Published online Oct 28, 2008. doi: 10.3748/wjg.14.6154
Revised: July 21, 2008
Accepted: July 28, 2008
Published online: October 28, 2008
AIM: To investigate the usefulness of transient elastography by Fibroscan (FS), a rapid non-invasive technique to evaluate liver fibrosis, in the management of chronic hepatitis B virus (HBV) carriers.
METHODS: In 297 consecutive HBV carriers, we studied the correlation between liver stiffness (LS), stage of liver disease and other factors potentially influencing FS measurements. In 87 chronic hepatitis B (CHB) patients, we monitored the FS variations according to the spontaneous or treatment-induced variations of biochemical activity during follow-up.
RESULTS: FS values were 12.3 ± 3.3 kPa in acute hepatitis, 10.3 ± 8.8 kPa in chronic hepatitis, 4.3 ± 1.0 kPa in inactive carriers and 4.6 ± 1.2 kPa in blood donors. We identified the cut-offs of 7.5 and 11.8 kPa for the diagnosis of fibrosis ≥ S3 and cirrhosis respectively, showing 93.9% and 86.5% sensitivity, 88.5% and 96.3% specificity, 76.7% and 86.7% positive predictive value (PPV), 97.3% and 96.3% negative predictive value (NPV) and 90.1% and 94.2% diagnostic accuracy. At multivariate analysis in 171 untreated carriers, fibrosis stage (t = 13.187, P < 0.001), active vs inactive HBV infection (t = 6.437, P < 0.001), alanine aminotransferase (ALT) (t = 4.740, P < 0.001) and HBV-DNA levels (t = -2.046, P = 0.042) were independently associated with FS. Necroinflammation score (t = 2.158, > 10/18 vs≤ 10/18, P = 0.035) and ALT levels (t = 3.566, P = 0.001) were independently associated with LS in 83 untreated patients without cirrhosis and long-term biochemical remission (t = 4.662, P < 0.001) in 80 treated patients. During FS monitoring (mean follow-up 19.9 ± 7.1 mo) FS values paralleled those of ALT in patients with hepatitis exacerbation (with 1.2 to 4.4-fold increases in CHB patients) and showed a progressive decrease during antiviral therapy.
CONCLUSION: FS is a non-invasive tool to monitor liver disease in chronic HBV carriers, provided that the pattern of biochemical activity is taken into account. In the inactive carrier, it identifies non-HBV-related causes of liver damage and transient reactivations. In CHB patients, it may warrant a more appropriate timing of control liver biopsies.
- Citation: Oliveri F, Coco B, Ciccorossi P, Colombatto P, Romagnoli V, Cherubini B, Bonino F, Brunetto MR. Liver stiffness in the hepatitis B virus carrier: A non-invasive marker of liver disease influenced by the pattern of transaminases. World J Gastroenterol 2008; 14(40): 6154-6162
- URL: https://www.wjgnet.com/1007-9327/full/v14/i40/6154.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.6154
| Chronic HBV carriers (n = 268) | Untreated HBV carriers (n = 188) | Treated HBV carriers (n = 80) | |
| Age (yr) | 48.2 ± 12.2 | 46.0 ± 11.8 | 53.3 ± 11.8 |
| Male/Female | 180/88 | 120/68 | 60/20 |
| HBeAg/anti-HBe | 31/237 | 22/166 | 9/71 |
| Alcohol intake > 60 g/d | 23 (8.6) | 14 (7.4) | 9 (11.3) |
| Diabetes | 12 (4.5) | 7 (3.7) | 5 (6.3) |
| Hyperlipaemia | 38 (14.2) | 34 (18.1) | 4 (5.0) |
| BMI | |||
| 25-30 kg/m2 | 95 (35.4) | 67 (35.6) | 28 (35.0) |
| > 30 kg/m2 | 7 (2.6) | 6 (3.2) | 1 (1.3) |
| ALT > 300 IU/L | 11 (4.1) | 9 (4.8) | 3 (3.8) |
| HBV-DNA (Log10 IU/mL) | 4.70 ± 2.17 | 4.97 ± 2.17 | 4.06 ± 2.05 |
| Inactive carriers | 68 (25.4) | 68 (36.2) | - |
| CHB Ishak score | |||
| S0-S2 | 85 (31.7) | 71 (37.8) | 14 (17.5) |
| S3-S4 | 19 (7.1) | 12 (6.4) | 7 (8.7) |
| S5-S6 | 30 (11.2) | 14 (7.4) | 16 (20.0) |
| US cirrhosis | 66 (24.6) | 23 (12.2) | 43 (53.8) |
| n | Fibroscan values (kPa) | |
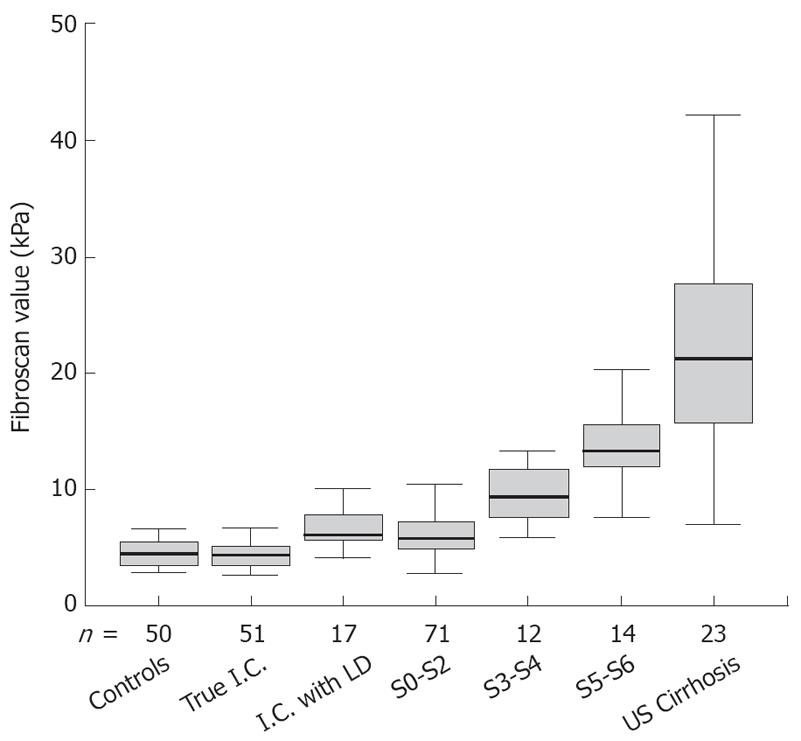
| Blood donors | 50 | 4.6 ± 1.2 |
| Acute Hepatitis1 | 9 | 12.3 ± 3.3 |
| Untreated HBsAg carriers overall1 | 188 | 8.9 ± 8.0 |
| Inactive carriers whithout LD2 | 51 | 4.3 ± 1.0 |
| Inactive carriers whit LD2 | 17 | 6.9 ± 2.3 |
| CHB S0-S2 | 71 | 6.4 ± 2.4 |
| CHB S3-S4 | 12 | 10.1 ± 3.8 |
| CHB S5-S6 | 14 | 15.7 ± 9.0 |
| US Cirrhosis3 | 23 | 23.6 ± 11.8 |
| Treated CHB overall1 | 80 | 13.4 ± 9.7 |
| CHB S0-S2 | 14 | 6.1 ± 1.7 |
| CHB S3-S4 | 7 | 8.5 ± 2.8 |
| CHB S5-S6 | 16 | 11.7 ± 5.2 |
| US Cirrhosis3 | 43 | 17.2 ± 11.4 |
| Variable | Univariate analysis | Multivariate analysis | |||
| P | B | 95% CI | P | ||
| Age | yr | 0.006 | NS | ||
| Sex | Male | 0.002 | NS | ||
| Phase of infection1 | CHB | < 0.001 | -9.939 | -12.989-6.889 | < 0.001 |
| Alcohol introduction | > 60 g/d | 0.076 | NS | ||
| Diabetes | Present | NS | |||
| Hyperlipemia | Present | 0.089 | NS | ||
| BMI | > 25 kg/m2 | < 0.001 | NS | ||
| ALT | Log10 IU/mL | < 0.001 | 5.713 | 3.333-8.094 | < 0.001 |
| Biochemical remission2 | Present | < 0.001 | NS | ||
| HBV-DNA serum levels | Log10 IU/mL | 0.051 | -0.470 | -0.924/-0.016 | 0.042 |
| Disease stage3 | Class | < 0.001 | 5.021 | 4.269-5.773 | < 0.001 |
| Variable | Univariate analysis | Multivariate analysis | |||
| P | B | 95% CI | P | ||
| Age | yr | NS | |||
| Sex | Male | 0.062 | NS | ||
| Alcohol introduction | >60 g/d | NS | |||
| Diabetes | Present | NS | |||
| Hyperlipemia | Present | NS | |||
| BMI | > 25 kg/m2 | NS | |||
| ALT | Log10 IU/mL | < 0.001 | 3.028 | 1.408-5.008 | 0.001 |
| Biochemical remission | Present | 0.017 | NS | ||
| HBV-DNA serum levels | Log10 IU/mL | 0.075 | NS | ||
| Necroinflammation score | ≥ 10/18 | < 0.001 | 1.611 | 0.117-3.104 | 0.035 |
| Disease stage1 | S3-S4 | < 0.001 | 3.054 | 1.318-4.789 | 0.001 |
| Variable | Univariate analysis | Multivariate analysis | |||
| P | B | 95% CI | P | ||
| Age | yr | NS | |||
| Sex | Male | NS | |||
| Alcohol introduction | > 60 g/d | NS | |||
| Diabetes | Present | NS | |||
| Hyperlipaemia | Present | NS | |||
| BMI | > 25 kg/m2 | NS | |||
| ALT | Log10 IU/mL | NS | |||
| Biochemical remission | Present | 0.001 | 8.705 | 5.277-12.133 | < 0.001 |
| HBV-DNA serum levels | Log10 IU/mL | NS | |||
| Disease stage1 | Class | < 0.001 | 4.374 | 2.982-5.766 | < 0.001 |
| Fibrosis stage≥S3 | Fibrosis S5-S6/US cirrhosis | |||
| IC + UT CHB | T CHB | IC + UT CHB | T CHB | |
| Sensitivity (%) | 93.9 | 78.8 | 86.5 | 54.2 |
| Specificity (%) | 88.5 | 71.4 | 96.3 | 90.5 |
| Positive predictive value (%) | 76.7 | 92.9 | 86.5 | 94.1 |
| Negative predictive value (%) | 97.3 | 41.7 | 96.3 | 41.3 |
| Diagnostic accuracy (%) | 90.1 | 77.5 | 94.2 | 63.8 |
| Likelihood ratio for pos. test | 8.18 | 2.76 | 23.18 | 5.69 |
| Likelihood ratio for neg. test | 0.07 | 0.30 | 0.14 | 0.51 |
Transient elastography by Fibroscan (FS)[1] has been proposed as a rapid, non-invasive technique to detect liver fibrosis[2], and many studies have confirmed its clinical usefulness, demonstrating good reproducibility and high correlation between FS and liver fibrosis at histology[3-7]. Nevertheless, liver stiffness (LS) is influenced by factors other than fibrosis, such as major variations of alanine aminotransferase (ALT) levels[8]. We showed that during hepatitis exacerbations, LS increased, paralleling the kinetics of ALT, whereas FS values were lower than expected according to the histological stage in patients with long-lasting (≥ 12 mo) ALT normalization[8]. Similar LS profiles have been reported in patients with acute viral hepatitis[9,10].
Thus, the biochemical status (ALT levels) of the patient has to be taken into account for an accurate interpretation of LS values in clinical practice. This might be highly relevant in chronic hepatitis B virus (HBV) infection where intervening phases of disease activity and remission and asymptomatic hepatitis reactivations are observed[11-14].
In order to assess the usefulness of FS in the clinical management of chronic HBV carriers, we studied prospectively LS and evaluated its variations according to the changes of the virological, biochemical and histological profiles of liver disease.
We studied 288 consecutive chronic HBV carriers (192 males, mean age 48.4 years, range 20-78 year) and nine patients with acute hepatitis B followed-up at the Hepatology Unit of the University Hospital of Pisa, Regional Reference Center for Chronic Liver Disease and Hepatocellular Carcinoma. The study was approved by the Ethical Committee of the hospital and patients gave their written informed consent.
HBV carriers were classified, after a monthly follow-up of at least 12 mo, as inactive or active according to their virological profile. Inactive carriers had serum HBV DNA persistently < 105 copies/mL (by COBAS Amplicor HBV Monitor, Roche, Basel, Switzerland) and IgM anti-HBc levels < 0.200 (by Core-M™ Axsym System, Abbott, Sligo, Ireland). Chronic hepatitis patients showed the presence of active viral replication (serum HBV-DNA levels persistently or intermittently ≥ 105 copies/mL during the follow-up), IgM anti-HBc ≥ 0.200 and liver histology consistent with chronic hepatitis. Exclusion criteria: hepatitis D virus (HDV) or hepatitis C virus (HCV) coinfections, Child B or C cirrhosis.
We studied the correlation between LS and the stage of liver disease with single point FS measurements in 297 HBV carriers (288 with chronic infection: 208 untreated and 80 treated; nine with acute hepatitis B) and 50 blood donors as controls. Transient elastography was performed within 6 mo (median 3 mo, 75% of cases between 0 and 4.6 mo) from liver biopsy in 157 patients with biochemical and/or virological signs of liver disease and 21 inactive carriers. In 47 HBV carriers with inactive infection, 63 patients with cirrhosis (with previous histological diagnosis and actual ultrasonographic signs of cirrhosis) and nine patients with acute hepatitis, who did not undergo liver biopsies, FS was performed within 1 wk from US and Doppler examinations of the liver.
To study the correlation between LS and spontaneous or treatment-induced variations of biochemical activity, we enrolled 87 patients who underwent monthly blood controls and FS measurements at least every 6 mo. In case of ALT flares (ALT values ≥ 300 IU/L with increments of at least 2 SD above previous values), patients were monitored with blood and FS test every 2 wk for the first month and monthly thereafter until flare resolution. Transaminases and virological markers (HBV DNA and IgM anti-HBc) were tested on the same day of FS measurements. In treated patients, FS was monitored every 3 mo.
Transient elastography was measured by Fibroscan (EchoSens, Paris, France). All measures were performed by trained physicians on the right liver lobe through intercostal spaces in the patient lying on his back, with right arm in maximal abduction. The US guide was used to identify a target liver area, at least 6 cm thick, without major vascular structures. The procedure was considered valid if at least 10 validated measurements were performed, with a success rate (ratio between numbers of validated and total measurements) ≥ 60% and interquartile range (IQR) < 20%. LS was recorded in kPa as the median value of all measurements.
Liver biopsies were obtained using 16 G disposable needles (Hepafix B; Braun, Melsungen, Germany). Liver specimens (median 27 mm, range 11-50 mm) were stained with hematoxylin and eosin. Necro-inflammatory activity and liver fibrosis were scored according to Ishak[15]. Steatosis was graded semiquantitatively, as reported previously[4]. Patients in whom liver biopsy yielded specimens shorter than 15 mm and/or with less than 11 portal tracts were excluded from the analysis.
The included variables were sex, age, virological profile (HBeAg/anti-HBe status), liver disease co-factors [alcohol intake (≤ 60 or > 60 g/d), iron overload (present, in case of staining at histology and serum iron > 150 g/L and/or ferritin > 400 μg/L), hyperlipemia (cholesterol > 240 mg/dL and/or triglycerides > 250 mg/dL), diabetes (fasting plasma glucose > 140 mg/dL), overweight [body mass index (BMI) > 25 kg/m2]. The biochemical profiles were defined as: (1) persistently elevated ALT; (2) biochemical remission (persistently normal ALT for at least 12 mo, at monthly controls); (3) ALT flares (when ALT values increased ≥ 300 IU/L, with increments of at least 2 SD above previous values). Virological profiles included HBV-DNA and IgM anti-HBc levels. Liver biopsy features were: length; number of fragments; portal tracts number; necro-inflammation, fibrosis and steatosis scores. Cirrhosis at ultrasound (US cirrhosis) was defined when enlargement of left/caudate lobes, nodular liver boundaries, and micro-macronodular liver structure were present. We recorded in addition: the signs of portal hypertension (portal vein diameter > 12 mm; spleen volume > 45 cm2; esophagus or gastric varices); the transient elastography performance (values, rate of successful measurements and IQRs); the characteristics of therapy (schedule, dose, duration and response).
Data are expressed as mean ± SD. The logarithmic transformation was used for quantitative data when their distributions were not normal. The Pearson’s correlation coefficient was used to analyze the correlations between values of liver elastometry and fibrosis. Differences between subgroups were analysed using one-way ANOVA, Mann-Whitney rank sum test or Kruskal-Wallis test when appropriate. To identify factors independently correlated with LS, variables with statistical associations (P < 0.05) or trends (P < 0.10) at univariate analysis were included in multiple regression analyses. The diagnostic performance of transient elastography was evaluated by receiver operating characteristic (ROC) curve. By using the cut-off values with the highest sensitivity + specificity sum, we defined two different cut-off values of liver transient elastography to identify patients with significant fibrosis (Ishak score ≥ 3/6) or cirrhosis. Statistical analysis was performed by SPSS (version 10.0, SPSS Inc., Chicago, IL, USA) software package.
Overall 277 of 297 (93.3%) HBV carriers were suitable for the analysis: nine had acute hepatitis, 68 inactive infection, and the remaining 200 had chronic hepatitis. Six patients (2.1%) were excluded because their liver biopsies were < 1.5 cm and 14 (4.9%) because their elastographic measures failed (seven cases had BMI > 28). Eighty patients were under treatment [61 nucleos(t)ides, NA; 19 interferon, IFN]. Demographic and clinical characteristics of the 268 chronic carriers are reported in Table 1.
FS values were 4.6 ± 1.2 kPa in 50 blood donors, 12.3 ± 3.3 kPa in nine patients with acute hepatitis and 10.3 ± 8.8 kPa in 268 chronic HBV carriers (P < 0.001) (Table 2).
In 68 inactive carriers, the mean FS value was 5.0 ± 1.8 kPa. Seventeen of them had abnormal ALT and at histology showed steatohepatitis or steatosis. Their mean LS values were significantly higher as compared to HBV carriers with normal ALT and without dysmetabolic profile (6.9 ± 2.3 kPa vs 4.3 ± 1.0 kPa, P < 0.001) (Figure 1). As a result of chronic liver damage caused by factors other than HBV, these 17 inactive carriers were excluded from further analysis.
In the 171 untreated chronic HBV carriers, LS correlated significantly with fibrosis stage (r = 0.706, P < 0.001). At univariate analysis, in the 171 untreated HBV carriers, LS significantly correlated with age, sex, phase of infection (inactive vs active), BMI, ALT levels, biochemical remission and fibrosis stage, showing a correlation trend for HBV-DNA levels, alcohol intake and hyperlipemia (Table 3). At multivariate analysis, the phase of HBV infection (P < 0.001), ALT levels (P < 0.001), HBV-DNA levels (P = 0.042) and fibrosis stage (P < 0.001) were independently associated with LS (Table 3). In the separate analysis of the 83 untreated patients with chronic hepatitis, but without cirrhosis, the factors independently associated with LS were ALT levels (P = 0.001), fibrosis stage (S3-S4 vs S0-S2, P = 0.001) and necroinflammation score (≥ 10/18 vs < 10/18; P = 0.035) (Table 4).
In 80 treated patients, LS correlated with fibrosis stage (r = 0.453, P < 0.001), but the mean values were lower than untreated patients with a comparable stage of fibrosis (6.1 vs 6.4 kPa in S0-S2 patients; 8.5 vs 10.1 kPa in S3-S4 patients; 11.7 vs 15.7 kPa in S5-S6 patients; 17.2 vs 23.6 kPa in US cirrhosis patients) (Table 2), and the difference reached the statistical significance in patients with US cirrhosis only (P = 0.035). Fifty of them were under long-term NA treatment and in long-term biochemical remission, which was independently associated with FS values (P < 0.001, Table 5).
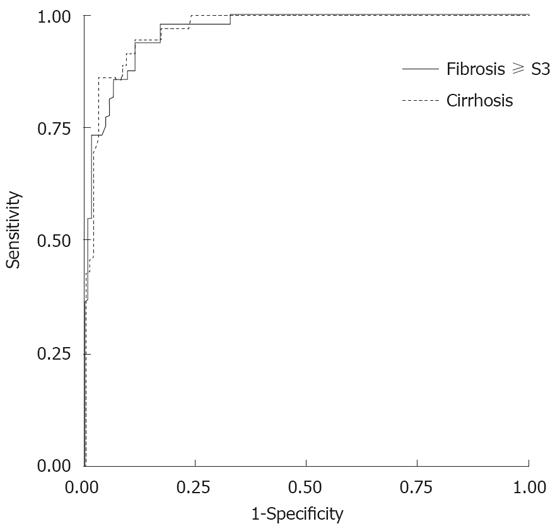
To identify the FS cut-offs for fibrosis ≥ S3 and cirrhosis, we analyzed untreated patients only. Area under ROC curve (AUROCs) for fibrosis ≥ S3 and cirrhosis were 0.966 and 0.973 (95% CI 0.942-0.989 and 0.952-0.994) (Figure 2) and their cut-off values were 7.5 and 11.8 kPa, respectively.
Fibrosis≥S3: The diagnostic performance of 7.5 kPa cut-off is reported in Table 6. Overall, 46 of 60 patients with elastography ≥ 7.5 kPa had fibrosis ≥ S3 (76.7% PPV) and 108 of 111 patients with FS < 7.5 kPa had S0-S2 fibrosis (97.3% NPV). Among the 14 patients with FS ≥ 7.5 kPa, but a fibrosis stage < S3, five patients had ALT levels > 300 UI/L at the time of FS measurement. None of the three patients with FS < 7.5 but fibrosis ≥ S3 had cirrhosis: one had S3 and two had S4 fibrosis at liver histology.
Cirrhosis: The diagnostic performance of 11.8 kPa cut-off is shown in Table 6. Thirty-two of 37 patients with elasticity ≥ 11.8 kPa had histological or US cirrhosis (86.5% PPV); 129 of 134 patients with FS values < 11.8 kPa did not have cirrhosis (96.3% NPV). All but one of the five non-cirrhotic (two with S3 and three with S4 fibrosis stage) patients with FS values ≥ 11.8 showed LS values ranging from 11.8 and 13.3 kPa; the remaining patient with 20 kPa FS value had S4 fibrosis and ALT levels > 300 UI/L at the time of FS measurement. Two of five cirrhotic patients with low FS (7.0 and 7.6 kPa respectively) were in prolonged spontaneous remission; the remaining three had elastometry values ranging between 8.9 kPa and 11.3 kPa.
In 87 patients, LS was monitored for a mean period of 19.9 ± 7.1 mo (range 6-36 mo): Seventy eight patients had chronic hepatitis (43 untreated and 35 treated) and nine had acute hepatitis. All patients underwent at least three FS measurements (mean 5.6, range 3-10).
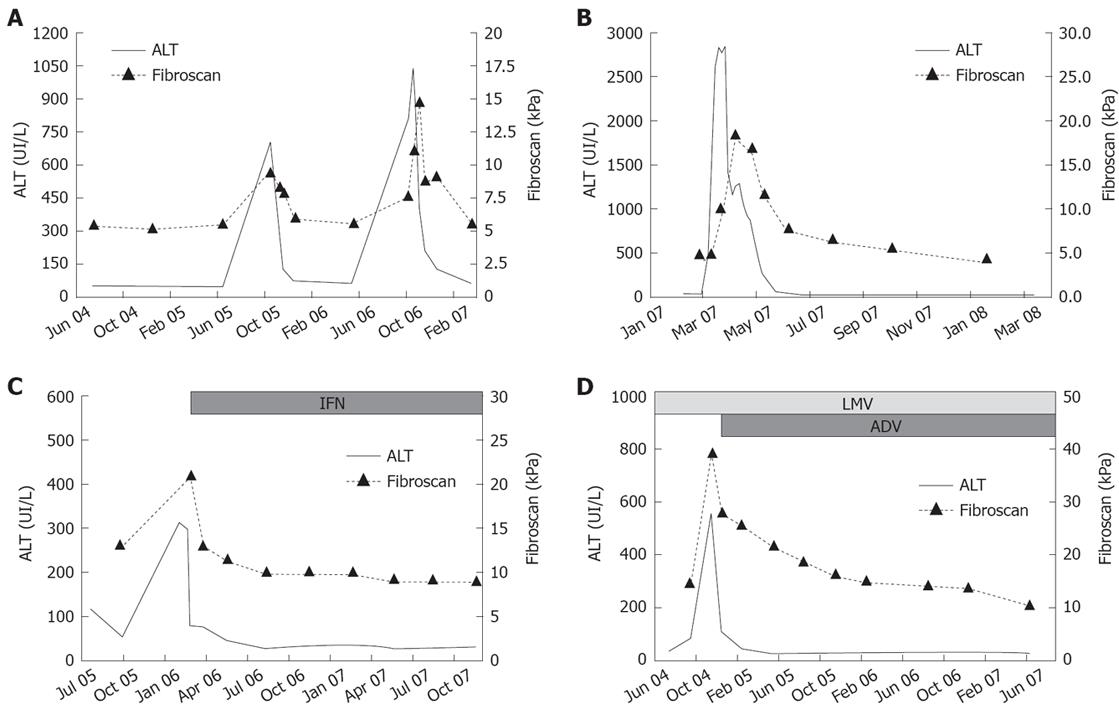
Untreated patients: Thirty patients showed stable biochemical and virological profiles without disease progression: their LS did not change, showing minor fluctuations (12 mo/baseline FS mean ratio 1.00 ± 0.20; 24 mo/baseline FS mean ratio 0.99 ± 0.26). The remaining 13 patients experienced hepatitis flares. During flares, FS values increased 1.2 to 4.4-fold as compared to baseline values (mean variation 2.1 ± 1.0-fold), mean FS value during flares being 20.7 ± 12.3 kPa (range 8.6-42 kPa). LS variations paralleled the dynamic profiles of ALT: FS values reached the peak simultaneously with ALT in eight patients (61.5%), later, with 15-30 d of delay, in the remaining five (38.5%). Thereafter, FS values decreased with a latency of 30 d from the initial ALT decrease and returned to baseline values within 3 to 6 mo (Figure 3A). Patients with disease profiles characterised by ALT flares intervened by complete biochemical remission showed major variations of FS values during their hepatitis exacerbations, as compared to patients with persistent ALT elevations between flares (FS variation ranging from 1.4 to 4.4 in the former and from 1.2 to 1.6-fold in the letters, P = 0.019).
Acute hepatitis: In nine patients with acute hepatitis B, FS values at presentation ranged from 8.2 to 16.6 (mean 12.3 ± 3.3) kPa and reached a peak of 11.8 to 45.7 kPa (20.0 ± 11.6 kPa) at the time of ALT peak. They then declined progressively to 5.6 ± 1.1 (range 4.4-6.9) kPa, in association with the reduction of ALT levels (Figure 3B).
Treated patients: The FS monitoring started with treatment in 18 patients, when treatment was already ongoing in the remaining 13. Overall, FS values showed progressive declines during therapy, with a mean on-treatment/baseline ratio of 0.9 ± 0.4 at 6 mo and 0.7 ± 0.2 at 12 mo. In patients with persistent response to long-term nucleoside/nucleotide analogues treatment, FS values decreased progressively during their follow up, with mean yearly reduction (ratio between two consecutive FS values registered at 12 mo intervals) of 0.8 ± 0.2 at 24 mo, 0.8 ± 0.1 at 36 mo and 0.7 ± 0.1 at 48 mo from the beginning of therapy.
All responders showed decreased FS values during therapy (Figure 3C and D): 0.8 ± 0.2 at 6 mo and 0.6 ± 0.2 at 12 mo, as compared to baseline values, respectively. FS value declines were similar in responders to IFN as compared to responders to NA: 0.7 ± 0.2 vs 0.8 ± 0.2 6 mo/baseline ratio and 0.6 ± 0.1 vs 0.7 ± 0.2 12 mo/baseline ratio, respectively. Two non-responder HBeAg-positive patients showed an increase of 1.1 and 2.4 times the FS values between 3 and 6 mo, during hepatitis flares, followed by a progressive decline that reached baseline values after 12 mo.
Transient elastography[1] is an easy to perform, reproducible method for the rapid and objective evaluation of LS in clinical practice[2,16] and it is proposed as a reliable, non-invasive, surrogate marker of fibrosis[3-7,17,18]. In fact, LS is a physical parameter that correlates primarily with fibrosis, but it is influenced also by other factors that modify the elasticity of the liver, such as significant variations of inflammatory infiltrate, edema and vascular congestion of the liver[8-10,19]. Accordingly, we showed that LS variations parallel ALT values during hepatitis exacerbations in the setting of both acute and chronic liver damage[8]. This evidence has important implications in clinical practice since the interpretation of the LS measure has to take into account the concurrent biochemical profile of the patient[8]. Thus, the interpretation of LS might be more difficult in the setting of CHB when major fluctuations of necrosis and inflammatory activity occur in a significant proportion of patients[11,12,18,19]. On the other hand, the availability of an easy to perform, non-invasive measure for fibrosis might improve the management of the HBV carrier. In the HBV carrier, the repeated measures of LS might help to identify the candidates for liver biopsy and to define both the phase of HBV infection and stage of liver disease that are mandatory to warrant the most appropriate treatment strategy, and to monitor liver disease progression in the single patient[20,21].
Addressing the issue of the clinical usefulness of LS in the management of the HBV carrier we found a highly significant correlation between transient elastography and fibrosis stages (P < 0.001). Using 7.5 and 11.8 kPa as cut-off values for fibrosis ≥ S3 and cirrhosis, the FS specificities were 88.5% and 96.3%, sensitivities 93.9% and 86.5%, and diagnostic accuracies 90.1% and 94.2%. These results confirm that FS is a reliable method to assess fibrosis in carriers with chronic HBV infection and disease[8].
Additional factors were independently associated with FS, such as active HBV infection (P < 0.001), HBV-DNA (P = 0.042) and ALT (P < 0.001) levels. In inactive HBV carriers, mean FS values were similar to normal controls and significantly lower than in CHB patients (4.3 ± 1.0 vs 4.6 ± 1.2 vs 11.2 ± 9 kPa; P < 0.001). These findings qualify LS as a promising tool to provide an important diagnostic assessment of the HBV carrier with inactive viral profile when the increased FS values suggest the presence of liver damage caused by factors other than HBV. In such cases, liver biopsy can be proposed for the precise characterization of liver disease. Indeed, in our study, 17 inactive carriers with metabolic liver disease had FS values higher (6.9 ± 2.3 kPa) than inactive carriers without liver disease.
In addition to the phase of HBV infection, only two other parameters, namely HBV DNA and ALT, were independently correlated with LS. Since both these parameters are linked with the extent of liver disease activity in the immune competent HBV carrier, our results further support the hypothesis that the extent of necrosis and inflammation influence LS significantly[20,22-24]. Accordingly, in the 83 untreated patients without cirrhosis, multivariate analysis showed that intra-hepatic necrosis and inflammation scores and ALT values were the only factors influencing FS (P = 0.035 and P < 0.001 respectively), in addition to the stage of liver disease.
The LS values identified as cut-offs for histological stage ≥ S3 and cirrhosis are lower than those proposed for chronic hepatitis C. A slight variable difference between cut-offs would not be surprising when different cohorts of patients are compared, but FS values in CHB patients with cirrhosis (11.8 kPa) are consistently and persistently lower than in chronic hepatitis C (CHC) cirrhosis[4-6,8,16]. Accordingly, lower values of LS cut-offs have been proposed in preliminary reports[25,26]. These findings are consistent with the specific features of histopathology of hepatitis C, in which the combination of portal lymphoid follicles, bile duct damage, lobular activity and steatosis may contribute to the different LS, as compared to hepatitis B histopathology[27].
In this prospective study of CHB patients, we observed 1.2 to 4.4-fold increases of FS values with ALT flares, and similarly, LS values fluctuated in parallel with ALT values in nine patients with acute hepatitis. Interestingly, the extent of FS fluctuations during the hepatitis exacerbations differed according to the biochemical patterns of CHB. The range of LS variations were significantly wider in patients with ALT flares intervened by complete biochemical remissions, as compared to patients with persistent ALT elevations between flares (FS variations ranged from 1.4 to 4.4-fold in the former and 1.2 to 1.6-fold in the latter group, P = 0.019). Altogether these findings confirm our original observation and other more recent reports on the major influence of the biochemical profile on LS in the setting of both acute and chronic liver damage[8-10]. Finally, we found that prolonged biochemical remissions were associated with progressive reductions of FS values. LS declined yearly at about 0.2-fold in treated patients followed-up prospectively for 48 mo, and a proportion of patients who maintained evidence of cirrhosis at US achieved values of FS < 11.8 kPa. This was responsible for the worse diagnostic performance of FS in treated patients in whom the sensitivity for detecting cirrhosis fell from 86.5% to 54.2% in untreated vs treated patients with fibrosis ≥ S5 (Table 6). Altogether, these data suggest a non-linear correlation between the overall kinetics of LS and histological staging during antiviral treatment. Future studies should be addressed to understand the relations among the reductions of LS, necrosis, inflammation and fibrosis in the separate settings of different fibrosis stages (i.e. ≥ S3/< S3 and presence/absence of cirrhosis) and liver disease etiology (i.e. HBV and HCV). In fact, much of the LS changes depend on the different elastic relations among fine blocks of the liver structure. Thus, the interplay between the extent and structure of the collagen septa within the fine liver block, and the different type and extent of liver inflammatory infiltrate within them, might account for both the different FS cut-offs between CHB and CHC patients and for the different kinetics of FS and fibrosis decline during antiviral therapy.
In conclusion, our study suggests that the LS provides a useful non-invasive tool to monitor liver disease in the chronic HBV carrier. In the inactive carrier, it helps to identify non-HBV-related causes of liver damage and transient reactivation of HBV liver disease. In the CHB patient, provided that the pattern of biochemical activity is taken into account, LS values < 7.5 exclude the presence of significant fibrosis (≥ S3) with a high NPV (97.3%) and low negative likelihood ratio (0.07). FS values ≥ 11.8 kPa are highly specific (96.3%) for cirrhosis and show good PPV (86.5%) and positive likelihood ratio (23.18). In the HBV carrier with LS values ranging from 7.5 to 11.8 kPa, which are indicative of significant liver disease, liver biopsy remains the gold standard for an accurate grading and staging of liver disease. Finally, in CHB patients the monitoring of LS appears useful to highlight major changes in intrahepatic liver disease and warrants a more appropriate timing for control liver biopsies.
The old measure of liver stiffness (LS) by hand palpation has had a new appraisal after the recent introduction of the objective measure of the speed of transmission of an elastic wave across the liver (transient elastography) registered by the new instrument Fibroscan® (EchoSens, Paris, France). Originally, the new technique was proposed in clinical practice as a non-invasive, surrogate marker of fibrosis and many studies demonstrated good reproducibility and a high correlation between LS and liver fibrosis at histology. However, liver elasticity is influenced not only by fibrosis, but also by the presence and extent of liquid, lipid and inflammatory infiltrates within the liver. The evidence that Fibroscan (FS) is significantly influenced by major variations of liver inflammation (as we previously showed), in addition to variations of staging, prompted the new frontier of testing FS values in the management of patients with chronic hepatitis.
The course of liver disease in a significant proportion of chronic hepatitis B (CHB) patients is characterized by hepatitis exacerbations, intervened by prolonged remissions whose biochemical and virologic patterns can be mistaken with those of chronic inactive carriers. Thus, measuring LS might be useful to distinguish active from inactive HBV carriers. We addressed this question and present here the results of the cross-sectional and prospective studies of a large cohort of pedigreed hepatitis B virus (HBV) carriers (68 inactive carriers, 200 CHB and nine acute hepatitis B patients).
FS correlates with fibrosis in CHB patients and FS provides a reliable method to assess the overall status of liver disease in the carrier with chronic HBV infection. The mean FS values of HBV-inactive carriers were comparable to those of normal controls and significantly lower than those of CHB patients. Interestingly, in HBV inactive carriers with metabolic liver disease FS values were significantly higher than in HBV-inactive carriers without liver disease. All factors stemming for activity of liver disease, namely the phase of infection (active or inactive), HBV-DNA and ALT levels influenced LS at multivariate analysis. Accordingly, in untreated patients without cirrhosis, histological necrosis and inflammation and ALT were the only factors influencing FS in addition to fibrosis. Thus both necrosis and inflammation influence LS that qualifies as a very promising tool for the non-invasive diagnostic assessment of the liver in the HBV carrier. The best cut-off values for fibrosis and cirrhosis were significantly lower than in chronic hepatitis C (CHC) patients, studied in identical conditions (same center, instrument, operators and test timing), suggesting that FS is influenced also by the different histopathology features of CHB and CHC. This prospective study on patients with hepatitis B exacerbations confirmed 1.2 to 4.4-fold increases of FS values at the time of ALT flares. Similarly, LS paralleled ALT fluctuations in patients with acute hepatitis B. Finally, in treated patients followed up for 48 mo, LS declined yearly at about 0.2-folds, reaching values below the cirrhosis cut-off (11.8 kPa) in a proportion of patients who maintained evidence of cirrhosis. This observation may explain the worse diagnostic performance of FS in treated versus untreated patients. Altogether, these data indicate the non-linear correlation between the kinetics of LS and histological staging during antiviral treatment.
This study suggests that LS provides a useful non-invasive tool to monitor not only fibrosis, but overall liver disease in the chronic HBV carrier. In monitoring CHB patients, LS appears useful to highlight major changes of liver disease and to warrant a more appropriate timing for control liver biopsies.
HBV-inactive carriers mean chronic HBV infection without liver damage caused by HBV, characterized by low HBV-DNA serum levels, persistently normal ALT and undetectable levels of IgM anti-HBc, a marker of HBV-induced liver disease (below the cut-off for CHB). Biochemical remission means transient ALT normalization (spontaneous or induced by antiviral treatment) in patients with CHB.
In this study, authors perform a cross-sectional and longitudinal analysis of LS in HBV carriers, correlating this variable with stage of disease, liver inflammation and other factors that could influence FS measurements. They found a good diagnostic accuracy to detect cirrhosis and fibrosis higher than S3. The work is well performed and conclusions are correctly sustained.
| 1. | Sandrin L, Tanter M, Gennisson JL, Catheline S, Fink M. Shear elasticity probe for soft tissues with 1-D transient elastography. IEEE Trans Ultrason Ferroelectr Freq Control. 2002;49:436-446. |
| 2. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. |
| 3. | Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Ledinghen V, Marcellin P, Dhumeaux D, Trinchet JC. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48-54. |
| 4. | Castera L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Ledinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. |
| 5. | Foucher J, Chanteloup E, Vergniol J, Castera L, Le Bail B, Adhoute X, Bertet J, Couzigou P, de Ledinghen V. Diagnosis of cirrhosis by transient elastography (FibroScan): a prospective study. Gut. 2006;55:403-408. |
| 6. | Colletta C, Smirne C, Fabris C, Toniutto P, Rapetti R, Minisini R, Pirisi M. Value of two noninvasive methods to detect progression of fibrosis among HCV carriers with normal aminotransferases. Hepatology. 2005;42:838-845. |
| 7. | Ganne-Carrie N, Ziol M, de Ledinghen V, Douvin C, Marcellin P, Castera L, Dhumeaux D, Trinchet JC, Beaugrand M. Accuracy of liver stiffness measurement for the diagnosis of cirrhosis in patients with chronic liver diseases. Hepatology. 2006;44:1511-1517. |
| 8. | Coco B, Oliveri F, Maina AM, Ciccorossi P, Sacco R, Colombatto P, Bonino F, Brunetto MR. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007;14:360-369. |
| 9. | Arena U, Vizzutti F, Corti G, Ambu S, Stasi C, Bresci S, Moscarella S, Boddi V, Petrarca A, Laffi G. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47:380-384. |
| 10. | Sagir A, Erhardt A, Schmitt M, Haussinger D. Transient elastography is unreliable for detection of cirrhosis in patients with acute liver damage. Hepatology. 2008;47:592-595. |
| 11. | Brunetto MR, Oliveri F, Coco B, Leandro G, Colombatto P, Gorin JM, Bonino F. Outcome of anti-HBe positive chronic hepatitis B in alpha-interferon treated and untreated patients: a long term cohort study. J Hepatol. 2002;36:263-270. |
| 12. | Hadziyannis SJ, Papatheodoridis GV. Hepatitis B e antigen-negative chronic hepatitis B: natural history and treatment. Semin Liver Dis. 2006;26:130-141. |
| 13. | Yuen MF, Yuan HJ, Hui CK, Wong DK, Wong WM, Chan AO, Wong BC, Lai CL. A large population study of spontaneous HBeAg seroconversion and acute exacerbation of chronic hepatitis B infection: implications for antiviral therapy. Gut. 2003;52:416-419. |
| 14. | Liaw YF. Hepatitis flares and hepatitis B e antigen seroconversion: implication in anti-hepatitis B virus therapy. J Gastroenterol Hepatol. 2003;18:246-252. |
| 15. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. |
| 16. | Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, Colombo M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968-973. |
| 17. | de Ledinghen V, Douvin C, Kettaneh A, Ziol M, Roulot D, Marcellin P, Dhumeaux D, Beaugrand M. Diagnosis of hepatic fibrosis and cirrhosis by transient elastography in HIV/hepatitis C virus-coinfected patients. J Acquir Immune Defic Syndr. 2006;41:175-179. |
| 18. | Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835-847. |
| 19. | Roulot D, Czernichow S, Le Clesiau H, Costes JL, Vergnaud AC, Beaugrand M. Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol. 2008;48:606-613. |
| 20. | Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507-539. |
| 21. | The EASL Jury. EASL International Consensus Conference on Hepatitis B: 13-14 September, 2002 Geneva, Switzerland. Consensus statement (Long version). J Hepatol. 2003;39:3-25. |
| 22. | van Nunen AB, Hansen BE, Suh DJ, Lohr HF, Chemello L, Fontaine H, Heathcote J, Song BC, Janssen HL, de Man RA. Durability of HBeAg seroconversion following antiviral therapy for chronic hepatitis B: relation to type of therapy and pretreatment serum hepatitis B virus DNA and alanine aminotransferase. Gut. 2003;52:420-424. |
| 23. | Manesis EK, Papatheodoridis GV, Sevastianos V, Cholongitas E, Papaioannou C, Hadziyannis SJ. Significance of hepatitis B viremia levels determined by a quantitative polymerase chain reaction assay in patients with hepatitis B e antigen-negative chronic hepatitis B virus infection. Am J Gastroenterol. 2003;98:2261-2267. |
| 24. | Feld JJ, Ayers M, El-Ashry D, Mazzulli T, Tellier R, Heathcote EJ. Hepatitis B virus DNA prediction rules for hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2007;46:1057-1070. |
| 25. | Marcellin P, de Ledinghen V, Dhumeaux D, Poupon R, Ziol M, Bedossa P. Non-invasive assessment of liver fibrosis in chronic hepatitis B using FibroScan (Abstract). Hepatology. 2005;42:715A. |
| 26. | Castera L, Foucher J, Bernard P, Bertet J, Couzigou P, de Ledinghen V. Transient elastography (FibroScan) and FibroTest to assess liver fibrosis in inactive hepatitis B carriers: a prospective controlled study (Abstract). Hepatology. 2006;44:489A. |
| 27. | Scheuer PJ, Davies SE, Dhillon AP. Histopathological aspects of viral hepatitis. J Viral Hepat. 1996;3:277-283. |
Peer reviewer: Juan Ramón Larrubia, PhD, Gastroenterology Unit and Liver Research Unit, Guadalajara University Hospital, University of Alcalá, Guadalajara 19002, Spain
S- Editor Li DL L- Editor Roberts SE E- Editor Lin YP