Published online Oct 28, 2008. doi: 10.3748/wjg.14.6244
Revised: October 6, 2008
Accepted: October 13, 2008
Published online: October 28, 2008
AIM: To construct a prokaryotic expression vector carrying Campylobacter jejuni peb1A gene and express it in Escherichia coli. Immunoreactivity and antigenicity of rPEB1 were evaluated. The ability of rPEB1 to induce antibody responses and protective efficacy was identified.
METHODS: peb1A gene was amplified by PCR, target gene and prokaryotic expression plasmid pET28a (+) was digested with BamHI and XhoI, respectively. DNA was ligated with T4 DNA ligase to construct recombinant plasmid pET28a(+)-peb1A. The rPEB1 was expressed in E. coli BL21 (DE3) and identified by SDS-PAGE. BALB/c mice were immunized with rPEB1. ELISA was used to detect the specific antibody titer and MTT method was used to measure the stimulation index of spleen lymphocyte transformation.
RESULTS: The recombinant plasmid pET28a (+)-peb1A was correctly constructed. The expression output of PEB1 protein in pET28a (+)-peb1A system was approximately 33% of total proteins in E. coli. The specific IgG antibody was detected in serum of BALB/c mice immunized with rPEB1 protein. Effective immunological protection with a lower sickness incidence and mortality was seen in the mice suffering from massive C. jejuni infection.
CONCLUSION: rPEB1 protein is a valuable candidate for C. jejuni subunit vaccine.
-
Citation: Du LF, Li ZJ, Tang XY, Huang JQ, Sun WB. Immunogenicity and immunoprotection of recombinant PEB1 in
Campylobacter-jejuni -infected mice. World J Gastroenterol 2008; 14(40): 6244-6248 - URL: https://www.wjgnet.com/1007-9327/full/v14/i40/6244.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6244
| Groups | Cases | Healthy | Sickness | Dead | Illness index | Protective rate (%) |
| Control | 6 | 0 | 2 | 4 | 9.14 ± 0.90 | 0 |
| J50 μg | 6 | 2 | 1 | 3 | 5.71 ± 0.49 | 33.3 |
| J100 μg | 6 | 4 | 1 | 1 | 3.00 ± 0.82 | 75 |
| J200 μg | 6 | 3 | 2 | 1 | 3.14 ± 0.90 | 66.7 |
Campylobacter jejuni is one of the leading causes of bacterial diarrhea in travelers, children, and military personnel in regions where water and food sources are commonly contaminated[1]. Moreover, C. jejuni is an infectious agent most often associated with Guillain-Barre syndrome (GBS), a post-infectious poly-neuropathy[2-5]. Campylobacter has been reported in many geographic regions and its incidence varies with the season. Campylobacter outbreak and sporadic cases occur in developed countries, but the risk of developing campylobacteriosis is greater in travelers, children, and military personnel in regions where water and food sources are commonly contaminated. Currently, no commercial vaccines are available for the prevention of campylobacter-induced diseases in humans or for the reduction/elimination of colonization in poultry.
The development of vaccines has been hampered because the pathogenesis of campylobacter infections is poorly understood. The live-attenuated or killed whole-cell campylobacter vaccine candidates have raised questions about its safety. The protein PEB1, encoded by peb1A genes, is considered a common antigen and a major cell adherence molecule of C. jejuni[6]. The peb1A gene contains 780 bases encoding a 259-residue polypeptide. The peptide sequence starting at residue 27 matches that determined from amino-terminal sequencing of mature PEB1 from C. jejuni. The molecular mass of mature PEBl (amino acids s 27-259) is 25.5 kDa. In this study, we constructed a prokaryotic expression vector carrying C. jejuni peb1A gene minus its signal sequence and expressed it in E. coli. These vaccine candidates were evaluated in mice for their ability to induce antibody responses specific to rPEB1 immunization and to protect the candidates against oral challenge with C. jejuni.
BALB/c mice, at the age of 6-8 wk, were purchased from Center of Experiment Animal of Sun Yat-sen University and housed in cages for 7 d before use.
C. jejuni was grown in brucella agar plates at 37°C in a microaerobic environment. E. coli JM109 used for amplification of the recombinant plasmid pET28a (+) was grown in a LB medium supplemented with kanamycin (50 μg/mL) at 37°C.
Primers were designed according to the sequence of the C. jejuni peb1A gene (Genbank, ATCC700819) minus its signal sequence. The sequence of up primer is 5'-GCGGATCCGCAGAAGGTAAACTTGAGTCTAT-3' and the sequence of down primer is 5'-CCGCTCGAGTTATAAACCCCATTTTTTCGCT-3'. The restriction sites of BamHI and XhoI (underline) were introduced into the sequences of up and down primers, respectively, for gene cloning.
As a first step in amplification of the C. jejuni peb1A gene, template DNA was extracted from the C. jejuni genome. In a 50-μL Eppendoff tube, 30.5 μL of ddH2O, 5 μL of 2 mmol/L dNTP, 5 μL 10 × PCR buffer, 0.5 μL of Taq polymerase, 1 μL of template DNA were added. The PCR product was subjected to electrophoresis on 1.5% agarose, purified using a DNA purification kit and then subjected to digestion with BamHI and XhoI. The digested PCR product was purified and inserted into pET28a (+) digested with the same restriction enzyme to construct pET28a (+)-peb1A. pET28a (+)-peb1A was transfected into E. coli JM109. After propagation, pET28a (+)-peb1A was identified with restriction enzyme by direct sequencing.
The peb1A gene from C. jejuni was expressed in E. coli as hexahistidine tagged proteins in pET-28a (+). E. coli BL21 (DE3) containing peb1A clone was grown in LB broth containing 30 μg/mL kanamycin. Cells were incubated at 37°C with shaking at 250 r/min for 3-4 h until the culture reached an OD of 0.3-0.4. Then, IPTG was added to the LB broth at a final concentration of 1 mmol/L to induce expression of the target protein PEB1. Culture was continued for 6 h and BL21(DE3) cells were harvested at 1, 2, 3, 4 and 6 h, respectively, by centrifugation. The pellet of BL21(DE3) cells was resuspended in 1 × LEW buffer containing 50 mmol/L NaH2PO4, 300 mmol/L NaCl, pH 8, and subjected to ultrasound in ice water. To evaluate the solubility and inclusion body formation, the resulting supernatant and sediments were separated by centrifugation at 12 000 r/min for 10 min at 4°C, and subjected to SDS-PAGE for expression of recombinant PEB1 (rPEB1), which was purified by nickel chromatography under native conditions.
BALB/c mice were injected with 100 μL of PBS or with PBS containing 25, 50 or 100 μg of rPEB1 protein emulsified with an equal volume of CFA or IFA. Mice in each vaccination group (n =10 mice) were immunized four times at 1-wk intervals by intramuscular and subcutaneous injection. Following vaccination, the mice were monitored for adverse effects. Blood was collected from mice at various time points before and after immunization, and allowed to clot. The tubes were spun at 3000 r/min for 10 min, and the serum was collected into a clean microcentrifuge tube. Serum samples were logged in and stored at -20°C.
ELISA was used to evaluate the level of antibody response to anti-PEB1. Briefly, rPEB1 was used as the solid phase. After blocking with PBST supplemented with 10% fetal calf serum, the serum from mice was added. After extensive washing, bound antibodies were detected with goat anti-mouse IgG labeled with horseradish peroxidase. Antibody titers were determined by the serial end-point dilution method. The titer of serum was expressed as group geometric mean ± SD of the mean of individual animal values, which represented the average of duplicate assays.
BALB/c mice immunized with rPEB1 or PBS (control) were sacrificed on day 60 after the first immunization. Splenocytes were harvested from the mice, co-cultured with rPEB1 (2 μg/mL) or with PHA in RPMI1640 for 54 h before addition of MTT (10 μL per well), and incubated at 37°C for 3 h. The supernatant was transferred into a new Eppendorf tube. Absorbance of the converted dye was measured at a wavelength of 570 nm with a spectrophotometer.
BALB/c mice at the age of 7-9 wk without specific pathogen were used in the study. The vaccinated mice were challenged with C. jejuni strain 81-176 in the oral model. We compared the protective efficacy of rPEB1 in immunized and non-immunized mice. Deaths occurred in challenged and control mice were recorded for more than 7 d. Illness index was scored as follows: 2 = dead, 1 = lethargic with ruffled fur and lower activity, and 0 = healthy.
A single band at the 720-bp site was well shown in C. jejuni genome amplified by PCR. Recombinant plasmid pET28a (+)-peb1A analyzed by restriction enzyme digestion and DNA sequence was correctly constructed.
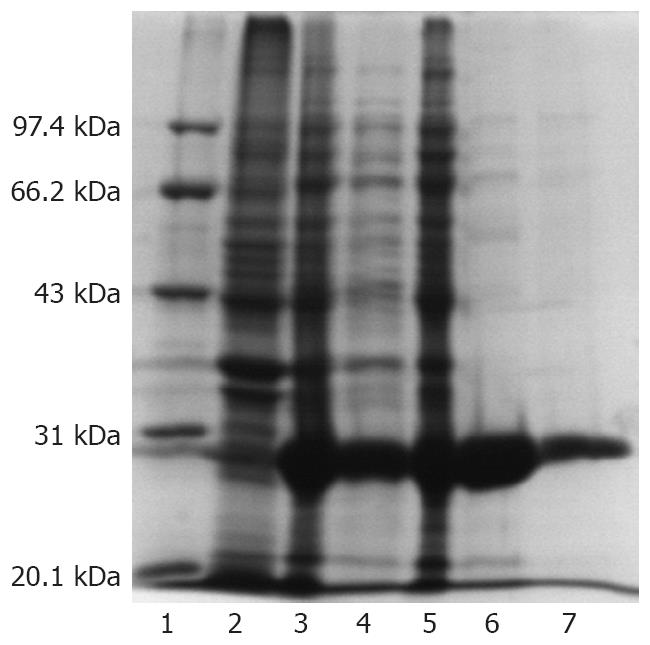
A rPEB1 protein with an expected molecular weight of 29kD was efficiently expressed in E. coli BL (DE3). The rPEB1 was mainly observed in supernatant of the E. coli BL (DE3) lysate and purified to approximately 96% purity by Ni-NTA resin after ultrasonication. The expression output of PEB1 protein in pET28a (+)-peb1A system was approximately 33% of total proteins of E. coli (Figure 1).
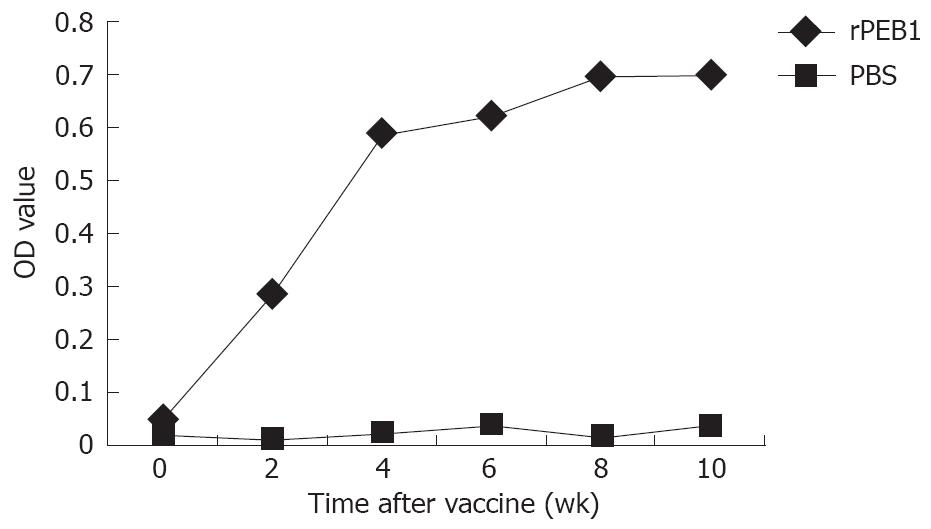
In subcutaneous and intramuscular injection groups, no apparent side effects were noted in mice and delivery of rPEB1 with CFA caused a ruffled fur appearance in all mice that lasted < 24 h, suggesting that injection of rPEB1 was safe. The mice in subcutaneous and intramuscular injection groups were immunized with rPEB1 interfused in CFA or IFA. PBS was substituted for rPEB1 in the control group. Anti- rPEB1 serum was detected 2 wk after the first immunization in both subcutaneous and intramuscular injection groups (Figure 2). Compared to the PBS group, significantly higher levels of serum IgG were detected in ≥ 90% of the animals when 50 μg or higher rPEB1 was delivered with the adjuvant. Vaccination with 100 μg rPEB1 with the adjuvant induced antigen specific serum IgG, which was indistinguishable from that in 200 μg recipients (Table 1). A clear vaccine dose-dependent response was seen for the response magnitude and a strong immune response was observed in mice after immunization with rPEB1. The highest end point dilution titer of anti- rPEB1 serum was 1:5600.
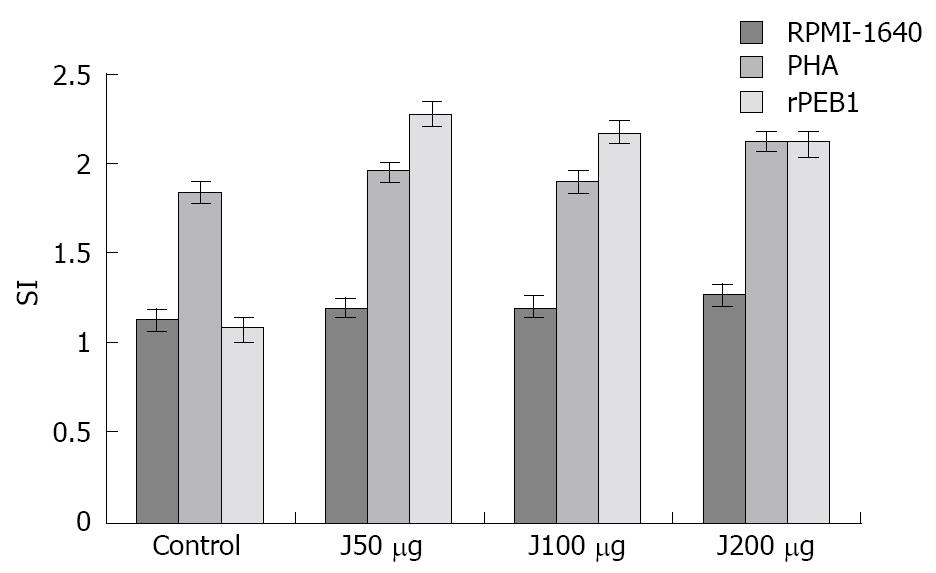
The proliferating response of splenocytes was generated in immunized mice when they were stimulated by PHA or rPEB1 protein. The stimulation index (SI) value for the immunized group was significantly higher than that for the control group, suggesting that proliferation of T cells from immunized mice could be stimulated by rPEB1. No difference in SI was observed in different groups immunized with different doses of rPEB1 compared with the PHA control group (Figure 3).
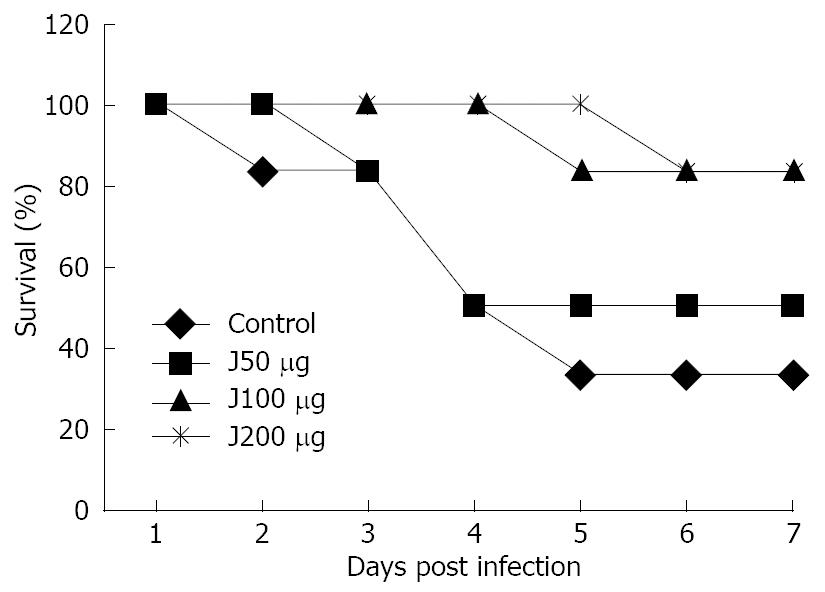
Fourteen days following vaccination, animals immunized with different doses of rPEB1 were challenged with wild-type C. jejuni. The results are summarized in Table 2. Fifty micrograms rPEB1 failed to protect mice against C. jejuni infection and no significant difference was observed in illness pattern of PBS recipients. The efficacy of rPEB1 vaccine was significantly higher in animals challenged with C. jejuni than in those of the control group, indicating that rPEB1 vaccine could eradicate C. jejuni infection (Figure 4).
PEB1, a surface-exposed conserved antigen in C. jejuni, is commonly recognized in convalescent sera from infected patients and involves binding of C. jejuni to eukaryotic cells[7]. Pei et al[8] have reported that PEB1 is a homolog of cluster 3 binding proteins of bacterial ABC transporters and a C. jejuni adhesion cell-binding factor 1. They determined the role of PEB1 in C. jejuni adherence and noted that the rate and duration of intestinal colonization by its mutants are significantly lower and shorter than those of the wild-type strain in mouse challenge test[8]. The adherence to epithelial cells is essential for the establishment of colonization in the gastrointestinal tract. It has also been shown that the PEB1 can adhere to Hela cells[9]. Inactivation of the peb1A locus significantly reduces C. jejuni adherence to Hela cells[10,11]. Moreover, the particulate PEB1 is the only antigen, known to elicit a prominent immune response[12,13]. We believe that PEB1 may be used as a vaccine for C. jejuni infection, which was confirmed by the fact that we successfully constructed a fusion gene containing C. jejuni peb1A gene and expressed rPEB1 protein in E. coil BL21 (DE3). rPEB1 with adjuvant CFA/IFA was used to immunize BALB/c mice, in which strong specific humoral immune responses were induced. High specific anti-rPEB1 and significantly higher specific T-cell proliferation were detected in BALB/c mice 3 wk after their first immunization. Furthermore, rPEB1 vaccination was found to have an effect on reducing the illness index of BALB/c mice after oral challenge with wild-type C. jejuni.
Sizemore et al[14] have reported that live and attenuated Salmonella Typhimurium strains expressing PEB1 can induce antibody responses specific to PEB1 following oral immunization, and have the ability to protect mice against infection with S. Typhimurium strains by reducing or eliminating systemic dissemination and intestinal colonization of wild-type C. jejuni strain 81-176. However, they noted that attenuated salmonella can stimulate production of serum IgG in mice and cannot protect mice against challenge with wild-type C. jejuni[14]. They believe that a small amount of antigen, available at the time of vaccination, may play a role in the absence of serum IgG[14]. Our results indicated that immunization with a low dose (50 μg) of rPEB1 could stimulate specific humoral immune responses and could not protect mice against challenge with wild-type C. jejuni.
The results of the animal protection test using 100 μg rPEB1 showed that most immunized mice remained healthy after C. jejuni challenge. Eighty percent of the control mice were lethargic with ruffled fur and lower activity and died 2 d post-challenge. The protective rate of rPEB1 immunization was 75% in the 100 μg rPEB1 group.
In conclusion, rPEB1, as a candidate vaccine, offers several advantages. It can lead to strong immune response, and provide a protective efficacy. Further study is needed to address its mechanism.
Despite the growing importance and widespread recognition of campylobacter enteritis as a major international public health problem, no commercial vaccines are available for the control of campylobacter-associated enteric disease in humans, or for the reduction/elimination of colonization in poultry.
This study looked for the best induction of protective immune responses when the immunogenic campylobacter protein PEB1 was expressed.
The results of this study showed that rPEB1 was successfully expressed in Escherichia coli and immunization of mice through systemic routes could induce strong and specific serum IgG responses and splenocyte proliferation.
Based on the results of our study, further investigation should be focused on mucosal immune responses, which may be more important to Campylobacter jejuni subunit vaccines.
The results of this study are interesting. The authors evaluated immunoreactivity and antigenicity of rPEB1, identified the ability of rPEB1 to induce systemic immune responses and its protective efficacy.
| 1. | Stutman HR. Salmonella, Shigella, and Campylobacter: common bacterial causes of infectious diarrhea. Pediatr Ann. 1994;23:538-543. |
| 2. | Hughes R. Campylobacter jejuni in Guillain-Barre syndrome. Lancet Neurol. 2004;3:644. |
| 3. | Tsugawa T, Nikaido K, Doi T, Koga M, Susuki K, Kubota T, Tsutsumi H. Guillain-Barre syndrome with meningoencephalitis after Campylobacter jejuni infection. Pediatr Infect Dis J. 2004;23:966-968. |
| 4. | Jacobs BC, van Doorn PA, Schmitz PI, Tio-Gillen AP, Herbrink P, Visser LH, Hooijkass H, van der Meche FG. Campylobacter jejuni infections and anti-GM1 antibodies in Guillain-Barre syndrome. Ann Neurol. 1996;40:181-187. |
| 5. | Yuki N, Ang CW, Koga M, Jacobs BC, van Doorn PA, Hirata K, van der Meche FG. Clinical features and response to treatment in Guillain-Barre syndrome associated with antibodies to GM1b ganglioside. Ann Neurol. 2000;47:314-321. |
| 6. | Pei Z, Blaser MJ. PEB1, the major cell-binding factor of Campylobacter jejuni, is a homolog of the binding component in gram-negative nutrient transport systems. J Biol Chem. 1993;268:18717-18725. |
| 7. | Pei ZH, Ellison RT 3rd, Blaser MJ. Identification, purification, and characterization of major antigenic proteins of Campylobacter jejuni. J Biol Chem. 1991;266:16363-16369. |
| 8. | Pei Z, Burucoa C, Grignon B, Baqar S, Huang XZ, Kopecko DJ, Bourgeois AL, Fauchere JL, Blaser MJ. Mutation in the peb1A locus of Campylobacter jejuni reduces interactions with epithelial cells and intestinal colonization of mice. Infect Immun. 1998;66:938-943. |
| 9. | Kervella M, Pages JM, Pei Z, Grollier G, Blaser MJ, Fauchere JL. Isolation and characterization of two Campylobacter glycine-extracted proteins that bind to HeLa cell membranes. Infect Immun. 1993;61:3440-3448. |
| 10. | Baqar S, Applebee LA, Bourgeois AL. Immunogenicity and protective efficacy of a prototype Campylobacter killed whole-cell vaccine in mice. Infect Immun. 1995;63:3731-3735. |
| 11. | Ang CW, Endtz HP, Jacobs BC, Laman JD, de Klerk MA, van der Meche FG, van Doorn PA. Campylobacter jejuni lipopolysaccharides from Guillain-Barre syndrome patients induce IgG anti-GM1 antibodies in rabbits. J Neuroimmunol. 2000;104:133-138. |
| 12. | Baqar S, Bourgeois AL, Schultheiss PJ, Walker RI, Rollins DM, Haberberger RL, Pavlovskis OR. Safety and immunogenicity of a prototype oral whole-cell killed Campylobacter vaccine administered with a mucosal adjuvant in non-human primates. Vaccine. 1995;13:22-28. |
| 13. | Baqar S, Bourgeois AL, Applebee LA, Mourad AS, Kleinosky MT, Mohran Z, Murphy JR. Murine intranasal challenge model for the study of Campylobacter pathogenesis and immunity. Infect Immun. 1996;64:4933-4939. |
Peer reviewer: Dr. Wang-Xue Chen, Institute for Biological Sciences, National Research Council of Canada, 100 Sussex Drive, Room 3100, Ottawa, Ontario K1A 0R6, Canada
S- Editor Xiao LL L- Editor Wang XL E- Editor Lin YP