Published online Jan 28, 2008. doi: 10.3748/wjg.14.647
Revised: October 12, 2007
Published online: January 28, 2008
This paper described a rare case of adenomyoma of common bile duct. The case is a 51-year-old man who was hospitalized for yellow color skin and sclera and itching for 2 mo without abdominal pain. Nothing special was found in physical examination except yellowish skin and sclera. The clinical presentation and Contrast-enhanced magnetic resonance imaging (MRI), Magnetic resonance cholangiopancreatography (MRCP), and ultrasonography suspected a tumor of the distal bile duct. The patient was treated successfully by pancreaticoduodenectomy. Histologically, the lesion consisted of adenoid and myofibrous tissue and moderate atypia. The immunophenotype of the epithelial component was cytokeratin 7+/cytokeratin 20-. The patient has been well without any evidence of recurrence for 12 mo since his operation.
- Citation: Shu GM, Wang YJ, Du Z, Li DY, Liu CL. Bile tract adenomyoma: A case report. World J Gastroenterol 2008; 14(4): 647-650
- URL: https://www.wjgnet.com/1007-9327/full/v14/i4/647.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.647
The benign biliary tract tumor is rarely found in clinical practice. It is only 0.1% among the total biliary tract operations and 6% of extrahepatic biliary tract tumors reported at home and abroad. Till the year 2000, less than 200 cases of benign biliary tract tumor had been reported in English literatures[1], including polyps, adenomatoid papilloma and adenoma, while adenomyoma was seldem found. One patient with biliary tract adenomyoma was admitted to our hospital and reported below.
The patient is a 51-year-old man admitted to our hospital in August 2005, who was presented with yellow color skin and sclera and complained of itching for 2 mo. He suffered from anorexia and occasional nausea and deep tea color urine, yellowish white feces and weight loss of 2.5 kg were found, but without vomiting, chill, fever, abdominal pain and distension. Nothing special was found in physical examination except yellowish skin and sclera.
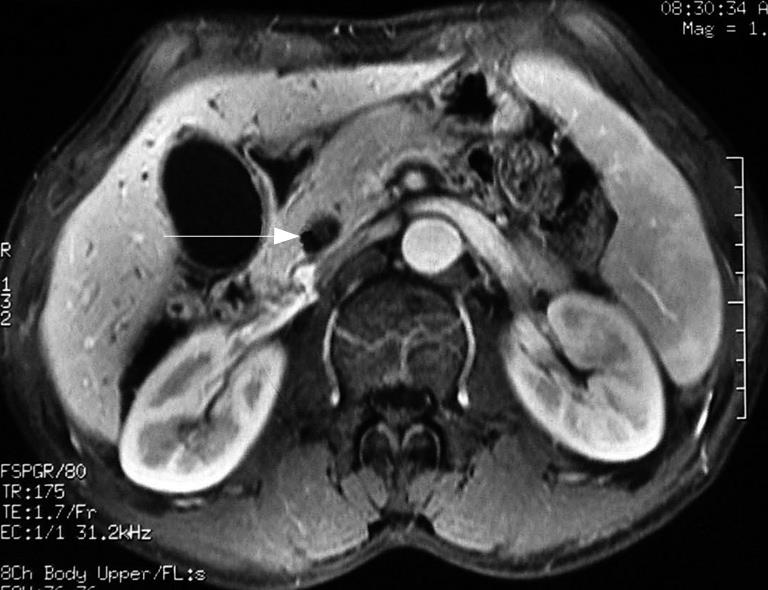
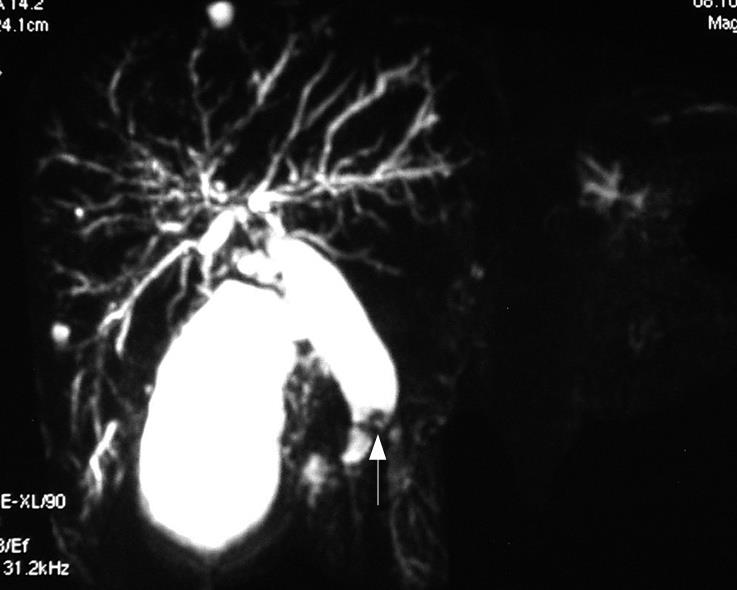
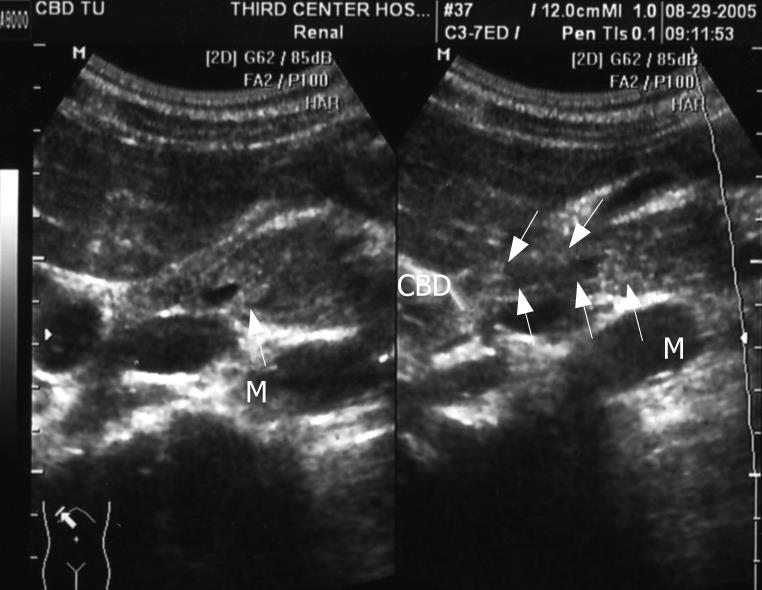
On admission, blood biochemical examination showed: alanine aminotransferase (ALT) 250 U/L, aspartate aminotransferase (AST) 102 U/L, alkaline phosphatase (ALP) 641 U/L, γ-glutamyltranspeptidase (γ-GT) 854 U/L, total bilirubin 32.9 &mgr;mol/L; direct bilirubin 22.8 &mgr;mol/L; Glucose 4.29 mmol/L, blood urea nitrogen (BUN) 6.56 mmol/L, creatinine (Cr) 67 &mgr;mol/L, carbohydrate antigen19-9 42.9 U/mL; carbohydrate antigen-50 57.86 U/mL; carcinoembryonic antigen (CEA) 6.8 ng/mL and γ-GT isoenzyme(-). α-fetal protein (AFP) 6.21 ng/mL, AFU 563 &mgr;mol/L.H, ferritin (Fer) 500 &mgr;g/L. Contrast-enhanced MRI revealed dilatation of the bile duct and pancreatic duct, eccentric stricture of the distal bile duct and multiple cysts of liver (Figure 1). Magnetic resonance cholangiopancreatograph (MRCP) showed dilatation of the bile duct and pancreas duct, irregular stricture of the bile duct (Figure 2). Ultrasonography showed distension of intrahepatic bile duct (0.32 cm), increase of gallbladder volume (11.1 cm × 4.4 cm) with smooth wall (0.3 cm thick), distension of common bile duct (1.27 cm) but stricture (0.4 cm) at pancreatic segment and a mass (1.6 cm × 0.27 cm) at the lumen of this segment (Figure 3).
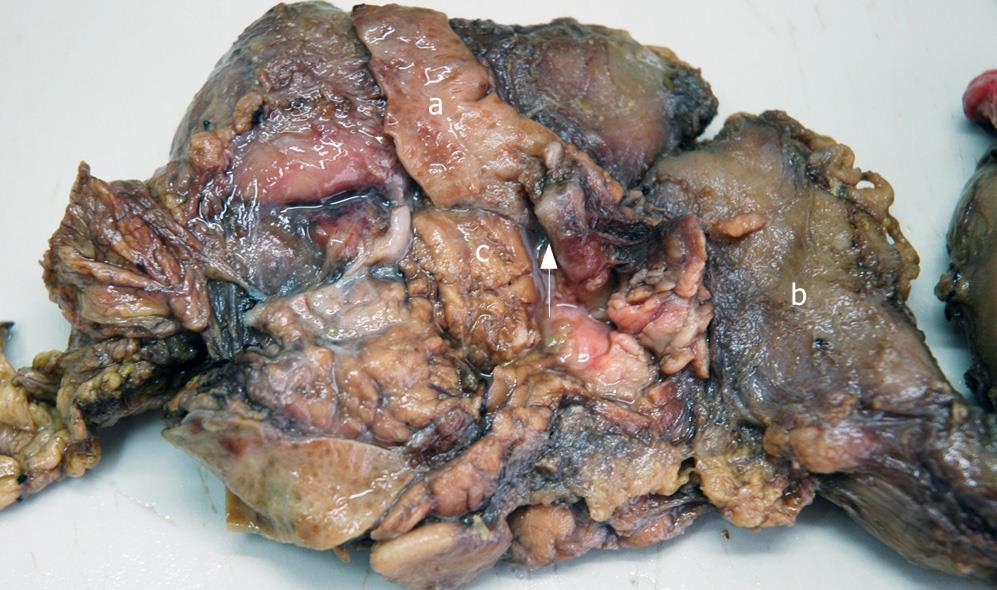
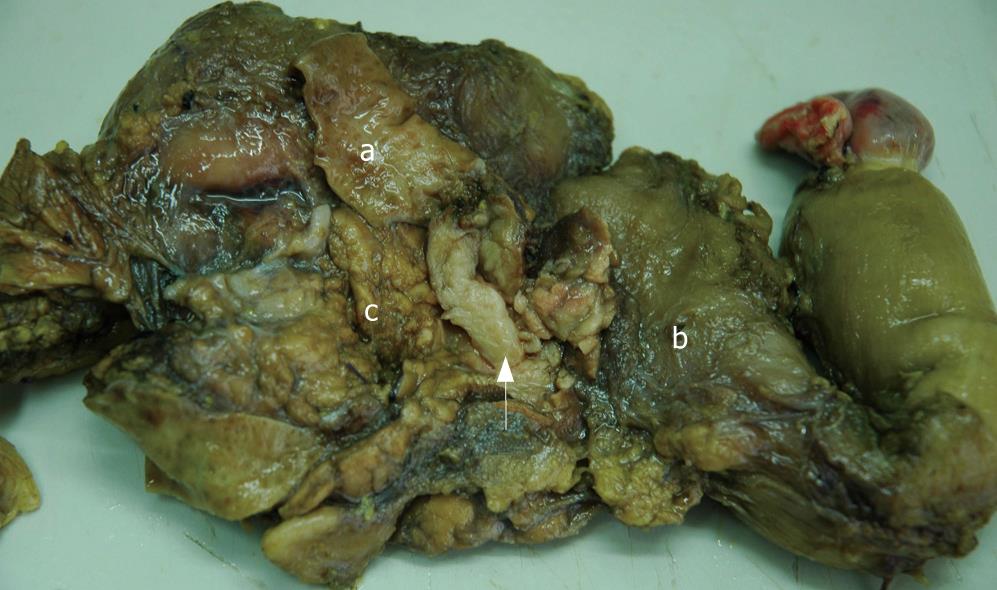
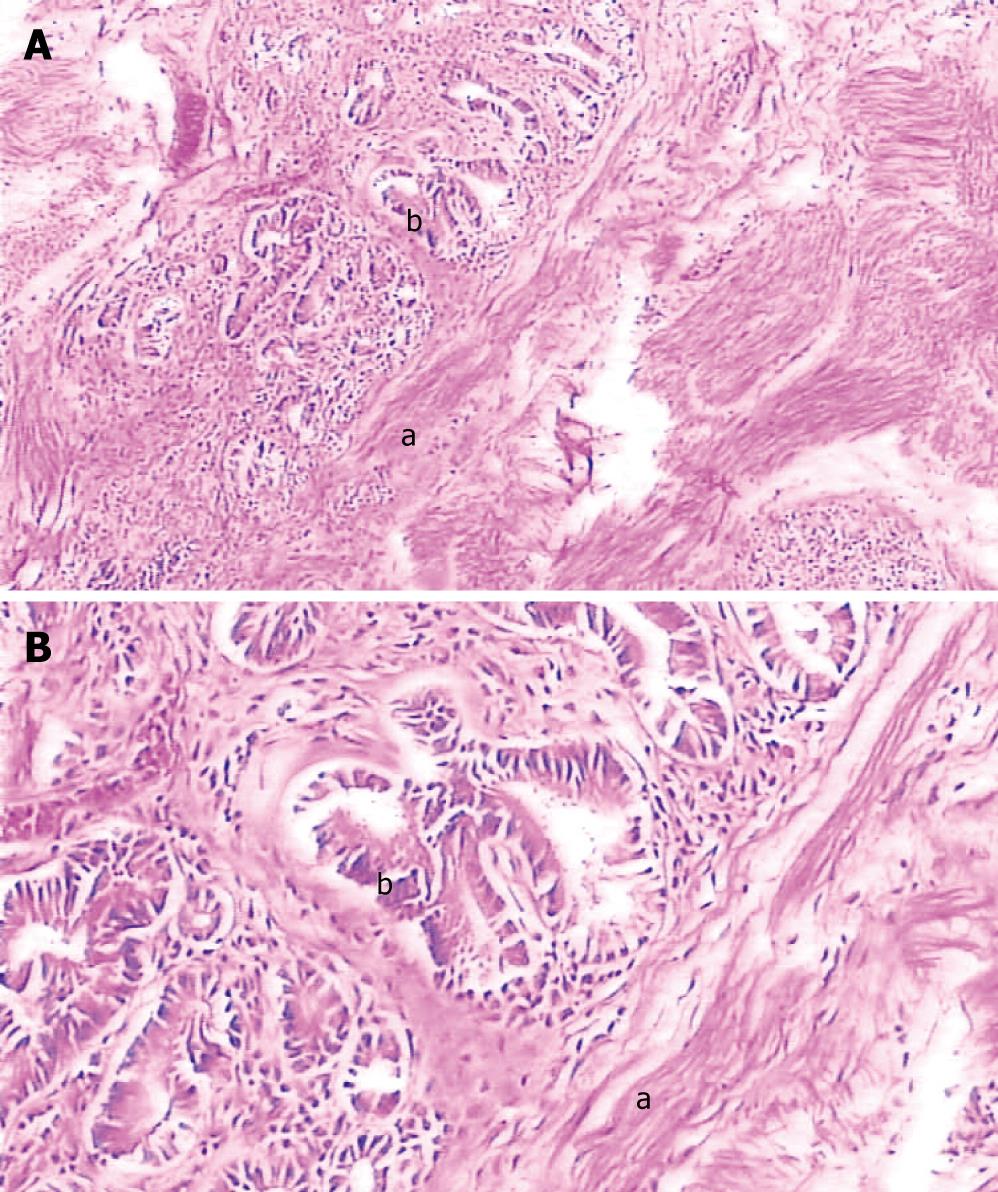
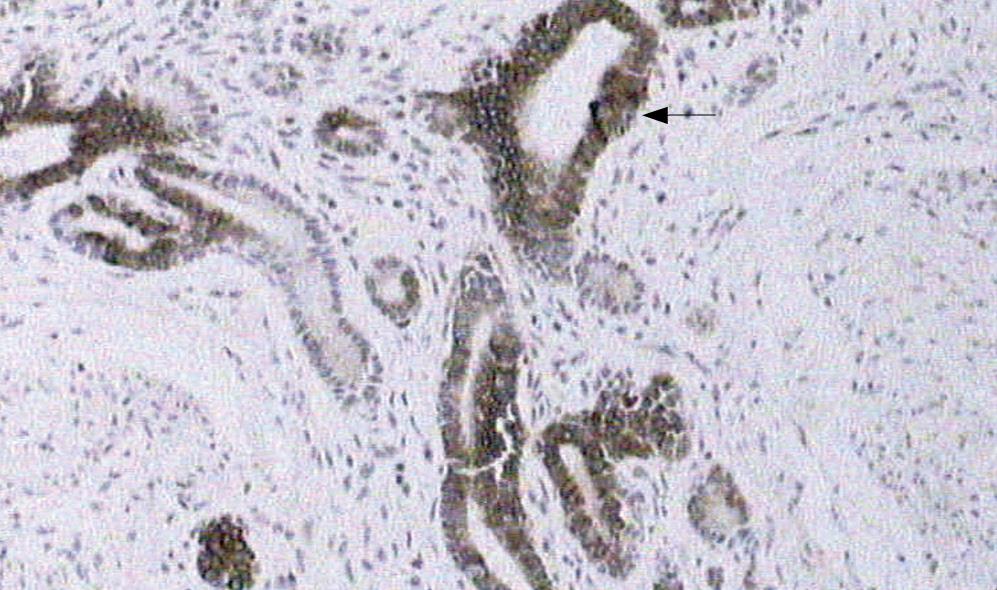
Gross observation revealed the tumor was a cord-like mass with smooth surface, white color in resecting surface, wide and immovable base (Figures 4 and 5). Pathological examination demonstrated that the tumor epithelium was adenomyomatosis with adenoid and myofibrous tissue and moderate atypia (Figure 6). Glandular epithelial component expressed cytokeratin 7 (Figure 7) but did not express cytokeratin 20. The stomach, intestine and pancreas were negative after surgery and lymphnodes aside the bile duct and in the hepatoduodenal ligament presented inflammatory hyperplasia. The digestive tract was reconstructed with Child operation. The patient has been well without any evidence of recurrence for 12 mo since his operation.
In the year 2000, Beazley and Blumgart[1] reviewed a total of 115 patients with benign polyps, adenomas or cystadenomas. The anatomical distribution of papillomas or adenomas was as follows: most tumors were located in the ampulla or close proximity of the Vaterian system (47%), followed by common bile duct (27%), common hepatic duct (15%), right and left hepatic duct (8%) and cholecyst duct (3%) in frequency. Adenomyoma of the biliary tract is a rare nonneoplastic lesion. Abdulkadir Bedirli and his colleagues[2] reviewed the literature from 1964 to 2000, and retrieved a total of 49 articles. Most were case reports of biliary tract adenomyoma. The lesions were located in the gallbladder (n = 35), extrahepatic biliary tree (n = 11), ampulla of Vater (n = 9) and duodenum (n = 4). Histology showed multiple glandular structures surrounded by a fibroblastic/myofibroblastic proliferation[3], and the immunophenotype of the epithelial component was cytokeratin 7+/cytokeratin 20-. The tumor in our case was developed in pancreatic segment of common bile duct, and the immunophenotype of the epithelial cells was cytokeratin 7+/cytokeratin 20-.
It is demonstrated that the adenomyoma is difficult to diagnose preoperatively. The ERCP image can be a useful diagnostic approach as it allows the inspection of the periampullary region and visualization of the bile duct and pancreatic duct. Moreover, a biopsy could be taken from the adenomyoma for further histopathological examination. However, the definite diagnosis is not easy to confirm before operation. Menzel and colleagues[4] showed that the overall accuracy for preoperative histopathological diagnosis was 62% for tumors in ampulla of Vater. The endoscopic retrograde cholangiopancreatography (ERCP) could not be performed in the patient because the mass was invisible in the duodenum[5].
Positron emission tomography with [18F] fluoro-2-deoxy-D-glucose was applied to diagnose bile duct cancer[6], and the median standard uptake value (SUV) was 3.8 [confidence interval (CI 3.56-5.01)] for the malignant lesions, 2.9 (CI 2.42-3.22) for the benign lesions, the sensitivity and specificity were 92.3% and 92.9%, respectively.
The adenomas are generally soft, hardly palpable and present little or no resistance in exploring ductal probes, thus being difficult to detect during the operation. They are more frequently detected by intraoperative cholangiography, ultrasonography or choledochoscopy. In the year 2000, Seo[7] reviewed 111 patients with benign or malignant bile duct tumors, analyzed the results of cholangioscopy examinations and classified the findings according to histology of the tumor. Since bile duct tumors exhibit characteristic cholangioscopic findings, cholangioscopy seems to be useful for differential diagnosis.
In a review[8] of 406 cases, brush cytology was used to assess the pancreatico-biliary stricture. There are three adenomas identified on brush cytology but could not be distinguished from adenocarcinoma morphologically.
Intraoperative frozen section from the mass may differentiate whether the lesion is benign or malignant[910]. Sato et al[11] defined the importance of intraoperative frozen section, Imai et al[12] stated that final histological examination should be required to confirm the definite diagnosis.
Therefore, according to the clinical presentation, biochemical, radiographic[13], and endoscopic investigations, and intraoperative frozen section may not be able to confirm whether the tumor is benign or malignant, pancreaticoduodenectomy was sometimes necessary[214–16].
In conclusion, preoperative endoscopic and radiological evaluations, and intraoperative section biopsies are insufficient for differentiating adenomyoma from other malignant tumors. Therefore, extensive surgery has to be performed for some non-neoplastic lesions which are suspected to be malignant.
| 1. | Beazley RM, Blumgart LH. Benign tumors and pseudotumors. In: Blumgart LH, Fong Y. Surgery of Liver and biliary tract. 3rd editor. Elsevier Science 2002; 977-991. |
| 2. | Bedirli A, Patiroglu TE, Sozuer EM, Sakrak O. Periampullary adenomyoma: report of two cases. Surg Today. 2002;32:1016-1018. |
| 3. | Handra-Luca A, Terris B, Couvelard A, Bonte H, Flejou JF. Adenomyoma and adenomyomatous hyperplasia of the Vaterian system: clinical, pathological, and new immunohistochemical features of 13 cases. Mod Pathol. 2003;16:530-536. |
| 4. | Menzel J, Poremba C, Dietl KH, Bocker W, Domschke W. Tumors of the papilla of Vater--inadequate diagnostic impact of endoscopic forceps biopsies taken prior to and following sphincterotomy. Ann Oncol. 1999;10:1227-1231. |
| 5. | Hai S, Yamamoto S, Tanaka H, Takemura S, Ichikawa T, Kodai S, Shinkawa H, Yamamoto T, Kubo S. Adenomyoma of the common hepatic duct mimicking bile duct cancer: report of a case. Surg Today. 2007;37:608-611. |
| 6. | Kluge R, Schmidt F, Caca K, Barthel H, Hesse S, Georgi P, Seese A, Huster D, Berr F. Positron emission tomography with [(18)F]fluoro-2-deoxy-D-glucose for diagnosis and staging of bile duct cancer. Hepatology. 2001;33:1029-1035. |
| 7. | Seo DW, Lee SK, Yoo KS, Kang GH, Kim MH, Suh DJ, Min YI. Cholangioscopic findings in bile duct tumors. Gastrointest Endosc. 2000;52:630-634. |
| 8. | Stewart CJ, Mills PR, Carter R, O'Donohue J, Fullarton G, Imrie CW, Murray WR. Brush cytology in the assessment of pancreatico-biliary strictures: a review of 406 cases. J Clin Pathol. 2001;54:449-455. |
| 9. | Kayahara M, Ohta T, Kitagawa H, Miwa K, Urabe T, Murata T. Adenomyomatosis of the papilla of Vater: a case illustrating diagnostic difficulties. Dig Surg. 2001;18:139-142. |
| 10. | Tsukamoto T, Kinoshita H, Hirohashi K, Kubo S, Tanaka H, Hamba H, Shuto T, Yamamoto T. Adenomyoma of the common bile duct. Hepatogastroenterology. 1999;46:1627-1630. |
| 11. | Sato Y, Shirai Y, Date K, Iwaya A, Hatakeyama K. Asymptomatic adenomyoma of the common hepatic duct discovered during a medical checkup: report of a case. Hepatogastroenterology. 2000;47:636-638. |
| 12. | Imai S, Uchiyama S, Suzuki T, Arita A, Yoshida K, Kodama H, Sato T, Akimaru K, Shibuya T, Kameda H. Adenomyoma of the common hepatic duct. J Gastroenterol. 1995;30:547-550. |
| 13. | Aoun N, Zafatayeff S, Smayra T, Haddad-Zebouni S, Tohme C, Ghossain M. Adenomyoma of the ampullary region: imaging findings in four patients. Abdom Imaging. 2005;30:86-89. |
| 14. | Ojima H, Takenoshita S, Nagamachi Y. Adenomyoma of the common bile duct: report of a case. Hepatogastroenterology. 2000;47:132-134. |
| 15. | Bill K, Belber JP, Carson JW. Adenomyoma (pancreatic heterotopia) of the duodenum producing common bile duct obstruction. Gastrointest Endosc. 1982;28:182-184. |