Published online Oct 28, 2008. doi: 10.3748/wjg.14.6218
Revised: July 14, 2008
Accepted: July 21, 2008
Published online: October 28, 2008
AIM: To examine the expression of thymidylate synthase (TS) and oncoprotein Bcl-2 in advanced colorectal cancer (CRC) patients, and to determine their mutual relationship, association to therapeutic response and impact on disease outcome.
METHODS: Tumor samples from 67 patients with CRC, who were treated at advanced stage with either irinotecan alone or in combination with 5-fluorouracil/leucovorin, were analyzed for expression of TS and Bcl-2 using immunohistochemistry.
RESULTS: A significant linear correlation between lower expression levels of Bcl-2 and lower levels of TS expression was found (P = 0.033). Patients with high levels of both TS and Bcl-2 expression had a significantly longer disease-free survival (DFS) (42.6 mo vs 5.4 mo, n = 25) than those with low TS/Bcl-2 index (P = 0.001). Tumors with low levels of both TS and Bcl-2 were associated with a longer survival with metastasis (WMS) interval in the whole patients group (n = 67, P = 0.035). TS/Bcl-2 index was not significantly related to disease-specific survival.
CONCLUSION: The present data suggest that CRC patients with low TS/Bcl-2 demonstrate a significantly shorter DFS and longer WMS.
- Citation: Bendardaf R, Ristämaki R, Syrjänen K, Pyrhönen S. Bcl-2 expression significantly correlates with thymidylate synthase expression in colorectal cancer patients. World J Gastroenterol 2008; 14(40): 6218-6223
- URL: https://www.wjgnet.com/1007-9327/full/v14/i40/6218.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6218
| Variable | Total patient group n (%) |
| Patients | 67 (100) |
| Female | 24 (36) |
| Male | 43 (64) |
| Age (yr) | |
| Mean (SD) | 57.6 (24-80) |
| Tumor localization | |
| Rectum | 14 (20.9) |
| Left colon | 31 (46.3) |
| Right colon | 15 (22.4) |
| Transverse colon | 7 (10.4) |
| Primary tumor status1 | |
| T1 | 0 (0) |
| T2 | 5 (7.5) |
| T3 | 48 (71.6) |
| T4 | 14 (20.9) |
| Primary nodal status1 | |
| N0 | 18 (26.9) |
| N1 | 35 (52.2) |
| No data | 14 (20.9) |
| Histological tumor grade | |
| GradeI | 10 (14.9) |
| Grade II | 45 (67.2) |
| Grade III | 12 (17.9) |
| Stage1 | |
| II | 11 (16.4) |
| III | 14 (20.9) |
| IV | 42 (62.7) |
| Localization of metastases | |
| Liver | 28 (41.8) |
| Lung | 1 (1.5) |
| Multiple | 34 (50.7) |
| Local | 4 (6.0) |
| Disease-specific survival (months) | |
| Mean (SD) | 34.4 (26.2) |
| Disease-specific outcome | |
| Alive | 3 (4.5) |
| Died of disease | 64 (95.5) |
Colorectal cancer (CRC) is the second most frequent cancer in Europe in 2004, responsible for 13% (376 400) of all incident cancer cases. It is also the second most frequent cause of cancer mortality in Europe, with an annual mortality of 11.9%, 203 700 annual deaths[1]. In the early stages, CRC is often a curable disease, but the overall prognosis is determined by the extent of local and particularly metastatic tumor spread. However, disease outlook is relatively poor for advanced disease and thus is a significant cause of worldwide cancer-related mortality[1]. For locally advanced and metastatic CRC, fluoropyrimidine, 5-fluorouracil (5-FU) has been the standard cytostatic drug for the last 50 years, in recent years used as modulated by leucovorin and in combination with irinotecan or oxaliplatin.
Fluoropyrimidine metabolites form a covalent complex with thymidylate synthase (TS). Formation of this complex prevents biosynthesis of intracellular thymidylate, which is essential for DNA biosynthesis. TS expression has been shown to be an independent prognostic factor in several other cancers. Higher TS levels in hepatic metastases and resection margin are independent predictors of disease progression and survival in patients with metastatic CRC[2]. Comparable results have been reported in other tumors e.g. gastric[3], cervical[4], ovarian, and head and neck cancers, for which TS+ tumors have demonstrated significantly worse outcome as compared to TS-negative tumors.
Increased expression of the proto-oncogene Bcl-2, a 24-kDa intracellular membrane protein that is able to inhibit programmed cell death without affecting cell proliferation, has been reported in gastrointestinal adenocarcinoma and its precursor lesions[5,6]. Bcl-2 has been shown to prolong cell survival by inhibiting apoptosis in several cell types[7,8]. Abnormal activation of the Bcl-2 gene appears to be an early event in colorectal tumorigenesis[6].
In this study, we examined the expression of TS and the oncoprotein Bcl-2 in locally advanced and metastatic CRC and determined their inter-relationships, as well as their impact on patient survival.
A series of 67 patients were diagnosed and treated for Stage II, III, IV CRC at the Department of Oncology and Radiotherapy, Turku University Hospital (TUH) and six other hospitals in the same hospital district, between January 1996 and August 2003. The key clinical characteristics of the patients are summarized in Table 1.
At the time of diagnosis, 11 patients had stage II, 14 had stage III and 42 had stage IV disease. When patients developed metastases or inoperable local recurrence, they were entered into the chemotherapy protocol. In the protocol, patients received one of two treatment regimens; 18 received irinotecan alone and 49 received a combination of irinotecan, 5-FU and folinic acid (FA) as first line treatment for metastatic disease. Irinotecan (350 mg/m2) was administered as a 60-90 min intravenous (i.v.) infusion every 3 wk. In the combination regimen, irinotecan (180-210 mg/m2) was administered as 60-90 min intravenous infusion and 5-FU (500 mg/m2, i.v. bolus) modulated with folinic acid (FA) (60 mg/m2, i.v. bolus). The 5-FU/FA administrations were repeated again on the following day. The cycle was repeated every 2 wk[9]. The mean duration of chemotherapy was 6.3 mo (SD, 3.4 mo). Treatment was continued until disease progression, or occurrence of unacceptable toxicity.
The patients were prospectively followed-up until the end of March 2007; mean follow-up time from diagnosis was 34.4 mo (± 26.2 mo). We used three endpoints to calculate the patient survival: (1) disease-free survival (DFS), which was calculated in 26 patients with stage IIor III diseaseat diagnosis; (2) overall disease-specific survival (DSS); and (3) survival with metastases (WMS). DFS is the time from diagnosis to the appearance of metastatic disease and relevant only for those patients with radically operated stage II and III patients at the time of diagnosis (n = 25). DSS is the time from diagnosis to death or to the time point when last seen alive at the clinic, and was calculable for all patients in the study. WMS was calculated from the date of recording the appearance of disease recurrence/metastases at the clinical visit, until to death or to the time point when last seen alive.
The study was approved by the Ethical Committee and was conducted in accordance with the Declaration of Helsinki. Samples were collected with the endorsement of the National Authority for Medico-legal Affairs.
Sixty-seven formalin-fixed, paraffin-embedded primary tumors were obtained from 67 patients. Sections were cut serially at 5 μm for routine hematoxylin and eosin staining and for immunohistochemical analysis. An experienced pathologist confirmed all histological diagnoses.
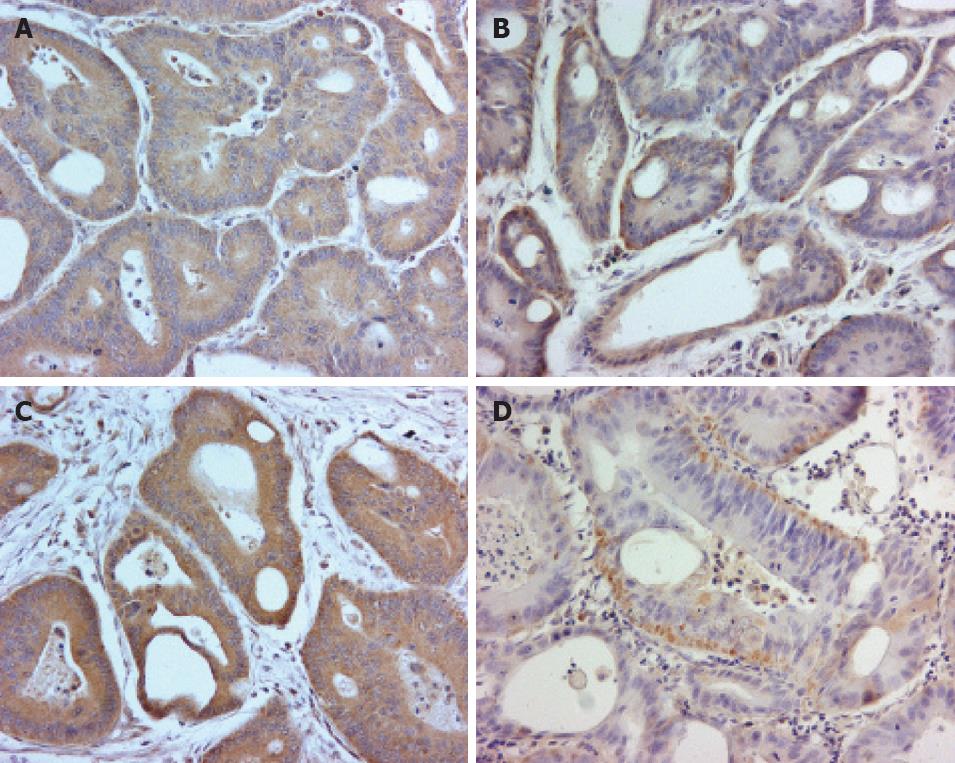
TS expression was studied immunohistochemically using monoclonal antibody (Mouse Clone TS 106) from Zymed Laboratory. Bcl-2 protein expression was studied using anti-Bcl-2 monoclonal mouse antibody, which recognizes a peptide comprising amino acids 41-54 of the human Bcl-2 protein (Clone 124, DAKO A/S, Glostrup, Denmark). Signal detection was performed using the streptavidin-biotin method (Vectastain ABC kit). Formalin-fized, paraffin-embedded sections were deparaffinized in xylene, rehydrated in graded alcohol, immersed in 0.01 mol/L citrate buffer (pH 6.0), heated in a domestic microwave oven at full power for 2 × 5 min, and left in the buffer to cool to room temperature. The sections were incubated in 0.3% hydrogen peroxide for 20 min to block endogenous peroxidase activity. Incubation with the primary antibody diluted in 1% bovine serum albumin/Tris-buffered saline, TS (1:25) and Bcl-2 (1:50), were carried out overnight in a humid chamber at 4°C. The following day, the slides were washed and incubated first with the biotinylated secondary antibody (30 min, 20°C), then with avidin-biotin-peroxidase complex (30 min, 20°C). Positive staining was visualized with 3,3’ diaminobenzidine (DAB) substrate solution and the sections were counterstained with Mayer’s hematoxylin. As negative controls, slides were processed with the omission of the primary antibody.
Expression of TS and Bcl-2 was assessed by an observer blinded to the clinical data. The slides were first screened for an overview of the general staining pattern. Four pictures of each slide, covering most of the tumor area, were taken with a light microscope (4 × magnification) connected to a camera and AnalySIS v 3.00 software (Soft Imaging System GmbH, Munster, Germany). Expression of TS in the four pictures was analyzed using Imaging Research Inc., St. Catharine’s, Ontario, Canada), which detected the brown color of the positively stained tumor cells and counted the area of those cells in pixels, and also counted in pixels the total tumor area. The percentage of positively stained tumor cells from the whole tumor area was counted and used in further analysis. This method of evaluating TS expression was able to distinguish between the presence of many cells expressing low amounts and a few cells expressing high amounts of TS, such that the percentage of TS expression reflected total TS expression in the tumor, which may be more relevant biologically.
For statistical purposes, expression profiles of each marker were treated as dichotomous variables, where tumors with negative or weak expression of Bcl-2 was one category (reduced expression), and all those with moderate or strong expression were grouped into the second category. For TS, we used median values as cut-off to build up the dichotomous variable of low- and high TS expression. In addition, combined TS-Bcl-2 indices were created, using the dichotomous Bcl-2 variables and TS variables (median cut-offs), resulting in four possible combinations of TS/Bcl-2: low/low (L/L); low/high (L/H); high/low (H/L); and high/high (H/H). Finally, these were converted to a 3-class index as follows: class 1, TS/Bcl-2, L/L; class 2, TS/Bcl-2, L/H or H/L; and class 3, TS/Bcl-2 H/H.
Statistical analyses were performed using the SPSS® (SPSS, Inc., Chicago, IL, USA) and STATA (Stata Corp., TX, USA) software packages (SPSS for Windows, version 14.0.1 and STATA/SE 10.1). Frequency tables were analyzed using the χ2 test, with likelihood ratio (LR) or Fisher’s exact test being used to assess the significance of the correlation between categorical variables. Differences in the means of continuous variables were analyzed using non-parametric tests (Mann-Whitney or Kruskal-Wallis tests) for two and multiple independent samples, respectively. ANOVA was only used for deriving the mean values in each stratum. Univariate survival (life-table) analysis for the outcome measure, DSS, DFS and WMS was based on Kaplan-Meier method, and the groups were compared with the log-rank (Mantel-Cox) test. In all tests, P < 0.05 was regarded as statistically significant.
Expression of Bcl-2 and staining of TS are shown in Figure 1. Bcl-2 index and TS index (absolute values) were significantly correlated (r = 0.286, Spearman rho, P = 0.019). Similarly, using the median-cut off values, there was a significant correlation between Bcl-2 and TS expression profile (P = 0.039, Spearman rho or P = 0.037, χ2). In pair-wise comparison, individual samples did not significantly deviate in their Bcl-2 and TS expression profile (median cut-off; P = 0.841, Wilcoxon signed ranks test), indicating that in individual tumors, co-detection of H/H and L/L of both markers was a frequent occurrence.
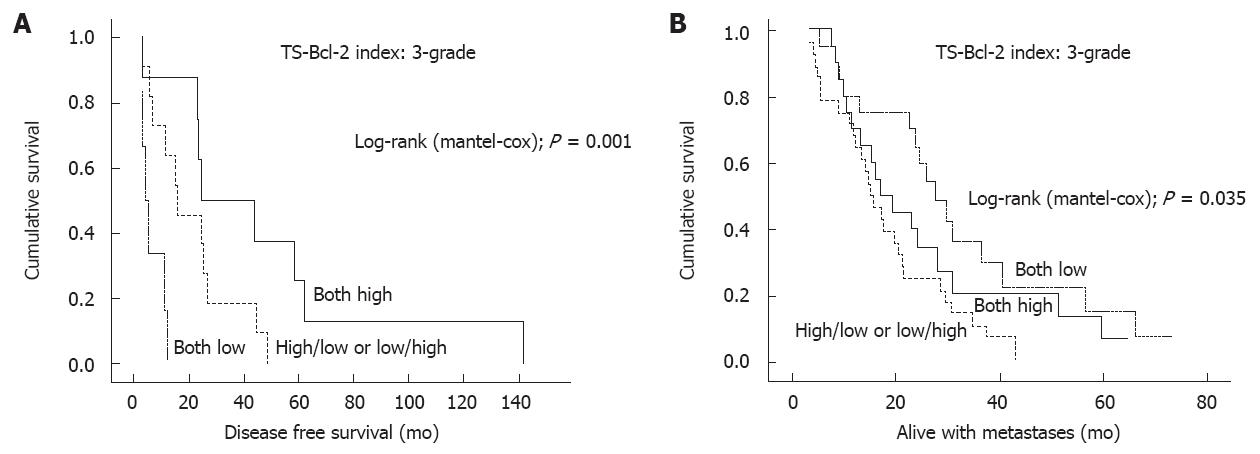
The combined TS-Bcl-2 index was evaluated in relation to all clinical variables recorded, including the response to treatment and survival (DSS, DFS and WMS). Interestingly, for the first time, a significant correlation between TS/Bcl- 2 index and DFS was observed. Among stage II and III disease (n = 25), tumors with H/H profile of TS and Bcl-2 (class 3) were associated with substantially longer DFS (42.6 mo) than those with L/L (5.4 mo) or those with H/L or L/H profile (20.7 mo; P = 0.031, Kruskal-Wallis), and this was even more evident in life-table analysis (P = 0.001, Mantel-Cox; Figure 2A).
There was a close correlation between TS/Bcl-2 expression and WMS; tumors with L/L profile of TS and Bcl-2 (class 1) were associated with a longer WMS (31.9 mo) than those with H/H (25.7 mo) or H/L and L/H (18.9 mo) in the whole series (n = 67, P = 0.076, Kruskal-Wallis and P =0.092 Mantel-Cox) (Figure 2B). There was no such difference in DSS among the three TS/Bcl-2 expression profiles, however. TS/Bcl-2 expression was not significantly associated with any other clinicopathological variables, including age, sex, TNM status, grade, stage or carcinoembryonic antigen (CEA) levels.
The treatment of CRC has become increasingly complex over recent years. With the emergence of new chemotherapy drugs and targeted agents, there has been a major improvement in the prognosis of patients with metastatic CRC. The identification of prognostic and predictive markers is clinically important, because CRC is a heterogeneous disease with various biological and clinical characteristics.
The present study analyzed the combined TS and Bcl-2 expression in CRC, with particular reference to disease outlook. An increase in TS levels has been suggested to be an important mechanism of resistance to fluoropyrimidine-based chemotherapy[10]. In several studies, TS is reported to be a predictor of both survival in CRC[11-14] and response to 5-FU therapy, with higher levels being associated with poorer prognosis and response to therapy[11,12,15,16]. This suggests that TS levels are not only of importance in predicting the natural course of the disease, but may also predict the sensitivity of cancer cells to 5-FU, which is widely used both in the adjuvant setting and in the treatment of metastases[17,18].
The role of the Bcl-2 family of proteins in chemoresponse has been evaluated extensively in in vitro models. Over-expression of Bcl-2 and Bcl-XL has been shown to induce drug resistance[19-21]. In relation to treatment, it has been demonstrated that elevated levels of Bcl-2 protein confers cytotoxic drug resistance to tumor cell lines[7,22]. As Bcl-2 blocks apoptosis in vitro and thus contributes to malignant cell accumulation, its over-expression is expected to be associated with more aggressive tumor biology. Indeed, genetic alteration of the Bcl-2 gene located on chromosome 18 is considered to be a key process in the pathogenesis and chemoresistance of human tumors, such as follicular lymphoma[23].
In our previous analysis of Bcl-2 expression in a subset of these tumors (n = 49), we found a weak association of lower levels of Bcl-2 expression with longer overall survival[24]. In another small series (n = 28)[25], we described that lower levels of Bcl-2 expression were significantly associated with lower levels of TS expression. The present study clearly confirmed this observation in a larger series of CRC patients (n = 67), for which a significant linear correlation was established between the two markers (P = 0.039, Spearman). High expression of TS (above the median) significantly correlated with higher Bcl-2 expression levels. In our previous study, some evidence has suggested that higher levels of TS and Bcl-2 are associated with shorter overall survival[24,25], implicating that elevated levels of TS and Bcl-2 may confer cytotoxic drug resistance among these patients.
To the best of our knowledge, our study shows for the first time the relationship between these two key molecules in a group of patients with locally advanced or metastatic CRC receiving similar treatment. As to the patient survival, there was a significant correlation of TS/Bcl-2 expression with DFS, in that the patients with high TS/Bcl-2 index had a longer DFS (Figure 2A). No such effect was shown for DSS, which was not significantly different among the patients with low- and high TS/Bcl-2 indices.
To conclude, the present data suggest that patients with CRC whose tumors have high TS and Bcl-2 demonstrate a significantly longer DFS and shorter WMS, as compared to patients with low expression of these markers.
Thymidylate synthase (TS) is reported to be a predictor of both survival in colorectal cancer (CRC) and response to 5-FU therapy, with higher levels being associated with poorer prognosis and response to therapy. Elevated levels of Bcl-2 protein also confer cytotoxic drug resistance to tumor cell lines. We here examined the expression of TS and oncoprotein Bcl-2 in advanced CRC patients, and determined their mutual relationship, association to therapeutic response and impact on disease outcome.
This study represents a translational study in which a clinical series of CRC samples were analyzed for two important molecular markers: TS and Bcl-2. Accordingly, this study represents a combined molecular analysis and a clinical study, whereby some key molecular pathways were analyzed and their relevance to clinical data was assessed in a series of 67 CRC patients with well-characterized treatment history and long-term follow-up data.
To the best of our knowledge, this is the first study to show the relationship between these two key molecules in a group of patients with locally advanced or metastatic CRC receiving similar treatment. As to the patient survival, there was a significant correlation of TS/Bcl-2 expression with DFS, in that the patients with high TS/Bcl-2 index had a longer DFS.
The present data suggest that patients with CRC whose tumors have high TS and Bcl-2 demonstrate a significantly longer DFS and shorter WMS, as compared to patients with low expression of these markers. This information should have potential clinical implications in the management of these patients.
The importance of this paper to the reader resides in the fact that this study is believed to be the first to suggest that CRC patients with low TS/Bcl-2 demonstrate a significantly shorter DFS, but keep alive longer with a metastatic disease. In relevant cases, one might consider utilizing this type of molecular marker data in more individualized tailoring of the appropriate therapies.
| 2. | Gonen M, Hummer A, Zervoudakis A, Sullivan D, Fong Y, Banerjee D, Klimstra D, Cordon-Cardo C, Bertino J, Kemeny N. Thymidylate synthase expression in hepatic tumors is a predictor of survival and progression in patients with resectable metastatic colorectal cancer. J Clin Oncol. 2003;21:406-412. |
| 3. | Suda Y, Kuwashima Y, Tanaka Y, Uchida K, Akazawa S. Immunohistochemical detection of thymidylate synthase in advanced gastric cancer: a prognostic indicator in patients undergoing gastrectomy followed by adjuvant chemotherapy with 5-fluoropyrimidines. Anticancer Res. 1999;19:805-810. |
| 4. | Suzuki M, Tsukagoshi S, Saga Y, Ohwada M, Sato I. Enhanced expression of thymidylate synthase may be of prognostic importance in advanced cervical cancer. Oncology. 1999;57:50-54. |
| 5. | Bronner MP, Culin C, Reed JC, Furth EE. The bcl-2 proto-oncogene and the gastrointestinal epithelial tumor progression model. Am J Pathol. 1995;146:20-26. |
| 6. | Sinicrope FA, Ruan SB, Cleary KR, Stephens LC, Lee JJ, Levin B. bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res. 1995;55:237-241. |
| 7. | Hockenbery D, Nunez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334-336. |
| 8. | Pidgeon GP, Barr MP, Harmey JH, Foley DA, Bouchier-Hayes DJ. Vascular endothelial growth factor (VEGF) upregulates BCL-2 and inhibits apoptosis in human and murine mammary adenocarcinoma cells. Br J Cancer. 2001;85:273-278. |
| 9. | Glimelius B, Ristamaki R, Kjaer M, Pfeiffer P, Skovsgaard T, Tveit KM, Linne T, Frodin JE, Boussard B, Oulid-Aessa D. Irinotecan combined with bolus 5-fluorouracil and folinic acid Nordic schedule as first-line therapy in advanced colorectal cancer. Ann Oncol. 2002;13:1868-1873. |
| 10. | Peters GJ, van der Wilt CL, van Groeningen CJ, Smid K, Meijer S, Pinedo HM. Thymidylate synthase inhibition after administration of fluorouracil with or without leucovorin in colon cancer patients: implications for treatment with fluorouracil. J Clin Oncol. 1994;12:2035-2042. |
| 11. | Johnston PG, Fisher ER, Rockette HE, Fisher B, Wolmark N, Drake JC, Chabner BA, Allegra CJ. The role of thymidylate synthase expression in prognosis and outcome of adjuvant chemotherapy in patients with rectal cancer. J Clin Oncol. 1994;12:2640-2647. |
| 12. | Lenz HJ, Hayashi K, Salonga D, Danenberg KD, Danenberg PV, Metzger R, Banerjee D, Bertino JR, Groshen S, Leichman LP. p53 point mutations and thymidylate synthase messenger RNA levels in disseminated colorectal cancer: an analysis of response and survival. Clin Cancer Res. 1998;4:1243-1250. |
| 13. | Yamachika T, Nakanishi H, Inada K, Tsukamoto T, Kato T, Fukushima M, Inoue M, Tatematsu M. A new prognostic factor for colorectal carcinoma, thymidylate synthase, and its therapeutic significance. Cancer. 1998;82:70-77. |
| 14. | Edler D, Hallstrom M, Johnston PG, Magnusson I, Ragnhammar P, Blomgren H. Thymidylate synthase expression: an independent prognostic factor for local recurrence, distant metastasis, disease-free and overall survival in rectal cancer. Clin Cancer Res. 2000;6:1378-1384. |
| 15. | Johnston PG, Lenz HJ, Leichman CG, Danenberg KD, Allegra CJ, Danenberg PV, Leichman L. Thymidylate synthase gene and protein expression correlate and are associated with response to 5-fluorouracil in human colorectal and gastric tumors. Cancer Res. 1995;55:1407-1412. |
| 16. | Leichman L, Lenz HJ, Leichman CG, Groshen S, Danenberg K, Baranda J, Spears CP, Boswell W, Silberman H, Ortega A. Quantitation of intratumoral thymidylate synthase expression predicts for resistance to protracted infusion of 5-fluorouracil and weekly leucovorin in disseminated colorectal cancers: preliminary report from an ongoing trial. Eur J Cancer. 1995;31A:1306-1310. |
| 17. | Edler D, Glimelius B, Hallstrom M, Jakobsen A, Johnston PG, Magnusson I, Ragnhammar P, Blomgren H. Thymidylate synthase expression in colorectal cancer: a prognostic and predictive marker of benefit from adjuvant fluorouracil-based chemotherapy. J Clin Oncol. 2002;20:1721-1728. |
| 18. | Shirota Y, Stoehlmacher J, Brabender J, Xiong YP, Uetake H, Danenberg KD, Groshen S, Tsao-Wei DD, Danenberg PV, Lenz HJ. ERCC1 and thymidylate synthase mRNA levels predict survival for colorectal cancer patients receiving combination oxaliplatin and fluorouracil chemotherapy. J Clin Oncol. 2001;19:4298-4304. |
| 19. | Miyashita T, Reed JC. bcl-2 gene transfer increases relative resistance of S49.1 and WEHI7.2 lymphoid cells to cell death and DNA fragmentation induced by glucocorticoids and multiple chemotherapeutic drugs. Cancer Res. 1992;52:5407-5411. |
| 20. | Minn AJ, Rudin CM, Boise LH, Thompson CB. Expression of bcl-xL can confer a multidrug resistance phenotype. Blood. 1995;86:1903-1910. |
| 21. | Walton MI, Whysong D, O’Connor PM, Hockenbery D, Korsmeyer SJ, Kohn KW. Constitutive expression of human Bcl-2 modulates nitrogen mustard and camptothecin induced apoptosis. Cancer Res. 1993;53:1853-1861. |
| 22. | Leahy DT, Mulcahy HE, O’Donoghue DP, Parfrey NA. bcl-2 protein expression is associated with better prognosis in colorectal cancer. Histopathology. 1999;35:360-367. |
| 23. | McDonnell TJ, Korsmeyer SJ. Progression from lymphoid hyperplasia to high-grade malignant lymphoma in mice transgenic for the t(14; 18). Nature. 1991;349:254-256. |
Peer reviewers: Shu Zheng, Professor, Scientific Director of Cancer Institute, Zhejiang University, Secondary Affiliated Hospital, Zhejiang University, 88# Jiefang Road, Hangzhou 310009, Zhejiang Province, China; Lars A Pahlman, Professor, Department of Surgery, Colorectal Unit, University Hospital, SE 751 85, Uppsala, Sweden; Damian Casadesus, MD, PhD, Calixto Garcia University Hospital, J and University, Vedado, Havana City, Cuba
S- Editor Zhong XY L- Editor Rippe RA E- Editor Ma WH