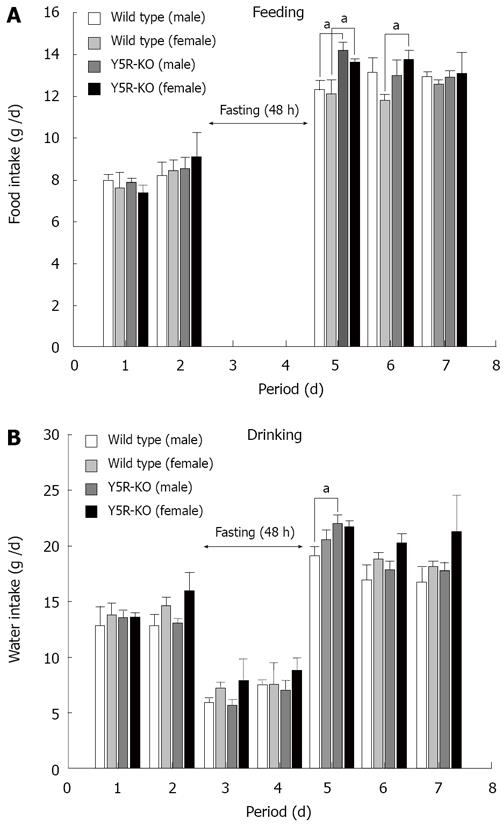
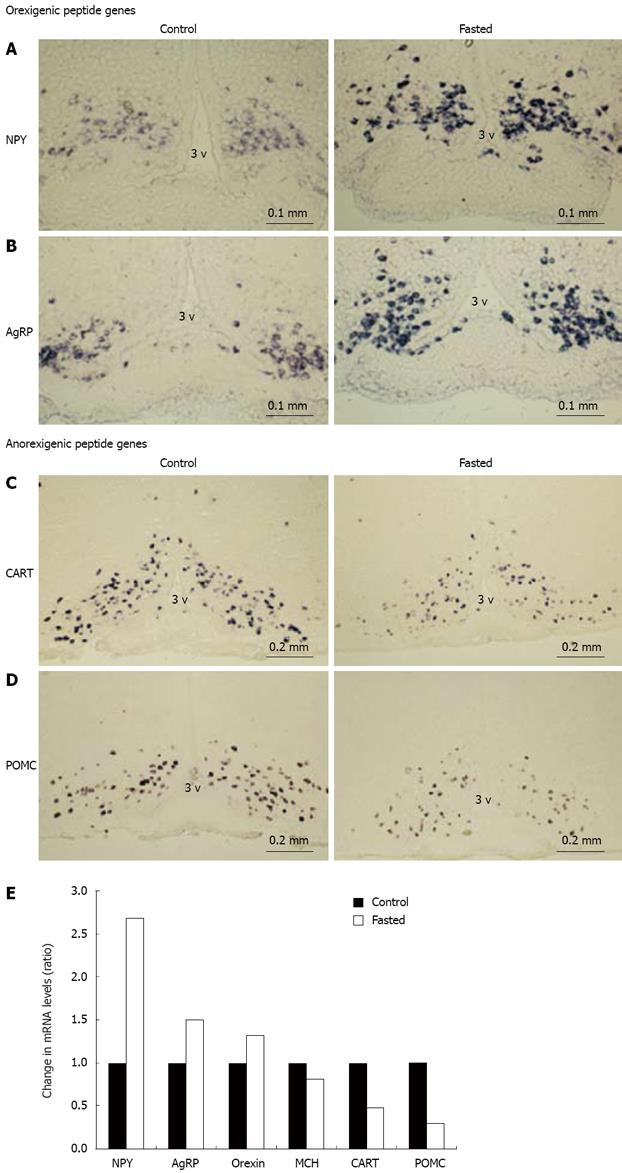
In our experiments with male 10-wk-old wild-type mice, fasting for 48 h increased daily food intake and daily water intake by 53% and 50%, respectively (Figure 1). The increase in food intake followed by the concomitant increase in the orexigenic NPY and AgRP gene expressions and decrease in the anorexigenic POMC gene expression in ARC[4]. RT-PCR indicated that NPY and AgRP gene expression is increased remarkably and galanin gene expression is increased moderately, while orexin, MCH, CART or POMC mRNA did not change significantly. These findings suggested that fasting-induced food intake is involved markedly in NPY/AgRP gene expression and moderately in galanin gene expression in ARC in wild-type mice. NPY and AgRP coexist in the same neurons in ARC, and NPY and AgRP gene expressions were increased simultaneously and remarkably by fasting[4,7,8].
In contrast to the concept that the NPY system plays a pivotal role in central feeding regulation, the following studies have performed that in the NPY-KO and NPY Y1-KO mice their body weights or feeding behaviors did not change or that in NPY Y5-KO mice the body weight and food intake increased conversely[17,18,20]. This suggested the existence of a compensatory mechanism other than the NPY system. The existence of multiple feeding-regulatory systems is very important for the homeostasis of body weight. Therefore, we tried to elucidate the compensatory system except for NPY by using NPY Y5-KO mice.
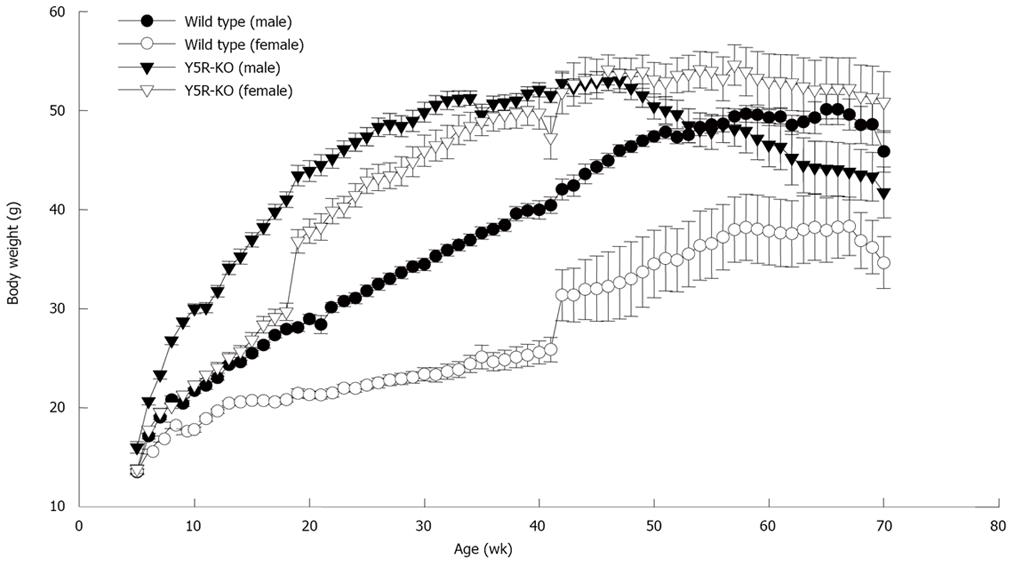
As shown in Figure 2, growth curve of wild-type and NPY Y5-KO mice from 4 to 69 wk old. Although NPY is an orexigenic peptide, obviously Y5-KO mice have obese phenotype. From 4 to 40 wk old, the body weight of Y5-KO mice was twice as much as that of wild-type mice. The increase was observed in both male and female gender but more remarkably in female gender. Because NPY is related with regulation of release of female gonadotropins from pituitary glands, and because female in human being tends to be fatty after menopause, activation through Y5 receptors may be involved in suppression of onset and progression of obesity in female.
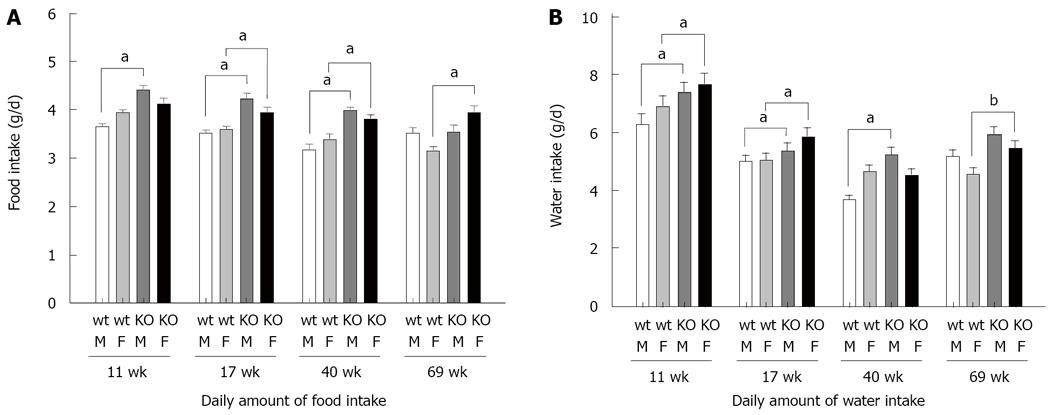
As shown in Figure 3, when Y5-KO mice were freely fed, their daily food intake and daily water intake were significantly higher than those of wild-type mice at any age. This suggested that overeating in Y5-KO mice produced obesity of the mice. Next, we measured the fasting (48 h)-induced change in food intake in Y5-KO mice (Figure 1A). Compared with fasting-induced food intake in wild-type mice, obviously fasting-induced feeding was augmented by about 2 times. The fasting-induced water intake in Y5-KO mice was significantly increased following increase in food intake (Figure 1B). Thus, obesity in NPY Y5-KO mice is probably due to overeating ad libitum and on fasting.
To deny the possibility that obesity might be due to decrease in energy consumption, the decrease rate in body weight was studied during fasting (48 h) in Y5-KO mice. The body weght loss in 2 d of Y5-KO mice was almost the same to that in wild-type mice (data not shown). This indicated that the energy consumption rate is not changed in Y5-KO mice, but the obesity in Y5-KO mice is simply due to increased food intake ad libitum and on fasting.
The compensatory feeding behavior might be due to change in gene expressions of appetite-related neuropeptides other than NPY. Therefore, the change in gene expression of feeding-regulating peptides in hypothalamus was investigated, first, when NPY Y5-KO mice were fed freely. Under ordinary conditions in NPY Y5-KO mice the NPY and AgRP gene expressions were diminished probably due to disuse[4]. The decrease in NPY and AgRP gene expression which expresses in the same neuron group in ARC may be due to increased leptin level following obesity in Y5-KO mice. The gene expression of orexin, MCH, or CART was not changed in hypothalamus; but the POMC gene expression was significantly decreased by RT-PCR and ISH, suggesting that the synthesis of anorexigenic POMC-derived peptides such as -MSH is decreased. This decrease in POMC gene expression appears to be the principal cause of the compensatory overeating.
Fasting for 48 h produces augmented the fasting-induced food intake, with the concomitant increase in the orexigenic NPY and AgRP gene expressions and decrease in the anorexigenic POMC gene expression in ARC in wild-type mice[4]. Obviously the fasting-induced feeding behavior is augmented in Y5-KO mice (Figure 1). Next we investigated whether changes in gene expressions of appetite-regulated neuropeptides in hypothalamus are involved in 48 h-fasting-induced induced feeding behavior (Figure 4). NPY and AgRP gene expressions were induced by 48 h-fasting more profoundly in NPY Y5-KO mice than those in wild-type mice (Figure 4A and B). In contrast, CART and POMC gene expression were conversely decreased more markedly by fasting in Y5-KO mice. In contrast orexin and MCH (melanin-concentrating hormone) gene expressions were not changed by fasting in the mouse hypothalamus (Figure 4E). Thus, the augmentation of fasting-induced feeding behavior in NPY Y5-KO mice was accompanied by the concomitant fasting-induced changes in NPY/AgRP (both increase) and POMC/CART (both decrease) gene expression. Because the NPY system probably dysfunctions in NPY Y5-KO mice, the concomitant increase in AgRP gene expression and decrease in POMC and CART gene expression are the cause of fasting-induced augmentation of feeding behavior in NPY Y5-KO mice. The compensatory mechanism of feeding is probably due to overfunction of compensation by POMC and partly CART gene expressions which results in the late-onset obesity in Y5-KO mice.
Feeding behavior and energy balance are regulated in complicated manner by networks of neurons with classical neurotransmitters and appetite-related peptides. In this article, we showed that the compensatory feeding behavior occurs in NPY Y5-KO mice when the NPY system is probably inhibited, so that the late-onset obesity has appeared. This is probably due to the compensatory change in POMC gene expression. At present the mechanism of compensatory change in POMC/AgRP gene expression is still unknown, and remains to be clarified. This compensatory mechanism is not dependent on the technical procedure of knockout mice. Questions regarding when the compensatory mechanism was been completed, and whether the compensation might occur essential only in adult brain[22,23].
The investigation about the regulation of NPY, AgRP and POMC gene expression in hypothalamic nuclei is useful for elucidation of central feeding regulation and also for development of novel anti-obesity drugs in future.
| 1. | Schwartz MW, Woods SC, Porte D Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661-671. |
| 2. | Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289-295. |
| 3. | Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444:854-859. |
| 4. | Higuchi H, Yamaguchi T, Niki T. [Regulation of hypothalamic neuropeptide expression and feeding behavior in NPY-Y5 knockout (KO) mice]. Nippon Yakurigaku Zasshi. 2006;127:92-96. |
| 5. | Kalra SP, Kalra PS. Neuropeptide Y: a physiological orexigen modulated by the feedback action of ghrelin and leptin. Endocrine. 2003;22:49-56. |
| 7. | Bertile F, Oudart H, Criscuolo F, Maho YL, Raclot T. Hypothalamic gene expression in long-term fasted rats: relationship with body fat. Biochem Biophys Res Commun. 2003;303:1106-1113. |
| 8. | Minami S, Kamegai J, Sugihara H, Suzuki N, Higuchi H, Wakabayashi I. Central glucoprivation evoked by admini-stration of 2-deoxy-D-glucose induces expression of the c-fos gene in a subpopulation of neuropeptide Y neurons in the rat hypothalamus. Brain Res Mol Brain Res. 1995;33:305-310. |
| 9. | Higuchi H, Nakano K, Kim CH, Li BS, Kuo CH, Taira E, Miki N. Ca2+/calmodulin-dependent transcriptional activation of neuropeptide Y gene induced by membrane depolarization: determination of Ca(2+)- and cyclic AMP/phorbol 12-myristate 13-acetate-responsive elements. J Neurochem. 1996;66:1802-1809. |
| 10. | Muraoka O, Xu B, Tsurumaki T, Akira S, Yamaguchi T, Higuchi H. Leptin-induced transactivation of NPY gene promoter mediated by JAK1, JAK2 and STAT3 in the neural cell lines. Neurochem Int. 2003;42:591-601. |
| 11. | Higuchi H, Hasegawa A, Yamaguchi T. Transcriptional regulation of neuronal genes and its effect on neural functions: transcriptional regulation of neuropeptide Y gene by leptin and its effect on feeding. J Pharmacol Sci. 2005;98:225-231. |
| 13. | Iyengar S, Li DL, Simmons RM. Characterization of neuropeptide Y-induced feeding in mice: do Y1-Y6 receptor subtypes mediate feeding? J Pharmacol Exp Ther. 1999;289:1031-1040. |
| 14. | Yokosuka M, Dube MG, Kalra PS, Kalra SP. The mPVN mediates blockade of NPY-induced feeding by a Y5 receptor antagonist: a c-FOS analysis. Peptides. 2001;22:507-514. |
| 15. | Gerald C, Walker MW, Criscione L, Gustafson EL, Batzl-Hartmann C, Smith KE, Vaysse P, Durkin MM, Laz TM, Linemeyer DL. A receptor subtype involved in neuropeptide-Y-induced food intake. Nature. 1996;382:168-171. |
| 16. | Kanatani A, Mashiko S, Murai N, Sugimoto N, Ito J, Fukuroda T, Fukami T, Morin N, MacNeil DJ, Van der Ploeg LH. Role of the Y1 receptor in the regulation of neuropeptide Y-mediated feeding: comparison of wild-type, Y1 receptor-deficient, and Y5 receptor-deficient mice. Endocrinology. 2000;141:1011-1016. |
| 17. | Marsh DJ, Hollopeter G, Kafer KE, Palmiter RD. Role of the Y5 neuropeptide Y receptor in feeding and obesity. Nat Med. 1998;4:718-721. |
| 18. | Pedrazzini T, Seydoux J, Kunstner P, Aubert JF, Grouzmann E, Beermann F, Brunner HR. Cardiovascular response, feeding behavior and locomotor activity in mice lacking the NPY Y1 receptor. Nat Med. 1998;4:722-726. |
| 19. | Naveilhan P, Hassani H, Canals JM, Ekstrand AJ, Larefalk A, Chhajlani V, Arenas E, Gedda K, Svensson L, Thoren P. Normal feeding behavior, body weight and leptin response require the neuropeptide Y Y2 receptor. Nat Med. 1999;5:1188-1193. |
| 20. | Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996;381:415-421. |
| 21. | Huang XF, Han M, Storlien LH. The level of NPY receptor mRNA expression in diet-induced obese and resistant mice. Brain Res Mol Brain Res. 2003;115:21-28. |
S- Editor Xiao LL E- Editor Ma WH