Published online Oct 21, 2008. doi: 10.3748/wjg.14.5945
Revised: September 16, 2008
Accepted: September 23, 2008
Published online: October 21, 2008
The striking gender disparity observed in the incidence of hepatocellular carcinoma (HCC) suggests an important role of sex hormones in HCC pathogenesis. Though the studies began as early as in 1980s, the precise role of sex hormones and the significance of their receptors in HCC still remain poorly understood and perhaps contribute to current controversies about the potential use of hormonal therapy in HCC. A comprehensive review of the existing literature revealed several shortcomings associated with the studies on estrogen receptor (ER) and androgen receptor (AR) in normal liver and HCC. These shortcomings include the use of less sensitive receptor ligand binding assays and immunohistochemistry studies for ERα alone until 1996 when ERβ isoform was identified. The animal models of HCC utilized for studies were primarily based on chemical-induced hepatocarcinogenesis with less similarity to virus-induced HCC pathogenesis. However, recent in vitro studies in hepatoma cells provide newer insights for hormonal regulation of key cellular processes including interaction of ER and AR with viral proteins. In light of the above facts, there is an urgent need for a detailed investigation of sex hormones and their receptors in normal liver and HCC. In this review, we systematically present the information currently available on androgens, estrogens and their receptors in normal liver and HCC obtained from in vitro, in vivo experimental models and clinical studies. This information will direct future basic and clinical research to bridge the gap in knowledge to explore the therapeutic potential of hormonal therapy in HCC.
- Citation: Kalra M, Mayes J, Assefa S, Kaul AK, Kaul R. Role of sex steroid receptors in pathobiology of hepatocellular carcinoma. World J Gastroenterol 2008; 14(39): 5945-5961
- URL: https://www.wjgnet.com/1007-9327/full/v14/i39/5945.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5945
| Subcellular localization | Method | ER subtype/Antibody source | n | Subjects | Positive cases | Country and area | Yr | Reference |
| Cytosolic | BA | NA | 5 | 5 M | 5 M | United States | 1982 | [30] |
| Cytosolic and nuclear | BA | NA | 5 | 3 M, 2 F | 3 M, 2 F | United Kingdom | 1983 | [31] |
| Cytosolic | IHC | NA, Anti ER Ab Hypolabs, Switzerland | 10 | NA | 1 | Singapore | 1984 | [39] |
| Cytosolic | BA | NA | 30 | 29 M, 1 F | 12 | Japan | 1986 | [13] |
| Cytosolic and nuclear | BA | NA | 8 | 6 M, 2 F | 1 M | Japan | 1986 | [17] |
| Cytosolic | BA | NA | 13 | 9 M, 4 F | 1 F | Japan | 1987 | [32] |
| Cytosolic | EIA | Abbot ER-EIA monoclonal kit | 13 | 9 M, 4 F | 3 M, 2 F | Japan | 1987 | [32] |
| Cytosolic | BA | NA | 19 | 19 F | 7 F | Japan | 1989 | [15] |
| Cytosolic | BA | NA | 66 | 52 M, 14 F | 23 M, 3 F | Japan | 1990 | [34] |
| Cytosolic | BA | NA | 6 | 4 M, 2 F | 1 F | Japan | 1990 | [35] |
| Cytosolic | BA | NA | 21 | 18 M, 3 F | 9 M, 1 F | Japan | 1991 | [36] |
| Cytosolic and nuclear | BA | NA | 9 | 6 M, 3 F | 6 M, 2 F | Italy, United States | 1991 | [37] |
| Cytosolic and nuclear | BA | NA | 9 | 6 M, 3 F | 6 M, 2 F | Italy, United States | 1991 | [33] |
| NA | IHC | NA, Abott, ER-ICA | 15 | 12 M, 3 F | 0 | Italy | 1993 | [40] |
| Cytosolic | EIA | NA, Abbot anti ER | 26 | 18 M, 8 F | 4 | Spain | 1993 | [41] |
| Cytosolic | EIA | NA, Abbot anti ER | 33 | 20 M, 13 F | 8 M, 5 F | Germany | 1997 | [42] |
| Cytosolic | IHC | NA, ER monoclonal Ab, Dako | 71 | 59 M, 12 F | 15 M, 2 F | Hong Kong | 1997 | [43] |
| Cytosolic and nuclear | IHC | ERα, Santacruz | 45 | 37 M, 8 F | 21 (cytosolic) | United States, Korea1 | 2004 | [44] |
| 11 (nuclear) | ||||||||
| NA | IHC | NA | 28 | NA | 11 | China | 2004 | [45] |
| NA | IHC | NA | 66 | 3 | Mexico | 2007 | [46] | |
| Nuclear | IHC | ERα, Dako (ID5) | 31 | 26 M, 5 F | 12 M, 4 F | Spain | 2007 | [47] |
| Method | ER subtype | n | Subjects | Positive cases | Country | Yr | Reference |
| ISH | ERα Wt | 15 | 12 M, 3 F | 11 | Italy | 1993 | [40] |
| RT-PCR | ERα Wt | 14 | 7 M, 7 F | 1 M, 7 F | Italy | 1995 | [51] |
| RT-PCR | ERα delta5 variant | 14 | 7 M, 7 F | 7 M, 3 F | Italy | 1995 | [51] |
| RT-PCR | ERα Wt | 40 | 25 M, 15 F | 16 M, 12 F | Italy | 1998 | [18] |
| RT-PCR | ERα delta5 variant | 40 | 25 M, 15 F | 20 M, 10 F | Italy | 1998 | [18] |
| RT-PCR | ERα Wt | 42 | 35 M, 7 F | 20 M, 5 F | Italy | 2003 | [19] |
| RT-PCR | ERα delta5 variant | 42 | 35 M, 7 F | 37 | Italy | 2003 | [19] |
| RT-PCR | ERβ Wt | 42 | 35 M, 7 F | 12 M, 4 F | Italy | 2003 | [19] |
| RT-PCR | ERα Wt | 28 | NA | 25 | China | 2004 | [45] |
| RT-PCR | ERα delta5 variant | 28 | NA | 27 | China | 2004 | [45] |
| RT-PCR | ERα Wt | 32 | 23 M, 9 F | 23 M, 9 F | Korea | 2006 | [52] |
| RT-PCR | ERα delta5 variant | 32 | 23 M, 9 F | 21 M, 9 F | Korea | 2006 | [52] |
| RT-PCR | ERβ Wt | 32 | 23 M, 9 F | 26 | Korea | 2006 | [52] |
| Receptor protein/mRNA | Type of liver tissue | Subjects | Positive cases | Method | Country | Yr | Reference |
| Estrogen Receptor (ER) | |||||||
| ER protein (cytosolic) | Normal liver tissue | 4 F | 4 F | BA | United Kingdom | 1978 | [28] |
| ER protein | Normal | 3 F | 3 F | BA | Germany | 1978 | [59] |
| ER protein | Normal | 2 M | 2 M | BA | United States | 1982 | [30] |
| ER protein | Normal | 1 M, 5 F | 1 M, 5 F | BA | Germany | 1982 | [60] |
| ER protein | Normal | 3 M, 3 F | 3 M, 3 F | BA | United States | 1983 | [61] |
| ER protein (cytosolic & nuclear) | Normal | 2 M, 2 F | 2 M, 2 F | BA | United Kingdom | 1983 | [31] |
| ER protein (cytosolic) | Surrounding liver tissue | 30 | 13 | BA | Japan | 1986 | [13] |
| ER protein (cytosolic & nuclear) | Non-cancerous tissue | 7 | 3 | BA | Japan | 1986 | [17] |
| ER protein (cytosolic & nuclear) | Normal | NA | NA | BA | United States | 1987 | [62] |
| ER protein (cytosolic) | Non-cirrhotic liver | 5 M, 7 F | 5 M, 7 F | BA, EIA | Japan | 1987 | [32] |
| ER protein | Normal | 2 | 2 | Japan | 1988 | [63] | |
| ER protein (cytosolic) | Surrounding liver tissue | 17 | 11 | BA | Japan | 1989 | [15] |
| ER protein | Surrounding non-cancerous tissue | 22 | 14 | NA | Japan | 1989 | [64] |
| ER protein (cytosolic) | Surrounding normal liver | 4 M, 1 F | 4 M, 1 F | BA | Japan | 1990 | [35] |
| ER protein | Adjacent normal tissue | 6 M, 3 F | 6 M, 3 F | BA | Italy, United States | 1991 | [33] |
| ER protein (cytosolic) | Non-tumoral liver | 18 M, 8 F | 9 M, 2 F | BA | Spain | 1993 | [41] |
| ER mRNA | Non-tumorous liver tissue | 13 | 7 | ISH | Italy | 1993 | [40] |
| ER mRNA | Peri-tumor tissue | 32 | 28 | RT-PCR | Korea | 2006 | [52] |
| Androgen Receptor (AR) | |||||||
| AR protein (cytosolic & nuclear) | Normal | 2 M, 2 F | 0 | BA | United Kingdom | 1983 | [31] |
| AR protein (cytosolic & nuclear) | Non-cancerous tissue | 6 | 1 | BA | Japan | 1986 | [17] |
| AR protein (cytosolic) | Non-neoplastic liver tissues | 17 | 11 | BA | Japan | 1989 | [15] |
| AR protein | Surrounding non-cancerous tissues | 21 | 7 | NA | Japan | 1989 | [64] |
| AR protein (cytosolic) | Surrounding liver | 9 M, 1 F | 7 M, 1 F | BA | Japan | 1990 | [35] |
| AR protein | Adjacent normal tissue | 6 M, 3 F | 6 M, 3 F | NA | Italy, United States | 1991 | [33] |
| AR mRNA | Peri-tumor tissue | 23 M, 9 F | 23 M, 9 F | RT-PCR | Korea | 2006 | [52] |
| Subcellular Organelle | Method | n | Subjects | Positive | Country | Yr | Reference | |
| AR protein | Cytosolic and nuclear | BA | 5 | 3 M, 2 F | 3 M, 2 F | United Kingdom | 1983 | [31] |
| Cytosolic | IHC | 10 | NA | 5 | Singapore | 1984 | [39] | |
| Cytosolic | BA | 19 | 19 M | 14 M | Japan | 1985 | [14] | |
| Cytosolic and nuclear | BA | 5 | 3 M, 2 F | 3 M, 2 F | United Kingdom | 1985 | [65] | |
| Cytosolic and nuclear | BA | 8 | 6 M, 2 F | 2 M, 2 F | Japan | 1986 | [17] | |
| Cytosolic | BA | 13 | 8 M, 5 F | 8 M, 5 F | United Kingdom | 1988 | [66] | |
| Cytosolic | BA | 19 | 19 F | 7 F | Japan | 1989 | [15] | |
| Cytosolic | BA | 45 | 31 M, 14 F | 25 M, 6 F | Japan | 1989 | [67] | |
| Cytosolic | BA | 11 | 9 M, 2 F | 6 M, 1 F | Japan | 1990 | [35] | |
| Cytosolic | BA | 21 | 18 M, 3 F | 18 | Japan | 1991 | [36] | |
| Cytosolic and nuclear | BA | 9 | 6 M, 3 F | 6 M, 3 F | Italy, United States | 1991 | [33] | |
| Cytosolic | BA | 5 | 3 M, 2 F | 3 M, 2F | Japan | 1992 | [68] | |
| Cytosolic | BA | 26 | 18 M, 8 F | 14 | Spain | 1993 | [41] | |
| Cytosolic | BA | 43 | 30 M, 13 F | 28 | Spain | 1995 | [69] | |
| NA | BA | 32 | China | 1998 | [70] | |||
| Nuclear | IHC | 31 | 26 M, 5 F | 18 M, 3 F | Spain | 2007 | [47] | |
| AR mRNA | ISH | 22 | 16 M, 6 F | 13 M, 3 F | Italy | 1994 | [16] | |
| RT-PCR | 38 | 24 M, 14 F | 21 M, 13 F | Italy | 2002 | [71] | ||
| RT-PCR | 32 | 23 M, 9 F | 23 M, 9 F | Korea | 2006 | [52] |
| Receptor protein/mRNA expression | Clinical parameter | n | Country | Salient findings | Yr | Reference |
| ER protein | Serum alpha-fetoprotein, carcinoembryonic antigen, HBV profile, tumor histology | 30 | Japan | No correlation with any parameter | 1986 | [13] |
| ER and AR protein | Serum alpha fetoprotein, HBV markers, histopathology | 19 | Japan | No correlation | 1989 | [15] |
| ER protein | Sex, age, alcohol abuse, underlying liver disease, hepatic functions | 66 | Japan | No correlation | 1990 | [34] |
| Tumor size, hepatic resection | Large tumor size and higher rate of resection in ER- | |||||
| Histopathology | No differences in ER+ and ER- | |||||
| Operative mortality, tumor recurrence, long-term survival rate | Similar in ER+ and ER- | |||||
| ER and AR protein | Intrahepatic recurrence | 78 | Japan | AR expression strongly associated with intrahepatic recurrence. Weak association with ER expression | 1995 | [72] |
| ER protein | Survival after curative resection | 28 | Germany | Negative effect of an ER+ tumor on patient survival after curative resection | 1997 | [42] |
| Wild type and variant ER mRNA | Survival | 96 | Italy | Significantly long survival in patients with wild type ERs than variant ERs | 2000 | [55] |
| AR protein | Recurrence rate | 45 | Japan | Significantly higher recurrence rates in AR+ group than AR- | 1989 | [15] |
| Survival rate | Significantly better survival rates in AR- patients than in AR+ | |||||
| AR protein | Tumor size | 43 | Spain | AR expression was significantly related to smaller tumor size | 1995 | [69] |
| Tumor recurrence | Higher tumor recurrence rates in surrounding tissues of AR+ than AR- | |||||
| AR protein | Tumor size and survival time | 32 | China | Survival rate correlated inversely with the levels of AR expression | 1998 | [70] |
| AR levels had positive correlation with the tumor size |
| Receptor protein/mRNA expression | Clinical parameter | Treatment | n | Country | Salient Findings | Yr | Reference |
| ER protein | Tumor growth | Progestin | 5 | United States | Tumor regression in 2 | 1982 | [30] |
| NA | Anti-tumor response | Tamoxifen 20 mg twice daily | 33 | United States | No complete or partial antitumor response | 1990 | [73] |
| Survival time | Long term survival (18+ to 39+ mo) in 4 patients | ||||||
| NA | Anti-tumoral effect | Tamoxifen 20 mg daily | 120 (placebo = 62) | Spain | No-antitumor effect | 1995 | [74] |
| Survival time | No significant differences in survival rate of placebo and treated groups | ||||||
| Wild type and variant ER mRNA | Tumor size and growth rate | Tamoxifen 80 mg daily or Megestrol 160 mg daily | 8 | Italy | Growth rate 4 times higher in tumors expression variant ER than wild type ERs. Tumor regression to half size in patients with wild type ER following tamoxifen treatment. Megestrol slowed down tumor growth in tumors with variant ERs | 1996 | [54] |
| ER protein | Mortality rates | Tamoxifen | 119 (placebo = 58) | China | No difference in 1 mo mortality rates and median survival in treated and control groups | 2000 | [75] |
| Survival | No effect of ER expression on survival | ||||||
| Variant ER mRNA | Tumor growth, survival | Megestrol 160 mg daily | 24 placebo, 21 treated | Italy | Significantly slowed down tumor growth and improved survival in treated patients than placebo group | 2001 | [76] |
| NA | Survival rates | Tamoxifen 120 mg daily or 60 mg daily | 329 | Singapore | No positive effect on survival and increasingly negative impact with increasing doses | 2005 | [77] |
Hepatocellular carcinoma (HCC) is one of the most lethal malignancies with limited treatment options. The major risk factors for HCC are chronic liver diseases with cirrhosis that include hepatitis B, hepatitis C, alcoholic liver disease and non-alcoholic steatohepatitis. Epidemiological reports indicate that regardless of etiologies, the incidence of HCC is higher in males than in females with the male: female ratio usually averaging between 2:1 and 4:1[1]. This male predominance is further supported by the clinical observations that chronic liver disease progresses more rapidly to cirrhosis in males than females and therefore cirrhosis that leads to HCC development is largely considered to be the disease of men and postmenopausal women[2]. In view of this remarkable gender disparity, various in vitro as well as in vivo studies have been initiated from time to time to explore the importance of sex hormones in HCC. However, the precise role of male and female sex hormones and their receptors in HCC remains still poorly understood. Androgens have been suggested to induce and promote HCC[3] and altered androgen metabolism has been reported to be associated with HCC[4]. In contrast, the role of estrogen in HCC has been controversial with evidence suggesting both carcinogenic and protective effects in the liver[3,5-9]. Very limited information is currently available regarding the mechanism of estrogen and androgen action in normal liver as well as in HCC.
It is well known that estrogen and androgen mediate their biological functions by binding with a high affinity to specific receptors, the estrogen receptor (ER) and the androgen receptor (AR). Both ER and AR belong to the family of nuclear receptors that act as transcription factors and regulate the expression of several genes. Our present day knowledge of structure and function of these receptors is primarily attributed to the extensive research on ER and AR in cancer of reproductive organs. However, recent advances in molecular research reveal that sex hormones do play a significant role in normal physiology of various organs other than the organs of the reproductive system. Both androgens and estrogens regulate transcriptional activation of various molecules involved in key cellular processes such as generation of immune responses, cell proliferation and apoptosis through functional receptors localized in various sub-cellular organelles[10-12].
The expression and functional status of AR and ER appear to play a significant role in the carcinogenesis of all hormone sensitive organs. However, liver has remained a less studied organ in the context of sex hormones and their receptors. Differential expression of wild type and variant forms of ER and AR has been reported in normal liver and HCC, indicating a strong link between sex hormones and pathogenesis of HCC[13-19]. Recent in vitro studies also provide further evidence in support of AR and ER involvement in various cellular events as well as interaction with viral proteins in hepatitis B virus (HBV) and hepatitis C virus (HCV)-induced HCC[20-25]. This review is focused on the compilation of the information so far available on the significance of AR and ERs in HCC and brings forth wide gaps in the existing knowledge to the notice of scientific world for future research.
The role of estrogen in modulating morphological and physiological features of liver became evident in early 1970s when a possible correlation between occurrence of hepatic neoplasms and use of oral contraceptives was suggested[26,27]. In the reproductive system, estrogen is known to act by binding to specific cytoplasmic and nuclear receptors. Hence, search began to identify such a receptor in the liver. In 1978, Duffy and Duffy first reported the presence of ER in normal human liver[28]. Subsequently, the presence of ERs in human HCC was demonstrated by Molteni et al[29] followed by Friedman et al[30] and Iqbal et al[31]. Since then, a number of studies have been reported addressing the expression of ERs in normal as well as neoplastic liver tissues. The early studies used indirect methods of receptor detection based on ligand binding assays. Table 1 gives the details of these studies[13,15,17,30-37]. These assays were quantitative and measured the amount of receptor in the samples as well as its affinity for the ligand in terms of dissociation constants. The percentage positivity for ER expression varied significantly among different studies. These variations may be attributed to the differences in sample size, methodologies, ethnicity of the population studied, stage of the disease and underlying etiologies. Earlier studies by Friedman et al[30] and Iqbal et al[31] showed that ER content is similar in HCC and normal liver. In contrast, later studies consistently showed that the expression of ER is decreased in HCC tissue specimens as compared to normal liver tissue specimens or the non-tumor part of the liver[17,32,38]. However, Eagon et al[33] documented elevated levels of cytosolic ER in 3 of the 9 tumors as compared to non-cancerous tissues. Nuclear ER expression was found to be suppressed in all HCC samples as compared to normal samples[33]. The major drawback of these studies was the use of binding assays for detection that do not provide any information on the subtype of ER, i.e. ERα and ERβ as known today. It is important to study the relative expression of both isoforms of ER since ERα and ERβ are known to have overlapping but quite distinct functions. There are few reports on direct detection of ER using specific antibodies. Table 1 gives the details of these studies[32,39-47]. However, all these studies have employed either immunohistochemistry (IHC) or enzyme-immuno assays (EIA) using antibodies specific for only ERα isoform. The ERβ isoform was identified later in 1996[48] and information on the expression of ERβ protein in HCC is lacking though few studies at mRNA levels have been documented.
Since the molecular characterization and cloning of ERα in the mid 1980s[49,50], attempts have been made to determine the expression of ER in liver tumors at mRNA level. Table 2 gives the details of these studies[18,19,40,45,51,52]. In situ hybridization using ER specific oligonucleotide sequence probe revealed that 11 out of 15 HCC tissue samples expressed ER mRNA[40]. Interestingly, the same samples were found to be negative for ER protein by IHC, suggesting the use of more sensitive methods and more specific antibodies for detecting ER at protein level. Subsequently, the mRNA expression of ER in HCC tissues was studied in different populations by a highly sensitive method of reverse transcriptase-polymerase chain reaction (RT-PCR). Villa et al[51] were the first to demonstrate the presence of wild type ERα in peritumoral and tumoral tissue of HCC patients using this technique. The use of RT-PCR further enabled the authors to detect a splice variant of ERα lacking exon 5 in the hormone binding domain[18,51]. A similar splice variant has been described in breast cancer tissues to be associated with tumor pathogenesis[53]. The significance of the variant ERα (vERα) in pathology, prognosis and treatment of HCC has also been studied. The presence of vER receptor is able to influence the natural history of patients with HCC by regulating tumor growth as well as patient survival. The presence of the liver vERα transcript in the tumor has been described to be the strongest negative predictor of survival in operable HCC patients[54,55]. Furthermore, the presence of vERα was found to correlate with a higher clinical aggressiveness of the tumor in comparison with the tumors characterized by wild-type ERα transcript. These tumors were responsive to megestrol and unresponsive to anti estrogen tamoxifen. High rates of vERα expression have been shown to be present in men at high risk of HCC development[56,57]. In patients with chronic hepatitis and cirrhosis, the expression of vERα has been associated with higher oxidative stress-induced DNA damage and c-myc mRNA expression, a factor indicating increased genomic instability, augmented cytoproliferation and carcinogenesis[5].
Using RT-PCR, in addition to ERα, the expression of the lately identified isoform of ER, i.e. ERβ, has also been studied in HCC patients. Iavarone et al[19] report that both ERβ and ERα wild type receptors either alone or together with vER are co-expressed more frequently in patients with chronic liver disease than in those with HCC. However, both ERs are similarly expressed in tumoral and extratumoral tissues of HCC patients[19]. In this study, HBV-related tumors either expressed wild type ERα and ERβ or expressed variant ER and ER delta 5 more often than HCV-related tumor, and HBV-related tumors showed a tendency towards loss of ERβ expression as the disease progressed from chronic inflammatory liver disease to HCC[19].
Breast cancer studies suggest that ERα:ERβ expression ratio changes during carcinogenesis and is believed to play a role in tumor development[58]. Recently, Wang et al[52] studied the expression of ERα and ERβ in HCC tissues of Korean population using RT-PCR, and assessed 32 tumoral and peritumoral tissues from HCC patients with underlying chronic HBV or HCV infection and observed that wild type and variant ERα are expressed in all the samples. However, the expression of vERα is stronger in tumor than in peritumor tissues. Interestingly, ERβ was found to be significantly over-expressed in HCV-infected HCC tissues as compared to HBV-infected HCC tissues. The differences in ER expression in HCV-infected HCC tissues compared to HBV-infected HCC tissues suggest different pathogenetic mechanisms. Overall from these studies there appears to be a change in co-expression pattern of ERα and ERβ from cirrhosis to HCC development in both HBV- and HCV- related tumors. Thus, these studies provide further evidence in support of importance of wild type and variant ERs in HCC, suggesting detailed investigations in this area.
Like estrogens, androgens have also been reported to play an important role in liver carcinogenesis. Iqbal et al[31] showed the presence of androgen receptors (AR) in HCC in 1983. However, it was not until 1985 that normal human liver was believed to express AR. In 1985, Nagasue and colleagues[14] demonstrated the presence of AR in normal human liver as well as in tumor and non-tumor parts of HCC tissues. Since then, several reports showing protein and mRNA expression of AR in liver have been published. Table 3[13,15,17,28,30-33,35,40,41,52,59-64] and Table 4[14-17,31,33-36,39,41,47,52,65-71] give the details of these studies. In general, AR is found to be over expressed in liver tumor compared to the adjacent normal tissue[15,17,33,36,38]. However, like ER expression studies, majority of the early studies employed indirect binding assays to detect AR in the liver tissues. More precise quantitative methods of direct detection of AR protein are needed. Reports using antibody-based detection of AR in liver tissues are very sparse.
The mRNA levels of AR have been assessed in the non-cancerous and HCC tissues primarily by RT-PCR (Tables 3 and 4). In 1994, Negro and colleagues[16] developed a non-radioisotopic in situ hybridization assay specific for human AR mRNA and found that 73% of HCC tissues could express variable amount of AR mRNA. However, normal hepatocytes were stained weakly in 42% of the non-neoplastic tissues. Though initial binding studies demonstrated higher AR levels in tumor tissues than in respective peri-tumoral part[15,17,33,36,38], more recent observations based on mRNA expression do not reveal any significant differences in tumor and peri-tumor tissues. Tavian et al[71] found higher AR mRNA levels in tumor than in the corresponding peri-tumoral tissue in a relatively small percentage of HCC samples, suggesting that AR mRNA levels are associated with the histological tumor differentiation showing a lower AR expression in poorly-differentiated HCC than in well-differentiated tumors. In contrast, AR levels in Korean population with HCC do not show differences between tumor and peri-tumor tissues using RT-PCR[52]. Due to these conflicting reports on AR expression in HCC, there is a need for detailed investigation of AR mRNA as well as protein levels using more sensitive and accurate detecting quantitative methods.
Despite a wide variability observed in studies of ER and AR expression in HCC, attempts have been made to determine the significance of these receptors by correlating their levels with clinical and pathological parameters. Table 5[13,15,34,42,55,69,70,72] and Table 6[30,54,73-77] present the salient findings of such clinical studies.
In few earlier studies using binding assays, no correlation was found between ER protein expression and sex, age, alcohol abuse, serum alpha-feto protein, carcinoembryonic antigen, HBV markers or tumor histology[15,34]. However, in subsequent reports, ER mRNA levels were shown to be associated with sex and viral etiology[19,51,52]. Increased vERα expression has been demonstrated more often in males than in females with HCC in Italian population, suggesting a strong link of ER with a higher incidence of HCC in males[19,51]. On the other hand, in Korean subjects no correlation has been found between the expression of vERα and HCC prevalence in males[52]. Interestingly, a distinct difference in ER expression pattern was observed in HBV- and HCV-infected HCC patients. Delta 5 deletion variants of ERα (vERα) and ERβ were found to be more often expressed in HBV-related tumors than in HCV-related tumors (67% vs 15%, P < 0.0007)[19]. In contrast, Wang et al[52] showed no remarkable difference in vERα levels in HCV- and HBV-infected HCC tissues (91.3% vs 100%). Nevertheless, a predominant expression of ERβ has been reported in HCV-infected than in HBV-infected patients with HCC (95.7% vs 44.4%, P < 0.05), suggesting that ERβ may play an important role in HCV-induced liver disease[52].
In addition to gender and etiological factors, tumor size, histopathology, operative mortality, tumor recurrence and survival after curative resection have also been studied in relation to ER expression in HCC[34,42,55]. Nagasue et al[34] showed that the large tumors are more commonly found in ER negative HCC patients and therefore the incidence of major hepatic resection is significantly higher in this group than in ER positive HCC patients. However, they did not report significant differences in histopathology of ER positive and ER negative tumors. Rates of mortality, tumor recurrence and long-term survival were also found to be similar in the two groups. In contrast to these observations, Jonas et al[42] showed that in patients undergoing curative resection, the 1- and 2-year survival rates in ER positive group are substantially lower than in ER negative group[42], suggesting that ER positive status has a negative effect on patient survival after curative resection of advanced HCC. Further, significantly longer survival rates have been reported in HCC patients with wild type ERs than in those expressing variant ERs[55].
In contrast with ER expression, AR levels are strongly associated with intra hepatic recurrence of tumors. The 5-year survival of recurrence free HCC patients was shown to be 55% for AR negative, 24% for ER negative, 10% for ER positive and 0% for AR positive tumors[67]. Similar findings have been reported by other researchers, suggesting a negative impact of AR positivity on tumor recurrence[69]. It was reported that AR negative patients show significantly better survival than AR positive patients[70]. Considering the tumor size, variable results have been documented in relation with AR expression. Boix et al[69] showed that AR expression is significantly related to smaller tumor size while Zhang et al[70] found that AR levels are positively correlated to tumor size.
The association of estrogens and androgens in HCC observed in basic and clinical studies has led to initiation of various clinical trials on hormonal treatment of HCC. Differential clinical outcome was reported in these trials that have resulted in continued debate about the use of hormonal therapy in HCC. Table 6 presents a list of few clinical studies that utilized hormonal therapy in HCC patients. A systematic review of these clinical trials on therapeutic evaluation of anti-estrogen and anti-androgen agents in liver cancer has been recently compiled by Di Maio et al[78]. The authors conclude that hormonal treatment should not be a part of the current management of HCC patients[78,79]. However, in most of these clinical studies, various inherent factors may have contributed to the observed inconclusive results. Few of these may include faulty patient subset selection criteria, no monitoring of tumor ER and AR expression at the time of recruitment and also during treatment of these patients and lastly the type of hormonal treatment given to the patient. Therefore, the debatable potential of hormone therapy in HCC may finally be attributed to the lack of complete understanding of ER and AR expression and hormonal responsiveness in the liver and their involvement in development of HCC.
Currently, limited information is available on the functional significance of ER and AR in HCC. In the following sections, we review the in vivo animal studies on liver carcinogenesis and in vitro studies on cell lines that have been conducted to understand the role of ER and AR in the liver.
Several attempts have been made to establish the role of estrogen and its receptors in hepatocarcinogenesis using animal models. Rat is the most extensively used model to study liver carcinogenesis. Rat hepatocytes are known to express ERs. ERα is the predominant isoform expressed in rat hepatocytes while cholangiocytes express both ERα and ERβ[80]. However, Inoue et al[81] showed that the levels of ERβ are higher than those of ER in cultured rat hepatocytes[81]. Hepatic stellate cells from rats appear to contain mainly ERβ[82]. Due to the lack of information about the existence of various ER isoforms, in most of the earlier studies, hepatic stellate cells did not differentiate ER into ERα and ERβ. In addition, majority of the in vivo studies have been conducted in animal models of chemical carcinogenesis. Diethylnitrosamine (DEN) is the most commonly used carcinogen in rat and mouse models of HCC. The pathogenesis of HCC in DEN-induced carcinogenesis in animal models differs from that in humans and therefore may not be directly comparable to human HCC[83]. Nevertheless, the histology and genetic signatures are similar to human HCC and a striking gender disparity with male predominance is also observed in these animal models as seen in humans[84]. In addition to DEN, acetylaminofluorene (AAF), di(2-ethylhexyl) phthalate (DEHP), peroxisome proliferator, arsenic and carbon tetrachloride have been used to induce HCC in various animal models[85-90].
Use of oral contraceptives and synthetic estrogens in women is reported to be a major risk factor for the development of hepatocellular adenoma, a benign liver tumor with malignant potential[26,27]. Shimomura et al[91] studied the role of ethinyl estradiol (EE) in inducing HCC in female rats, following EE treatment for 12 mo, 8% of rats developed HCC, revealing that EE causes mutations in hepatocytes leading to DNA adduct formation and induces HCC development in affected cells. The initial events in HCC, i.e. DNA adduct formation by EE, appear to be carried out in an ER independent manner since tamoxifen, a known selective estrogen receptor modulator (SERM), inhibited ER expression and suppressed HCC, but did not affect DNA adduct formation. Exogenous estrogens have also been shown to promote hepatocarcinogenesis induced by other agents[92,93]. Campen et al[92] documented that administration of 17-alpha ethinylestradiol in ovariectomized rats promotes DEN-induced carcinogenesis in a dose dependent manner. Further, it was reported that synthetic female hormones act synergistically with ethanol to increase HCC incidence[93]. Alcohol could affect HCC development due to EE by promoting changes in ER kinetics and expression as well as in DNA adduct formation.
Liver is the major site of estrogen metabolism[94]. Alterations in sex hormone metabolism are also considered a critical factor determining the significance of sex hormones in the process of liver carcinogenesis. Eagon et al[86,87] reported that the activity of male estrogen-metabolizing enzyme, estrogen 2-hydroxylase and male specific estrogen sequestering protein is reduced in liver, which explains the raised serum estradiol levels but the decreased hepatic activity of cytosolic and nuclear ER observed in DEHP-induced HCC in male Fischer 344 rats. The expression of cytochrome P450 enzymes that play an important role in estrogen metabolism have also been shown to be affected during hepatocarcinogenesis. Waalkes and colleagues[88] have described the feminized pattern of P450 genes in male mice with HCC induced by exposure to arsenic in utero. The expression of female dominant CYP2A4 and CYP2B9 is increased whereas levels of male dominant CYP7B1 gene gets reduced in arsenic treated animals. Recent findings suggest that cytochrome P450 (CYP) is regulated by estrogen itself through the involvement of estrogen receptors[94]. Nonetheless, elucidation of the exact mechanism of regulation of CYP isoforms by estrogen in liver needs further investigation.
In contrast with earlier animal studies that support estrogens in promoting and inducing carcinogenesis, recent studies highlighted the protective role of estrogens in HCC development. Shimizu et al[8] showed that estrogen can suppress chemical hepatocarcinogenesis induced by dimethylnitrosamine (DEN)-2-acetylaminofluorene (AAF) in partial hepatectomy (PH) model of hepatocarcinogenesis. In addition, estrogen has been shown to prevent the progression of liver disease to HCC. Estradiol treatment could reduce hepatic steatosis and restore the impairment in mitochondrial and peroxisomal fatty acid β-oxidation in aromatase-deficient mice which lack intrinsic ability to produce estrogen[95]. Furthermore, estradiol treatment was also shown to result in a dose dependent suppression of hepatic fibrosis in hepatic fibrosis models of male rats[96,97]. The mechanism of protective action of estrogens against progression of chronic liver disease has been recently reviewed by Shimizu and Ito[7].
Recently, using a mouse model of DEN-induced hepatic carcinogenesis, Naugler et al[83] described a molecular mechanism explaining the lower HCC susceptibility in females and the anti-inflammatory role of estrogen in preventing HCC development. The authors investigated the relationship between HCC development and gender dependent expression of interleukin-6 (IL-6). IL-6 is a proinflammatory cytokine that plays an important role in chronic hepatitis, the prerequisite for progression to cirrhosis and HCC. The serum IL-6 levels were higher in male mice than in female mice after administration of DEN, leading to a higher rate of liver cell proliferation in male mice. This effect can be further mediated by ERα, suggesting that ERβ plays a little role in modulating the expression of IL-6.
The role of AR has also been studied in animal models of chemical induced carcinogenesis[87,98-100]. In DEN treated Wistar rats, a 20-fold increase in hepatic AR concentration was reported in females, suggesting that increased hepatic AR concentration is correlated with accelerated tumor development in these animals, in which male rats showed a slower tumor development with no change in AR concentrations[98]. Subsequent studies in the same model revealed that removal of ovary increases AR levels in the liver of female rats but testosterone treatment does not further enhance AR levels[99]. On the other hand, normal adult males with intact testis or testosterone treatment maintain high levels of AR but in castrated rats estrogen treatment reduces AR expression[99]. Animal studies demonstrated that the expression of both AR and ER increases during preneoplastic stages and that progression towards cancer development can suppress ER and maintain AR expression levels[94,100].
Interestingly, anti-androgen treatment has been shown to reduce AR levels in liver as well as the size and number of tumors in male Spargue Dawley rat model of hepatocarcinogenesis[101]. It has been shown that inhibition of AR positive HCC with anti-androgen cypertone acetate in male mice involves cell cycle arrest and to some extent induction of apoptosis due to increased synthesis of transforming growth factor-β 1 (TGF-β1)[102]. In another model of chemical-induced liver carcinogenesis, inhibition of androgens using 5-alpha reductase inhibitors significantly suppressed HCC development in rats[103]. Recent studies in a xenograft model of hepatocarcinogenesis in nude mice suggested that AR expression remains elevated until development of tumor and starts declining as the size of tumor increases[104]. It is therefore proposed that androgen therapy may be ineffective after establishment of the tumor. Nevertheless, for better understanding and rationale design of hormone-based therapies, it is mandatory to study the role of ERs and AR in animal models mimicking the natural course of disease progression to HCC development as in humans. Currently available HBV and HCV transgenic mice depicting features close to human HCC pathogenesis, appear to be promising models for future in vivo studies.
Estrogen action and the role of ERs in carcinogenesis have been well documented in mammary carcinoma and the studies have revealed the involvement of estrogens in key cellular processes such as apoptosis, cell cycle, proliferation, oxidative stress and inflammation. The progress in understanding the role of estrogen in regulating various cellular events in liver carcinogenesis has been rather slow. However, the research conducted over recent years provides key insights in this direction.
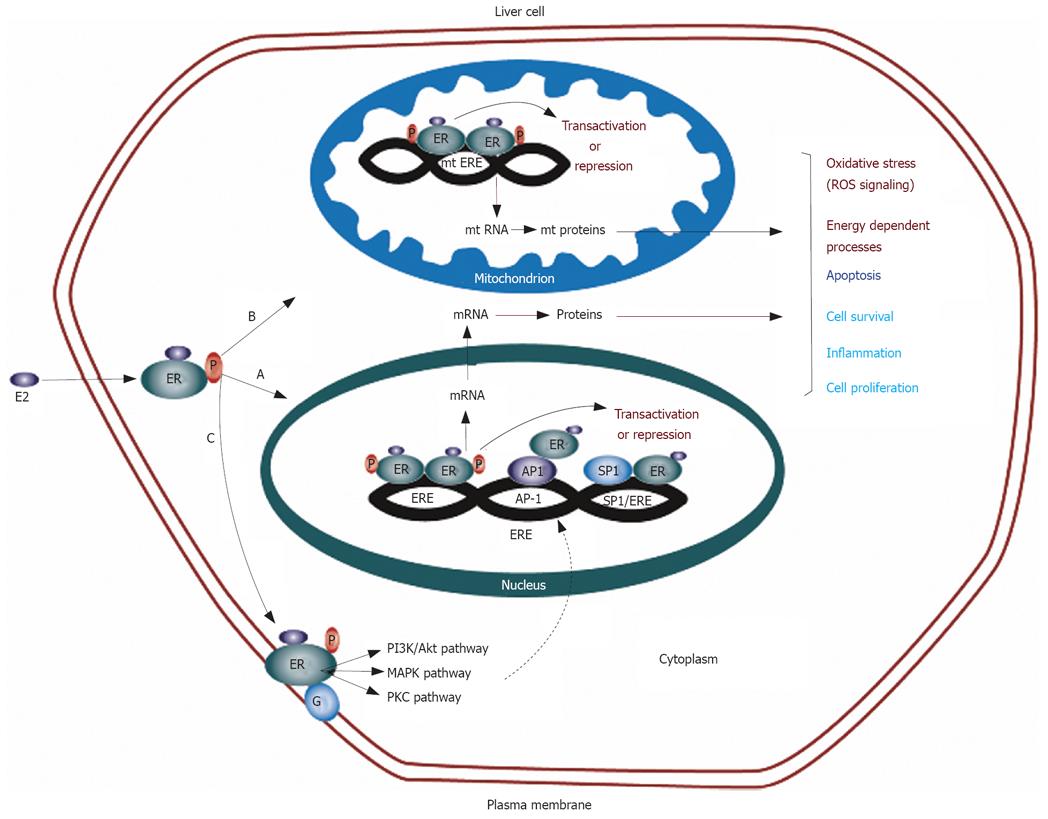
The classical mode of estrogen action is the genomic mechanism in which ERs function as ligand-activated transcription factors[105]. Activated ERs translocate to the nuclei and regulate the expression of specific target genes. These transcriptional regulations are achieved through interaction with estrogen responsive element (ERE) sequences located in the promoter region of the target gene[106]. However, one third of the genes regulated by ERs in humans do not contain ERE-like sequences[107]. ERs can also regulate the transcription of such genes without binding to DNA through protein-protein interactions with other transcription factors, such as AP-1 and Sp-1 in the nuclei[108]. In addition, this transcriptional control at alternate response elements is also facilitated by non-genomic actions of estrogen. The non-genomic functions of estrogen are initiated by membrane-localized ERs and are associated with activation of various signaling pathways especially protein kinases[109]. The functions of many transcription factors are regulated through protein kinase-mediated phosphorylation including CREB, NF-κB and AP-1 and these transcription factors may thus be targets for non-genomic actions of estrogens. This possible convergence of genomic and non-genomic actions at multiple response elements provides an extremely fine degree of control for the regulation of transcription for ERs (Figure 1)[105,110]. In the following section, we discuss the findings of recent in vitro studies highlighting the significance of ER in mediating genomic and non-genomic actions of estrogen in liver cells to modulate the expression of a number of genes involved in cellular processes central to carcinogenesis.
Telomerase activation has been implicated in hepatocarcinogenesis and the expression of human telomerase reverse transcriptase (hTERT) that encodes for the catalytic subunit of the multicomponent enzyme telomerase hTERT is the prerequisite for telomerase activation[111,112]. Several studies indicated that estrogen regulates transactivation of the hTERT gene by direct interaction of activated ER with an imperfect ERE sequence in the hTERT promoter[113]. Estrogen treatment has been shown to up-regulate the expression of hTERT mRNA and protein in three normal human hepatic cell lines (hc-cells, hNheps and WRL-68) expressing ERα to varying degrees[90]. Furthermore, estrogen exposure prevents shortening of telomeres and decreases the number of cells undergoing senescence, indicating that estradiol acts as a positive modulator of the hTERT gene in the liver[90]. However, the mechanism of ER-mediated transactivation of hTERT in the liver is not well understood. In contrast, in HepG2 cells, estrogen modulation of telomerase activity has been found to be regulated post-transcriptionally via the IP3/PKC pathway[114,115]. IP3 production has been shown to be up-regulated by estrogens in HepG2 cells[114]. Furthermore, estradiol-induced IP3/PKC-alpha production is dependent on either ERα or ERβ expression in both HepG2 and Hela cells[114]. It is hypothesized that membrane ER-mediated IP3/PKC-alpha pathway represents an alternative signaling pathway utilized by cells when low ER levels are unable to activate classic ER-mediated genomic mechanisms as in HepG2 cells[114].
A similar regulatory mechanism has been observed in case of estrogen modulation of expression of cyclin D1 gene in hepatoma cells. Cyclin D1, important for progression of cells through G1 phase of cell cycle, is a well defined target for estrogen action in mammary carcinoma[116,117], although no detectable estrogen responsive element like sequence in the cyclin D1 gene promoter has been reported in these cells[118]. The cyclin D1 mechanism identified in mammary carcinoma cells involves direct interaction of ERα and Sp1 or ERα and Ap-1[119]. Interestingly, Marino et al[23] demonstrated that in HepG2 cells, estrogen-induced activation of cyclin D1 transcription can occur independently of the transcriptional activity of ER. They further showed that the effect of 17-beta estradiol on HepG2 cells is mediated by activation of the MAPK/ERK pathway by membrane-localized ER that increases the expression of cyclin D1 gene through activation of AP-1 transcription factor[23], suggesting that non-genomic signaling pathways play an the pivotal role in estrogen-mediated regulation of gene expression at multiple response elements.
Besides, modulating the molecules involved in cell cycle control and cell proliferation, estrogen has also been shown to regulate the expression of genes crucial for apoptosis of hepatocytes and dysregulation of apoptosis in hepatic cells is reported to be a significant factor in accelerating hepatocarcinogenesis or tumor progression in HCC[120]. The Bcl-2 family of proteins regulates one of the key steps in the conserved apoptotic pathway. Among the members of this family, Bcl-2 and Bcl-xL act as inhibitors of apoptosis where as Bax and Bak promote apoptosis[121,122]. Ethinyl estradiol is known to increase the levels of Bcl-2 protein in cultured female rat hepatocytes[123]. Estradiol and idoxifene, two selective estrogen receptor modulators, are known to induce the expression of Bcl-2 protein in male rat liver tissues[124]. Omoya et al[9] and Inoue et al[81] also demonstrated that estradiol is able to stimulate the expression of Bcl-2 and Bcl-xL and to suppress Bad expression in oxidative stress-induced early apoptotic rat hepatocytes. Similar findings have been recently documented in response to estradiol treatment of Huh-7 human hepatoma cells describing a dose dependent increase in expression of Bcl-2 and Bcl-xL and a reduction in Bad levels[22]. No change was observed in expression of pro-apoptotic protein Bax. The regulation of Bcl-2 gene expression by estrogen in mammary carcinoma cells has been shown to be mediated indirectly through activation of Sp-1 transcription factor[125]. However, the precise mechanism of Bcl-2 transactivation in hepatocytes has not been clearly understood.
One of the most interesting mechanisms of transcriptional regulation at alternate response elements by estrogen is through inhibition of transcription factor NF-κB. Studies demonstrating a mutually antagonistic cross-talk between these families of transcription factors have been recently reviewed[126]. The ER has been shown to mediate opposition of NF-κB functions at various levels by inhibiting the activation of signaling pathways, preventing nuclear translocation, blocking DNA binding or inhibiting recruitment of co-activators for transcription[126]. Estrogen has been shown to bring about its anti-inflammatory and anti-oxidant effects on liver cells by suppressing the NF-κB activity as evident from the following studies. It was reported that 17 beta-estradiol-bound ERα interferes with cytokine-induced activation of a NF-κB reporter in HepG2 cells, suggesting that estrogen exerts its anti-inflammatory and protective effects on human liver cells[127]. Moreover, in an in vivo model, estrogen treatment has been shown to block the induction of hepatic expression of inflammatory vascular cell adhesion molecule-1 (VCAM-1), tumor necrosis factor-α (TNF-α), and regulate normal T-cell expression and secretion upon activation[128]. In a mouse model of DEN-induced HCC, ERα was suggested to suppress IL-6 production, a pro-inflammatory molecule, through the involvement of the NF-κB pathway[83]. Estrogen has also been reported to suppress oxidative stress-induced reactive oxygen species (ROS) generation, lipid peroxidation, activation of AP-1 and NF-κB as well as loss of Cu-Zn SOD activity in cultured rat hepatocytes[2,9].
In addition to genomic and non-genomic actions of estrogen mediated by nuclear and membrane ER, mitochondria have also recently been identified as important targets of estrogen and ERs[110]. Early binding studies on sub-cellular fractions indicated that ER is present in rat liver mitochondria[129]. Both ERα and ERβ have been reported to be present in the mitochondria of human HepG2 cells[130-132]. The mitochondrial genome has been shown to contain sequences that have partial homology to the estrogen responsive elements[132-134]. Both ERα and ERβ bind to mitochondrial DNA and the binding can be increased by estradiol using mobility shift assays and surface plasmon resonance[135]. These results suggest that estradiol is directly involved in the regulation of mitochondrial DNA transcription (Figure 1). Regulation of apoptosis and oxidative metabolism by estrogens in mitochondria may be important in the normal liver and in the development of HCC.
Ethinyl estradiol treatment has been shown to elevate the expression levels of mitochondrial DNA-encoded cytochrome C oxidase subunit III (CO III) and ATP synthase 6 in vivo as well as in HepG2 cells[136]. This increased expression of mitochondrial transcripts is accompanied by increased mitochondrial superoxide production and respiratory chain activity that require cytochrome P450-mediated biotransformation of ethinyl estradiol and 17-beta estradiol to catechol metabolites[136,137]. In addition to CO III, the levels of CO I and CO II encoded by mitochondrial DNA have also been found to be elevated in ethinyl estradiol treated female rat hepatocytes. This effect is accompanied by increased mitochondrial superoxide production, high ATP levels and increased Bcl2 production, and is suggested to play a role in ethinyl estradiol-mediated inhibition of apoptosis[123]. In contrast, 17-beta estradiol and 17-beta estradiol like compounds, diethylstilbestrol (DES), tamoxifen and genistein, have been found to induce apoptotic effects in human hepatoma Hep3B cell line[138]. These compounds cause the leaking of cytochrome C from mitochondria and activation of caspase-3 in an ER dependent manner. In another study, the two isoforms, ERα and ERβ, showed their opposing actions on apoptosis in a poorly differentiated HCC cell line HA22T[139]. Over-expressed ERβ but not ERα induces the expression of caspase-8 and TNF-α in HA22T cells in response to estradiol treatment, indicating that the death receptor-mediated apoptotic pathway is activated[139].
Differential roles of ERα and ERβ have also been observed in non-genomic actions of estrogen in the liver[21,23]. There is indirect evidence that membrane ER may exist in human liver as the binding of gold tagged estrogen-BSA conjugate on the surface of clathrin-coated pits in HepG2 cells has been demonstrated by electron microscopic visualization[140]. The non-genomic mechanism of action of sex steroids on the plasma membrane involves the activation of protein kinase cascades (Figure 1). Two major cascades, protein kinase C, and mitogen-activated protein (MAP) kinase are active and important in carcinogenic liver cells. Protein kinase C cascade and its second messenger IP3 are important in cell proliferation and have been discussed in this review in context of transcriptional regulation of hTERT expression by estrogen. The mitogen-activated protein (MAP) kinase cascade is another pathway that is regulated by the action of sex steroids at the plasma membrane. This complex signaling cascade involves three major pathways: ERK, p38, and JNK[141]. In HepG2 cells, estradiol has been found to rapidly increase the phosphorylation of ERK[21,23]. Naringenin, an anti-estrogenic flavonone, induces the activation of p38 in ERα containing HepG2 cells or in ERβ containing human colon adenocarcinoma DLD-1 cells[142], suggesting that naringenin has an antiestrogenic effect only on the ERα expressing cells, whereas it mimicks the estradiol effects on ERβ expressing cells. The role of ERα and ERβ in the regulation of MAP kinase cascade has been further studied in cell lines expressing either ERα or ERβ[21]. It was found that estrogen-bound ERα can rapidly activate the ERK and AKT signal transduction pathways leading to cell cycle progression and inhibition of apoptosis, whereas estrogen-complexed ERβ can induce rapid phosphorylation of p38 leading the cells to the apoptotic cycle and cell death. These studies further support the functional antagonism between ERα and ERβ with respect to estrogen-induced cell proliferation and emphasize the need to study the independent and interactive role of both isoforms in hepatocarcinogenesis.
In comparison with ER, there is limited information about genomic and non-genomic functions of AR in the liver. Like ER, AR has also been shown to regulate gene transcription by binding to androgen responsive sequences (ARE)[143,144]. Yoon et al[145] demonstrated that androgen can directly regulate the expression of transformation growth factor-beta 1 (TGF-β1) through binding of AR to ARE in TGF-β1 promoter, suggesting that such activation might regulate the progression of HCC in both human and animal models[145]. Furthermore, AR has been shown to interact with a newly identified transcription factor, paternally expressed gene 10 (PEG 10) in hepatoma cell line[146]. PEG 10 has growth promoting properties and is implicated in hepatocarcinogenesis[147,148]. Dihydrotestosterone (DHT) promotes hepatoma formation in nude mice through PEG 10 activation. In addition, DHT treatment is shown to up-regulate hTERT expression in hepatoma cell lines in a PEG-10 dependent manner[146]. These studies indicate that PEG-10-mediated transactivation of target genes by AR has an essential role in hepatocarcinogenesis.
To the best of our knowledge, AR has not been detected in the liver mitochondria. The information about the membrane localization of AR in human liver cells is also lacking. However, AR has been reported to occur in the plasma membranes of male rat liver[149]. Androgens are also involved in the regulation of the MAP kinase signaling pathways as orchiectomy of H-ras 12V transgenic mice decreases phospho-MEK and phospho-ERK in liver tissues. In addition, orchiectomy reduces hepatotumorigenesis in male mice while ovariectomy increases phospho-MEK and phospho-ERK in liver tissue from female mice, but ovariectomy does not affect the incidence of tumorigenesis[150]. Detailed investigations are urgently needed to confirm the existence of non-genomic signaling actions of androgens in liver and the role of AR in mediating these functions.
Chronic infection with HBV and HCV is the major cause of increasing incidence of HCC worldwide. Several reports support the role of HBV and HCV proteins in disturbing cellular homeostasis and causing malignant transformation of hepatocytes[151,152]. Recent studies in hepatoma cell lines suggest the interactive role of ERs and AR with HBV and HCV proteins in viral pathogenesis.
Han et al[24] recently reported that HBV protein (HBx) interacts with ERα. HBx is a multifunctional protein involved in neoplastic transformation in cultured cells and can induce HCC in transgenic mice. HBx associates with both ERα and delta 5 deletion variant of ERα (vERα) and inhibits ERα transcriptional activity by recruiting histone deacetylase enzyme, HDAC-1[24]. HDAC-1 belongs to the family of enzymes involved in deacetylation of hyperacetylated histone tails, leading to compaction of chromatin and transcritptional repression[153]. Both HBx and vERα have additive effects on suppression of ERα transactivation[24].
ERα has also been shown to interact with nonstructural (NS) 5B protein of HCV[25]. NS5B is a RNA-dependent RNA polymerase, which plays a central role in viral genome replication[154]. HCV replication takes place in a replication complex consisting of viral RNA and non structural proteins including NS5B[155]. The replication complex forms on the surface of intracellular membranes including endoplasmic reticulum membrane and is associated with lipid rafts rich in caveolin 2 (CAV 2) on these membranes[156-158]. Using chemical biology approach, Watashi et al[25] demonstrated that ERα facilitates the interaction of NS5B with CAV 2 in lipid rafts and hence promotes the participation of NS5B in HCV replication complex. However, they did not find that ERβ affects HCV replication in the same study[25]. An important observation of the study is that tamoxifen inhibits ERα actions and suppresses HCV genome replication, further supporting the potential for anti-ER drugs in developing new anti-HCV strategies.
Like ER, HBx protein has also been shown to interact with AR. HBx functions as a positive transcriptional co-regulator to increase AR-mediated transcriptional activity. This transcription enhancement is increased in the presence of androgen in a concentration-responsive manner. However, HBx does not physically associate with ligand-bound AR in the nuclei, suggesting that HBx augments AR activity by increasing the phosphorylation of AR through HBx-mediated activation of the c-Src kinase signaling pathway[159]. In contrast, Zheng et al[160] demonstrated that HBx can physically bind to AR in the liver and alter the subcellular localization of AR both in the presence and absence of dihydrotestosterone (DHT). Further studies indicated that HBx can enhance the gene transactivation activity of AR by enhancing its DNA binding activity in a DHT-dependent manner.
Taken together, studies on hepatoma cell lines, HCC tissues and animal models of hepatocarcinogenesis, highlight the importance of sex hormones and their receptors in HCC pathogenesis. Further investigations are urgently needed to elucidate the precise mechanism of action of estrogens, androgens and their receptors in regulating normal liver physiology and pathophysiology of chronic liver diseases resulting in HCC.
A thorough re-examination of studies conducted so far to detect the expression of ER and AR in liver tissues is needed using newer specific, sensitive and quantitative methods. With the emerging significance of ERβ and the availability of isoform specific antibodies, the relative levels of both ERα and ERβ can be determined. The studies on mRNA expression in liver tissues have demonstrated the presence of deletion mutants (variant forms) that need to be further validated at protein levels for establishing their significance in diagnosis and prognosis of HCC. Considering the male predominance of HCC and the wide gap in the information available on AR in liver, detailed mechanistic studies need to be conducted to reveal the mechanism of androgen function in normal liver and HCC. In addition, evaluation of ER and AR status at premalignant stages of chronic liver disease due to different etiological factors is required for critical understanding of their role in HCC pathogenesis.
Recent in vitro studies focusing on molecular interaction of hormonal receptors with viral proteins need to be further confirmed in in vivo animal models. Currently available HBV and HCV transgenic mouse models as well as human hepatocyte xenograft models can serve as a valuable preclinical tools to validate the importance of sex hormone receptors in chronic liver disease development and progression to HCC. Thus, with the availability of state of the art technologies, the time is ripe to embark on to move this important field forward. Well designed, systematic studies employing adequate tools to study ERs and ARs in chronic liver disease and HCC may contribute to the development of novel therapeutics or prognostic markers. These studies may also be further helpful in resolving controversies about the use of hormonal therapy for HCC.
| 1. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. |
| 3. | De Maria N, Manno M, Villa E. Sex hormones and liver cancer. Mol Cell Endocrinol. 2002;193:59-63. |
| 4. | Granata OM, Carruba G, Montalto G, Miele M, Bellavia V, Modica G, Blomquist CH, Castagnetta LA. Altered androgen metabolism eventually leads hepatocellular carcinoma to an impaired hormone responsiveness. Mol Cell Endocrinol. 2002;193:51-58. |
| 5. | Farinati F, Cardin R, Bortolami M, Grottola A, Manno M, Colantoni A, Villa E. Estrogens receptors and oxidative damage in the liver. Mol Cell Endocrinol. 2002;193:85-88. |
| 6. | Farinati F, De Maria N, Marafin C, Fagiuoli S, Della Libera G, Naccarato R. Hepatocellular carcinoma in alcoholic cirrhosis: is sex hormone imbalance a pathogenetic factor? Eur J Gastroenterol Hepatol. 1995;7:145-150. |
| 7. | Shimizu I, Ito S. Protection of estrogens against the progression of chronic liver disease. Hepatol Res. 2007;37:239-247. |
| 8. | Shimizu I, Yasuda M, Mizobuchi Y, Ma YR, Liu F, Shiba M, Horie T, Ito S. Suppressive effect of oestradiol on chemical hepatocarcinogenesis in rats. Gut. 1998;42:112-119. |
| 9. | Omoya T, Shimizu I, Zhou Y, Okamura Y, Inoue H, Lu G, Itonaga M, Honda H, Nomura M, Ito S. Effects of idoxifene and estradiol on NF-kappaB activation in cultured rat hepatocytes undergoing oxidative stress. Liver. 2001;21:183-191. |
| 10. | Gavrilova-Jordan LP, Price TM. Actions of steroids in mitochondria. Semin Reprod Med. 2007;25:154-164. |
| 11. | Boonyaratanakornkit V, Edwards DP. Receptor mechanisms mediating non-genomic actions of sex steroids. Semin Reprod Med. 2007;25:139-153. |
| 12. | Pettersson K, Gustafsson JA. Role of estrogen receptor beta in estrogen action. Annu Rev Physiol. 2001;63:165-192. |
| 13. | Nagasue N, Ito A, Yukaya H, Ogawa Y. Estrogen receptors in hepatocellular carcinoma. Cancer. 1986;57:87-91. |
| 14. | Nagasue N, Ito A, Yukaya H, Ogawa Y. Androgen receptors in hepatocellular carcinoma and surrounding parenchyma. Gastroenterology. 1985;89:643-647. |
| 15. | Nagasue N, Kohno H, Chang YC, Hayashi T, Utsumi Y, Nakamura T, Yukaya H. Androgen and estrogen receptors in hepatocellular carcinoma and the surrounding liver in women. Cancer. 1989;63:112-116. |
| 16. | Negro F, Papotti M, Pacchioni D, Galimi F, Bonino F, Bussolati G. Detection of human androgen receptor mRNA in hepatocellular carcinoma by in situ hybridisation. Liver. 1994;14:213-219. |
| 17. | Ohnishi S, Murakami T, Moriyama T, Mitamura K, Imawari M. Androgen and estrogen receptors in hepatocellular carcinoma and in the surrounding noncancerous liver tissue. Hepatology. 1986;6:440-443. |
| 18. | Villa E, Dugani A, Moles A, Camellini L, Grottola A, Buttafoco P, Merighi A, Ferretti I, Esposito P, Miglioli L. Variant liver estrogen receptor transcripts already occur at an early stage of chronic liver disease. Hepatology. 1998;27:983-988. |
| 19. | Iavarone M, Lampertico P, Seletti C, Francesca Donato M, Ronchi G, del Ninno E, Colombo M. The clinical and pathogenetic significance of estrogen receptor-beta expression in chronic liver diseases and liver carcinoma. Cancer. 2003;98:529-534. |
| 20. | Acconcia F, Kumar R. Signaling regulation of genomic and nongenomic functions of estrogen receptors. Cancer Lett. 2006;238:1-14. |
| 21. | Acconcia F, Totta P, Ogawa S, Cardillo I, Inoue S, Leone S, Trentalance A, Muramatsu M, Marino M. Survival versus apoptotic 17beta-estradiol effect: role of ER alpha and ER beta activated non-genomic signaling. J Cell Physiol. 2005;203:193-201. |
| 22. | Cheng X, Shimizu I, Yuan Y, Wei M, Shen M, Huang H, Urata M, Sannomiya K, Fukuno H, Hashimoto-Tamaoki T. Effects of estradiol and progesterone on tumor necrosis factor alpha-induced apoptosis in human hepatoma HuH-7 cells. Life Sci. 2006;79:1988-1994. |
| 23. | Marino M, Acconcia F, Bresciani F, Weisz A, Trentalance A. Distinct nongenomic signal transduction pathways controlled by 17beta-estradiol regulate DNA synthesis and cyclin D(1) gene transcription in HepG2 cells. Mol Biol Cell. 2002;13:3720-3729. |
| 24. | Han J, Ding L, Yuan B, Yang X, Wang X, Li J, Lu Q, Huang C, Ye Q. Hepatitis B virus X protein and the estrogen receptor variant lacking exon 5 inhibit estrogen receptor signaling in hepatoma cells. Nucleic Acids Res. 2006;34:3095-3106. |
| 25. | Watashi K, Inoue D, Hijikata M, Goto K, Aly HH, Shimotohno K. Anti-hepatitis C virus activity of tamoxifen reveals the functional association of estrogen receptor with viral RNA polymerase NS5B. J Biol Chem. 2007;282:32765-32772. |
| 26. | Fechner RE. Benign hepatic lesions and orally administered contraceptives. A report of seven cases and a critical analysis of the literature. Hum Pathol. 1977;8:255-268. |
| 27. | Vana J, Murphy GP, Aronoff BL, Baker HW. Primary liver tumors and oral contraceptives. Results of a survey. JAMA. 1977;238:2154-2158. |
| 28. | Duffy MJ, Duffy GJ. Estradiol receptors in human liver. J Steroid Biochem. 1978;9:233-235. |
| 29. | Molteni A, Bahu RM, Battifora HA, Fors EM, Reddy JK, Rao MS, Scarpelli DG. Estradiol receptor assays in normal and neoplastic tissues. A possible diagnostic acid for tumor differentiation. Ann Clin Lab Sci. 1979;9:103-108. |
| 30. | Friedman MA, Demanes DJ, Hoffman PG Jr. Hepatomas: hormone receptors and therapy. Am J Med. 1982;73:362-366. |
| 31. | Iqbal MJ, Wilkinson ML, Johnson PJ, Williams R. Sex steroid receptor proteins in foetal, adult and malignant human liver tissue. Br J Cancer. 1983;48:791-796. |
| 32. | Kohigashi K, Fukuda Y, Imura H. Estrogen receptors in hepatocellular carcinoma: is endocrine therapy for hepatocellular carcinoma likely to be effective? Gastroenterol Jpn. 1987;22:322-330. |
| 33. | Eagon PK, Francavilla A, DiLeo A, Elm MS, Gennari L, Mazzaferro V, Colella G, Van Thiel DH, Strazl TE. Quantitation of estrogen and androgen receptors in hepatocellular carcinoma and adjacent normal human liver. Dig Dis Sci. 1991;36:1303-1308. |
| 34. | Nagasue N, Kohno H, Chang YC, Yamanoi A, Nakamura T, Yukaya H, Hayashi T. Clinicopathologic comparisons between estrogen receptor-positive and -negative hepatocellular carcinomas. Ann Surg. 1990;212:150-154. |
| 35. | Nagasue N, Kohno H, Chang Y, Hayashi T, Nakamura T. Specificity of androgen receptors of hepatocellular carcinoma and liver in humans. Hepatogastroenterology. 1990;37:474-479. |
| 36. | Nagasue N, Kohno H, Yamanoi A, Kimoto T, Chang YC, Nakamura T. Progesterone receptor in hepatocellular carcinoma. Correlation with androgen and estrogen receptors. Cancer. 1991;67:2501-2505. |
| 37. | Francavilla A, Panella C, Amoruso A, Giangaspero A, Gennari L, Mazzaferro V, Colella G, Van Thiel DH, Starzl TE. Role of estrogens and epidermal growth factor in hepatocellular carcinoma (HCC). Dig Dis Sci. 1991;36:1299-1302. |
| 38. | Eagon PK, Elm MS, Stafford EA, Porter LE. Androgen receptor in human liver: characterization and quantitation in normal and diseased liver. Hepatology. 1994;19:92-100. |
| 39. | Wong LY, Chan SH, Oon CJ, Rauff A. Immunocytochemical localization of testosterone in human hepatocellular carcinoma. Histochem J. 1984;16:687-692. |
| 40. | Pacchioni D, Papotti M, Andorno E, Bonino F, Mondardini A, Oliveri F, Brunetto M, Bussolati G, Negro F. Expression of estrogen receptor mRNA in tumorous and non-tumorous liver tissue as detected by in situ hybridization. J Surg Oncol Suppl. 1993;3:14-17. |
| 41. | Boix L, Bruix J, Castells A, Fuster J, Bru C, Visa J, Rivera F, Rodes J. Sex hormone receptors in hepatocellular carcinoma. Is there a rationale for hormonal treatment? J Hepatol. 1993;17:187-191. |
| 42. | Jonas S, Bechstein WO, Heinze T, Kling N, Lobeck H, Tullius SG, Steinmueller T, Neuhaus P. Female sex hormone receptor status in advanced hepatocellular carcinoma and outcome after surgical resection. Surgery. 1997;121:456-461. |
| 43. | Ng IO, Ng M, Fan ST. Better survival in women with resected hepatocellular carcinoma is not related to tumor proliferation or expression of hormone receptors. Am J Gastroenterol. 1997;92:1355-1358. |
| 44. | Moon WS, Chang K, Tarnawski AS. Overexpression of metastatic tumor antigen 1 in hepatocellular carcinoma: Relationship to vascular invasion and estrogen receptor-alpha. Hum Pathol. 2004;35:424-429. |
| 45. | Xing BC, Wang JH, Wang Y, Hao CY, Huang XF, Wang Y. [Expression of wild type and variant estrogen receptors in human hepatocellular carcinoma.]. Beijing Da Xue Xue Bao. 2004;36:620-622. |
| 46. | Meza-Junco J, Montano-Loza AJ, Gamboa-Dominguez A, Green-Renner D. Expression of oestrogen and growth factor receptors in hepatocellular carcinoma. Clin Oncol (R Coll Radiol). 2007;19:801-802. |
| 47. | Vizoso FJ, Rodriguez M, Altadill A, Gonzalez-Dieguez ML, Linares A, Gonzalez LO, Junquera S, Fresno-Forcelledo F, Corte MD, Rodrigo L. Liver expression of steroid hormones and Apolipoprotein D receptors in hepatocellular carcinoma. World J Gastroenterol. 2007;13:3221-3227. |
| 48. | Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925-5930. |
| 49. | Walter P, Green S, Greene G, Krust A, Bornert JM, Jeltsch JM, Staub A, Jensen E, Scrace G, Waterfield M. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci USA. 1985;82:7889-7893. |
| 50. | Green S, Walter P, Greene G, Krust A, Goffin C, Jensen E, Scrace G, Waterfield M, Chambon P. Cloning of the human oestrogen receptor cDNA. J Steroid Biochem. 1986;24:77-83. |
| 51. | Villa E, Camellini L, Dugani A, Zucchi F, Grottola A, Merighi A, Buttafoco P, Losi L, Manenti F. Variant estrogen receptor messenger RNA species detected in human primary hepatocellular carcinoma. Cancer Res. 1995;55:498-500. |
| 52. | Wang AG, Lee KY, Kim SY, Choi JY, Lee KH, Kim WH, Wang HJ, Kim JM, Park MG, Yeom YI. The expression of estrogen receptors in hepatocellular carcinoma in Korean patients. Yonsei Med J. 2006;47:811-816. |
| 53. | Murphy LC, Dotzlaw H, Leygue E, Coutts A, Watson P. The pathophysiological role of estrogen receptor variants in human breast cancer. J Steroid Biochem Mol Biol. 1998;65:175-180. |
| 54. | Villa E, Dugani A, Fantoni E, Camellini L, Buttafoco P, Grottola A, Pompei G, De Santis M, Ferrari A, Manenti F. Type of estrogen receptor determines response to antiestrogen therapy. Cancer Res. 1996;56:3883-3885. |
| 55. | Villa E, Moles A, Ferretti I, Buttafoco P, Grottola A, Del Buono M, De Santis M, Manenti F. Natural history of inoperable hepatocellular carcinoma: estrogen receptors' status in the tumor is the strongest prognostic factor for survival. Hepatology. 2000;32:233-238. |
| 56. | Villa E, Colantoni A, Camma C, Grottola A, Buttafoco P, Gelmini R, Ferretti I, Manenti F. Estrogen receptor classification for hepatocellular carcinoma: comparison with clinical staging systems. J Clin Oncol. 2003;21:441-446. |
| 57. | Villa E, Colantoni A, Grottola A, Ferretti I, Buttafoco P, Bertani H, De Maria N, Manenti F. Variant estrogen receptors and their role in liver disease. Mol Cell Endocrinol. 2002;193:65-69. |
| 58. | Leygue E, Dotzlaw H, Watson PH, Murphy LC. Altered estrogen receptor alpha and beta messenger RNA expression during human breast tumorigenesis. Cancer Res. 1998;58:3197-3201. |
| 59. | Macdonald JS, Lippman ME, Woolley PV, Petrucci PP, Schein PS. Hepatic estrogen and progesterone receptors in an estrogen-associated hepatic neoplasm. Cancer Chemother Pharmacol. 1978;1:135-138. |
| 60. | Bojar H, Schutte J, Staib W, Broelsch C. Does human liver contain estrogen receptors? A comparative study of estrogen binding in human and rodent liver. Klin Wochenschr. 1982;60:417-425. |
| 61. | Porter LE, Elm MS, Van Thiel DH, Dugas MC, Eagon PK. Characterization and quantitation of human hepatic estrogen receptor. Gastroenterology. 1983;84:704-712. |
| 62. | Porter LE, Elm MS, Van Thiel DH, Eagon PK. Hepatic estrogen receptor in human liver disease. Gastroenterology. 1987;92:735-745. |
| 63. | Ohnami S, Nakata H, Nagafuchi Y, Zeze F, Eto S. [Estrogen receptors in human gastric, hepatocellular, and gallbladder carcinomas and normal liver tissues]. Gan To Kagaku Ryoho. 1988;15:2923-2928. |
| 64. | Hamazaki K, Miura H, Sakai H, Sato S, Yunoki M, Miichi N, Noda T, Mori M, Orita K. [Estrogen and androgen receptors in hepatocellular carcinoma and in noncancerous liver tissue]. Gan No Rinsho. 1989;35:1109-1113. |
| 65. | Wilkinson ML, Iqbal MJ, Williams R. Characterisation of high affinity binding sites of androgens in primary hepatocellular carcinoma. Clin Chim Acta. 1985;152:105-113. |
| 66. | Bannister P, Meystre CM, Losowsky MS. Androgen receptor concentrations in needle biopsy specimens of human liver. Liver. 1988;8:28-31. |
| 67. | Nagasue N, Chang YC, Hayashi T, Galizia G, Kohno H, Nakamura T, Yukaya H. Androgen receptor in hepatocellular carcinoma as a prognostic factor after hepatic resection. Ann Surg. 1989;209:424-427. |
| 68. | Nagasue N, Yamanoi A, Kohno H, Kimoto T, Chang Y, Taniura H, Uchida M, Nakamura T. Androgen receptor in cirrhotic liver, adenomatous hyperplastic nodule and hepatocellular carcinoma in the human. Hepatogastroenterology. 1992;39:455-460. |
| 69. | Boix L, Castells A, Bruix J, Sole M, Bru C, Fuster J, Rivera F, Rodes J. Androgen receptors in hepatocellular carcinoma and surrounding liver: relationship with tumor size and recurrence rate after surgical resection. J Hepatol. 1995;22:616-622. |
| 70. | Zhang X, He L, Lu Y, Liu M, Huang X. Androgen receptor in primary hepatocellular carcinoma and its clinical significance. Chin Med J (Engl). 1998;111:1083-1086. |
| 71. | Tavian D, De Petro G, Pitozzi A, Portolani N, Giulini SM, Barlati S. Androgen receptor mRNA under-expression in poorly differentiated human hepatocellular carcinoma. Histol Histopathol. 2002;17:1113-1119. |
| 72. | Nagasue N, Yu L, Yukaya H, Kohno H, Nakamura T. Androgen and oestrogen receptors in hepatocellular carcinoma and surrounding liver parenchyma: impact on intrahepatic recurrence after hepatic resection. Br J Surg. 1995;82:542-547. |
| 73. | Engstrom PF, Levin B, Moertel CG, Schutt A. A phase II trial of tamoxifen in hepatocellular carcinoma. Cancer. 1990;65:2641-2643. |
| 74. | Castells A, Bruix J, Bru C, Ayuso C, Roca M, Boix L, Vilana R, Rodes J. Treatment of hepatocellular carcinoma with tamoxifen: a double-blind placebo-controlled trial in 120 patients. Gastroenterology. 1995;109:917-922. |
| 75. | Liu CL, Fan ST, Ng IO, Lo CM, Poon RT, Wong J. Treatment of advanced hepatocellular carcinoma with tamoxifen and the correlation with expression of hormone receptors: a prospective randomized study. Am J Gastroenterol. 2000;95:218-222. |
| 76. | Villa E, Ferretti I, Grottola A, Buttafoco P, Buono MG, Giannini F, Manno M, Bertani H, Dugani A, Manenti F. Hormonal therapy with megestrol in inoperable hepatocellular carcinoma characterized by variant oestrogen receptors. Br J Cancer. 2001;84:881-885. |
| 77. | Chow PK, Tai BC, Tan CK, Machin D, Win KM, Johnson PJ, Soo KC. High-dose tamoxifen in the treatment of inoperable hepatocellular carcinoma: A multicenter randomized controlled trial. Hepatology. 2002;36:1221-1226. |
| 78. | Di Maio M, De Maio E, Morabito A, D'Aniello R, De Feo G, Gallo C, Perrone F. Hormonal treatment of human hepatocellular carcinoma. Ann N Y Acad Sci. 2006;1089:252-261. |
| 79. | Di Maio M, Daniele B, Pignata S, Gallo C, De Maio E, Morabito A, Piccirillo MC, Perrone F. Is human hepatocellular carcinoma a hormone-responsive tumor? World J Gastroenterol. 2008;14:1682-1689. |
| 80. | Alvaro D, Alpini G, Onori P, Perego L, Svegliata Baroni G, Franchitto A, Baiocchi L, Glaser SS, Le Sage G, Folli F. Estrogens stimulate proliferation of intrahepatic biliary epithelium in rats. Gastroenterology. 2000;119:1681-1691. |
| 81. | Inoue H, Shimizu I, Lu G, Itonaga M, Cui X, Okamura Y, Shono M, Honda H, Inoue S, Muramatsu M. Idoxifene and estradiol enhance antiapoptotic activity through estrogen receptor-beta in cultured rat hepatocytes. Dig Dis Sci. 2003;48:570-580. |
| 82. | Zhou Y, Shimizu I, Lu G, Itonaga M, Okamura Y, Shono M, Honda H, Inoue S, Muramatsu M, Ito S. Hepatic stellate cells contain the functional estrogen receptor beta but not the estrogen receptor alpha in male and female rats. Biochem Biophys Res Commun. 2001;286:1059-1065. |
| 83. | Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121-124. |
| 84. | Lee JS, Chu IS, Mikaelyan A, Calvisi DF, Heo J, Reddy JK, Thorgeirsson SS. Application of comparative functional genomics to identify best-fit mouse models to study human cancer. Nat Genet. 2004;36:1306-1311. |
| 85. | Mishkin SY, Farber E, Ho RK, Mulay S, Mishkin S. Evidence for the hormone dependency of hepatic hyperplastic nodules: inhibition of malignant transformation after exogenous 17 beta-estradiol and tamoxifen. Hepatology. 1983;3:308-316. |
| 86. | Eagon PK, Chandar N, Epley MJ, Elm MS, Brady EP, Rao KN. Di(2-ethylhexyl)phthalate-induced changes in liver estrogen metabolism and hyperplasia. Int J Cancer. 1994;58:736-743. |
| 87. | Eagon PK, Elm MS, Epley MJ, Shinozuka H, Rao KN. Sex steroid metabolism and receptor status in hepatic hyperplasia and cancer in rats. Gastroenterology. 1996;110:1199-1207. |
| 88. | Waalkes MP, Liu J, Chen H, Xie Y, Achanzar WE, Zhou YS, Cheng ML, Diwan BA. Estrogen signaling in livers of male mice with hepatocellular carcinoma induced by exposure to arsenic in utero. J Natl Cancer Inst. 2004;96:466-474. |
| 89. | Waalkes MP, Liu J, Ward JM, Diwan BA. Enhanced urinary bladder and liver carcinogenesis in male CD1 mice exposed to transplacental inorganic arsenic and postnatal diethylstilbestrol or tamoxifen. Toxicol Appl Pharmacol. 2006;215:295-305. |
| 90. | Sato R, Maesawa C, Fujisawa K, Wada K, Oikawa K, Takikawa Y, Suzuki K, Oikawa H, Ishikawa K, Masuda T. Prevention of critical telomere shortening by oestradiol in human normal hepatic cultured cells and carbon tetrachloride induced rat liver fibrosis. Gut. 2004;53:1001-1009. |
| 91. | Shimomura M, Higashi S, Mizumoto R. 32P-postlabeling analysis of DNA adducts in rats during estrogen-induced hepatocarcinogenesis and effect of tamoxifen on DNA adduct level. Jpn J Cancer Res. 1992;83:438-444. |
| 92. | Campen D, Maronpot R, Lucier G. Dose-response relationships in promotion of rat hepatocarcinogenesis by 17 alpha-ethinylestradiol. J Toxicol Environ Health. 1990;29:257-268. |
| 93. | Yamagiwa K, Higashi S, Mizumoto R. Effect of alcohol ingestion on carcinogenesis by synthetic estrogen and progestin in the rat liver. Jpn J Cancer Res. 1991;82:771-778. |
| 94. | Tsuchiya Y, Nakajima M, Yokoi T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005;227:115-124. |
| 95. | Nemoto Y, Toda K, Ono M, Fujikawa-Adachi K, Saibara T, Onishi S, Enzan H, Okada T, Shizuta Y. Altered expression of fatty acid-metabolizing enzymes in aromatase-deficient mice. J Clin Invest. 2000;105:1819-1825. |
| 96. | Yasuda M, Shimizu I, Shiba M, Ito S. Suppressive effects of estradiol on dimethylnitrosamine-induced fibrosis of the liver in rats. Hepatology. 1999;29:719-727. |
| 97. | Shimizu I, Mizobuchi Y, Yasuda M, Shiba M, Ma YR, Horie T, Liu F, Ito S. Inhibitory effect of oestradiol on activation of rat hepatic stellate cells in vivo and in vitro. Gut. 1999;44:127-136. |
| 98. | Ostrowski JL, Ingleton PM, Underwood JC, Parsons MA. Increased hepatic androgen receptor expression in female rats during diethylnitrosamine liver carcinogenesis. A possible correlation with liver tumor development. Gastroenterology. 1988;94:1193-1200. |
| 99. | Tejura S, Rodgers GR, Dunion MH, Parsons MA, Underwood JC, Ingleton PM. Sex-steroid receptors in the diethylnitrosamine model of hepatocarcinogenesis: modifications by gonadal ablation and steroid replacement therapy. J Mol Endocrinol. 1989;3:229-237. |
| 100. | Liang L, Lu M, Huang J. [Antiandrogen treatment for nude mice model with ectopic transplanted human HCC]. Zhonghua Yixue Zazhi. 1998;78:299-300. |
| 101. | Matsuura B, Taniguchi Y, Ohta Y. Effect of antiandrogen treatment on chemical hepatocarcinogenesis in rats. J Hepatol. 1994;21:187-193. |
| 102. | Nagasue N, Yu L, Yamaguchi M, Kohno H, Tachibana M, Kubota H. Inhibition of growth and induction of TGF-beta 1 in human hepatocellular carcinoma with androgen receptor by cyproterone acetate in male nude mice. J Hepatol. 1996;25:554-562. |
| 103. | Maruyama S, Nagasue N, Dhar DK, Yamanoi A, El-Assal ON, Satoh K, Okita K. Preventive effect of FK143, a 5alpha-reductase inhibitor, on chemical hepatocarcinogenesis in rats. Clin Cancer Res. 2001;7:2096-2104. |
| 104. | Liang L, Zhou Z, Lu M, Peng B, Lu H, Yin X, He Q, Huang J. [Dynamic change of androgen-receptor in tumor and surrounding liver tissue of nude mice model with transplanted human HCC]. Zhonghua Waike Zazhi. 1998;36:525-527. |
| 105. | Björnström L, Sjöberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19:833-842. |
| 106. | Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson JA. Mechanisms of estrogen action. Physiol Rev. 2001;81:1535-1565. |
| 107. | O'Lone R, Frith MC, Karlsson EK, Hansen U. Genomic targets of nuclear estrogen receptors. Mol Endocrinol. 2004;18:1859-1875. |
| 108. | Göttlicher M, Heck S, Herrlich P. Transcriptional cross-talk, the second mode of steroid hormone receptor action. J Mol Med. 1998;76:480-489. |
| 109. | Lösel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol. 2003;4:46-56. |
| 110. | Chen JQ, Yager JD, Russo J. Regulation of mitochondrial respiratory chain structure and function by estrogens/estrogen receptors and potential physiological/pathophysiological implications. Biochim Biophys Acta. 2005;1746:1-17. |
| 111. | Hytiroglou P, Theise ND. Telomerase activation in human hepatocarcinogenesis. Am J Gastroenterol. 2006;101:839-841. |
| 112. | Rudolph KL, Chang S, Millard M, Schreiber-Agus N, DePinho RA. Inhibition of experimental liver cirrhosis in mice by telomerase gene delivery. Science. 2000;287:1253-1258. |
| 113. | Kyo S, Takakura M, Kanaya T, Zhuo W, Fujimoto K, Nishio Y, Orimo A, Inoue M. Estrogen activates telomerase. Cancer Res. 1999;59:5917-5921. |
| 114. | Marino M, Distefano E, Trentalance A, Smith CL. Estradiol-induced IP(3) mediates the estrogen receptor activity expressed in human cells. Mol Cell Endocrinol. 2001;182:19-26. |
| 115. | Brandt S, Heller H, Schuster KD, Grote J. The tamoxifen-induced suppression of telomerase activity in the human hepatoblastoma cell line HepG2: a result of post-translational regulation. J Cancer Res Clin Oncol. 2005;131:120-128. |
| 116. | Altucci L, Addeo R, Cicatiello L, Dauvois S, Parker MG, Truss M, Beato M, Sica V, Bresciani F, Weisz A. 17beta-Estradiol induces cyclin D1 gene transcription, p36D1-p34cdk4 complex activation and p105Rb phosphorylation during mitogenic stimulation of G(1)-arrested human breast cancer cells. Oncogene. 1996;12:2315-2324. |
| 117. | Prall OW, Sarcevic B, Musgrove EA, Watts CK, Sutherland RL. Estrogen-induced activation of Cdk4 and Cdk2 during G1-S phase progression is accompanied by increased cyclin D1 expression and decreased cyclin-dependent kinase inhibitor association with cyclin E-Cdk2. J Biol Chem. 1997;272:10882-10894. |
| 118. | Herber B, Truss M, Beato M, Muller R. Inducible regulatory elements in the human cyclin D1 promoter. Oncogene. 1994;9:1295-1304. |
| 119. | Foster JS, Henley DC, Ahamed S, Wimalasena J. Estrogens and cell-cycle regulation in breast cancer. Trends Endocrinol Metab. 2001;12:320-327. |
| 120. | Park YN, Chae KJ, Kim YB, Park C, Theise N. Apoptosis and proliferation in hepatocarcinogenesis related to cirrhosis. Cancer. 2001;92:2733-2738. |
| 121. | Finucane DM, Bossy-Wetzel E, Waterhouse NJ, Cotter TG, Green DR. Bax-induced caspase activation and apoptosis via cytochrome c release from mitochondria is inhibitable by Bcl-xL. J Biol Chem. 1999;274:2225-2233. |
| 122. | Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609-619. |
| 123. | Chen J, Delannoy M, Odwin S, He P, Trush MA, Yager JD. Enhanced mitochondrial gene transcript, ATP, bcl-2 protein levels, and altered glutathione distribution in ethinyl estradiol-treated cultured female rat hepatocytes. Toxicol Sci. 2003;75:271-278. |
| 124. | Lu G, Shimizu I, Cui X, Itonaga M, Tamaki K, Fukuno H, Inoue H, Honda H, Ito S. Antioxidant and antiapoptotic activities of idoxifene and estradiol in hepatic fibrosis in rats. Life Sci. 2004;74:897-907. |
| 125. | Dong L, Wang W, Wang F, Stoner M, Reed JC, Harigai M, Samudio I, Kladde MP, Vyhlidal C, Safe S. Mechanisms of transcriptional activation of bcl-2 gene expression by 17beta-estradiol in breast cancer cells. J Biol Chem. 1999;274:32099-32107. |
| 126. | Kalaitzidis D, Gilmore TD. Transcription factor cross-talk: the estrogen receptor and NF-kappaB. Trends Endocrinol Metab. 2005;16:46-52. |
| 127. | Harnish DC, Scicchitano MS, Adelman SJ, Lyttle CR, Karathanasis SK. The role of CBP in estrogen receptor cross-talk with nuclear factor-kappaB in HepG2 cells. Endocrinology. 2000;141:3403-3411. |
| 128. | Evans MJ, Eckert A, Lai K, Adelman SJ, Harnish DC. Reciprocal antagonism between estrogen receptor and NF-kappaB activity in vivo. Circ Res. 2001;89:823-830. |
| 129. | Pietras RJ, Szego CM. Partial purification and characterization of oestrogen receptors in subfractions of hepatocyte plasma membranes. Biochem J. 1980;191:743-760. |
| 130. | Chen JQ, Yager JD. Estrogen's effects on mitochondrial gene expression: mechanisms and potential contributions to estrogen carcinogenesis. Ann N Y Acad Sci. 2004;1028:258-272. |
| 131. | Solakidi S, Psarra AM, Sekeris CE. Differential subcellular distribution of estrogen receptor isoforms: localization of ERalpha in the nucleoli and ERbeta in the mitochondria of human osteosarcoma SaOS-2 and hepatocarcinoma HepG2 cell lines. Biochim Biophys Acta. 2005;1745:382-392. |
| 132. | Psarra AM, Solakidi S, Sekeris CE. The mitochondrion as a primary site of action of steroid and thyroid hormones: presence and action of steroid and thyroid hormone receptors in mitochondria of animal cells. Mol Cell Endocrinol. 2006;246:21-33. |
| 133. | Sekeris CE. The mitochondrial genome: a possible primary site of action of steroid hormones. In Vivo. 1990;4:317-320. |
| 134. | Demonacos CV, Karayanni N, Hatzoglou E, Tsiriyiotis C, Spandidos DA, Sekeris CE. Mitochondrial genes as sites of primary action of steroid hormones. Steroids. 1996;61:226-232. |
| 135. | Chen JQ, Eshete M, Alworth WL, Yager JD. Binding of MCF-7 cell mitochondrial proteins and recombinant human estrogen receptors alpha and beta to human mitochondrial DNA estrogen response elements. J Cell Biochem. 2004;93:358-373. |
| 136. | Chen J, Gokhale M, Li Y, Trush MA, Yager JD. Enhanced levels of several mitochondrial mRNA transcripts and mitochondrial superoxide production during ethinyl estradiol-induced hepatocarcinogenesis and after estrogen treatment of HepG2 cells. Carcinogenesis. 1998;19:2187-2193. |
| 137. | Chen J, Li Y, Lavigne JA, Trush MA, Yager JD. Increased mitochondrial superoxide production in rat liver mitochondria, rat hepatocytes, and HepG2 cells following ethinyl estradiol treatment. Toxicol Sci. 1999;51:224-235. |
| 138. | Huang EJ, Wu CC, Huang HP, Liu JY, Lin CS, Chang YZ, Lin JA, Lin JG, Chen LM, Lee SD. Apoptotic and anti-proliferative effects of 17beta-estradiol and 17beta-estradiol-like compounds in the Hep3B cell line. Mol Cell Biochem. 2006;290:1-7. |
| 139. | Huang EJ, Wu CC, Lee SD, Chen JH, Liu JY, Ko JL, Lin JA, Lu MC, Chen LM, Huang CY. Opposing action of estrogen receptors alpha and beta on tumor necrosis factor-alpha gene expression and caspase-8-mediated apoptotic effects in HA22T cells. Mol Cell Biochem. 2006;287:137-145. |
| 140. | Moats RK 2nd, Ramirez VD. Electron microscopic visualization of membrane-mediated uptake and translocation of estrogen-BSA:colloidal gold by hep G2 cells. J Endocrinol. 2000;166:631-647. |
| 141. | Shimada K, Nakamura M, Ishida E, Konishi N. Molecular roles of MAP kinases and FADD phosphorylation in prostate cancer. Histol Histopathol. 2006;21:415-422. |
| 142. | Totta P, Acconcia F, Leone S, Cardillo I, Marino M. Mechanisms of naringenin-induced apoptotic cascade in cancer cells: involvement of estrogen receptor alpha and beta signalling. IUBMB Life. 2004;56:491-499. |
| 143. | Claessens F, Verrijdt G, Haelens A, Callewaert L, Moehren U, d'Alesio A, Tanner T, Schauwaers K, Denayer S, Van Tilborgh N. Molecular biology of the androgen responses. Andrologia. 2005;37:209-210. |
| 144. | Gelmann EP. Molecular biology of the androgen receptor. J Clin Oncol. 2002;20:3001-3015. |
| 145. | Yoon G, Kim JY, Choi YK, Won YS, Lim IK. Direct activation of TGF-beta1 transcription by androgen and androgen receptor complex in Huh7 human hepatoma cells and its tumor in nude mice. J Cell Biochem. 2006;97:393-411. |
| 146. | Jie X, Lang C, Jian Q, Chaoqun L, Dehua Y, Yi S, Yanping J, Luokun X, Qiuping Z, Hui W. Androgen activates PEG10 to promote carcinogenesis in hepatic cancer cells. Oncogene. 2007;26:5741-5751. |
| 147. | Okabe H, Satoh S, Furukawa Y, Kato T, Hasegawa S, Nakajima Y, Yamaoka Y, Nakamura Y. Involvement of PEG10 in human hepatocellular carcinogenesis through interaction with SIAH1. Cancer Res. 2003;63:3043-3048. |
| 148. | Tsou AP, Chuang YC, Su JY, Yang CW, Liao YL, Liu WK, Chiu JH, Chou CK. Overexpression of a novel imprinted gene, PEG10, in human hepatocellular carcinoma and in regenerating mouse livers. J Biomed Sci. 2003;10:625-635. |
| 149. | Konoplya EF, Popoff EH. Identification of the classical androgen receptor in male rat liver and prostate cell plasma membranes. Int J Biochem. 1992;24:1979-1983. |
| 150. | Wang AG, Moon HB, Chun SY, Lee TH, Yu DY, Lee DS. Orchiectomy reduces hepatotumorigenesis of H-ras12V transgenic mice via the MAPK pathway. Life Sci. 2006;79:1974-1980. |
| 151. | Murakami S. Hepatitis B virus X protein: structure, function and biology. Intervirology. 1999;42:81-99. |
| 152. | Levrero M. Viral hepatitis and liver cancer: the case of hepatitis C. Oncogene. 2006;25:3834-3847. |
| 153. | Xue Y, Wong J, Moreno GT, Young MK, Cote J, Wang W. NURD, a novel complex with both ATP-dependent chromatin-remodeling and histone deacetylase activities. Mol Cell. 1998;2:851-861. |
| 154. | Bartenschlager R, Lohmann V. Novel cell culture systems for the hepatitis C virus. Antiviral Res. 2001;52:1-17. |
| 155. | Lohmann V, Korner F, Koch J, Herian U, Theilmann L, Bartenschlager R. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science. 1999;285:110-113. |
| 156. | Aizaki H, Lee KJ, Sung VM, Ishiko H, Lai MM. Characterization of the hepatitis C virus RNA replication complex associated with lipid rafts. Virology. 2004;324:450-461. |
| 157. | Miyanari Y, Hijikata M, Yamaji M, Hosaka M, Takahashi H, Shimotohno K. Hepatitis C virus non-structural proteins in the probable membranous compartment function in viral genome replication. J Biol Chem. 2003;278:50301-50308. |
| 158. | Shi ST, Lee KJ, Aizaki H, Hwang SB, Lai MM. Hepatitis C virus RNA replication occurs on a detergent-resistant membrane that cofractionates with caveolin-2. J Virol. 2003;77:4160-4168. |
| 159. | Chiu CM, Yeh SH, Chen PJ, Kuo TJ, Chang CJ, Chen PJ, Yang WJ, Chen DS. Hepatitis B virus X protein enhances androgen receptor-responsive gene expression depending on androgen level. Proc Natl Acad Sci USA. 2007;104:2571-2578. |
Peer reviewers: Dr. Henning Schulze-Bergkamen, First Medical Department, University of Mainz, Langenbeckstrasse 1, Mainz 55101, Germany; Dr. Ursula M Gehling, Department of Hepatobiliary Surgery and Visc, University Hospital Hamburg-Eppendorf, Martinistrasse 52, Hamburg 20246, Germany
S- Editor Zhong XY L- Editor Wang XL E- Editor Yin DH