Published online Oct 14, 2008. doi: 10.3748/wjg.14.5868
Revised: July 13, 2008
Accepted: July 20, 2008
Published online: October 14, 2008
AIM: To investigate the tight junction protein expressions of intestinal mucosa in an experimental model of cardiopulmonary bypass (CPB) in rats.
METHODS: Thirty anesthetized rats were randomly divided into two groups: Group S (n = 10) served as sham operation and group C (n = 20) served as CPB which underwent CPB for 1 h. Expression of occludin and zonula occludens-1 (ZO-1) were determined by Western blotting and immunocytochemistry, respectively. Plasma levels of diamine oxidase (DAO) and d-lactate were determined using an enzymatic spectrophotometry.
RESULTS: Immunohistochemical localization of occludin and ZO-1 showed disruption of the tight junctions in enterocytes lining villi at the end of CPB and 2 h after CPB. The intensities of the occludin and ZO-1 at the end of CPB were lower than those of control group (76.4% ± 22.5% vs 96.5% ± 28.5% and 62.4% ± 10.1% vs 85.5% ± 25.6%, P < 0.05) and were further lower at 2 h after CPB (50.5% ± 10.5% and 45.3% ± 9.5%, P < 0.05). Plasma d-lactate and DAO levels increased significantly (8.688 ± 0.704 vs 5.745 ± 0.364 and 0.898 ± 0.062 vs 0.562 ± 0.035, P < 0.05) at the end of CPB compared with control group and were significantly higher at 2 h after CPB than those at the end of CPB (9.377 ± 0.769 and 1.038 ± 0.252, P < 0.05). There were significant negative correlations between occludin or ZO-1 expression and DAO (r2 = 0.5629, r2 = 0.5424, P < 0.05) or d-lactate levels (r2 = 0.6512, r2 = 0.7073, P < 0.05) both at the end of CPB and 2 h after CPB.
CONCLUSION: CPB markedly down-regulates the expression of occludin and ZO-1 proteins in intestinal mucosa of rats. The close correlation between expression of tight junctions (TJs) and plasma levels of DAO or d-lactate supports the hypothesis that intestinal permeability increases during and after CPB because of decreases in the expressions of TJs.
- Citation: Sun YJ, Chen WM, Zhang TZ, Cao HJ, Zhou J. Effects of cardiopulmonary bypass on tight junction protein expressions in intestinal mucosa of rats. World J Gastroenterol 2008; 14(38): 5868-5875
- URL: https://www.wjgnet.com/1007-9327/full/v14/i38/5868.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5868
| Variables | Groups | Pre-CPB | 30 min after CPB | 60 min after CPB | 2 h after CPB |
| MAP (mmHg) | CPB | 84.50 ± 7.05 | 62.14 ± 15.23ac | 67.08 ± 19.12ac | 72.18 ± 17.39ac |
| Sham | 86.33 ± 16.82 | 84.26 ± 14.55 | 85.45 ± 10.36 | 83.63 ± 11.24 | |
| HR (/min) | CPB | 315 ± 30 | 286 ± 28 | 305 ± 42 | 310 ± 37 |
| Sham | 325 ± 34 | 320 ± 25 | 315 ± 20 | 324 ± 15 | |
| pH | CPB | 7.41 ± 0.03 | 7.34 ± 0.05ac | 7.33 ± 0.06ac | 7.39 ± 0.02 |
| Sham | 7.40 ± 0.02 | 7.41 ± 0.03 | 7.39 ± 0.02 | 7.40 ± 0.02 | |
| PaO2 (mmHg) | CPB | 399 ± 23 | 209 ± 94ac | 125 ± 40ac | 366 ± 40 |
| Sham | 402 ± 22 | 396 ± 17 | 396 ± 14 | 402 ± 20 | |
| BE | CPB | -1.86 ± 0.35 | -3.36 ± 1.22ac | -4.30 ± 1.84ac | -2.30 ± 1.50 |
| Sham | -1.96 ± 0.45 | -2.36 ± 0.75 | -1.54 ± 0.85 | -1.95 ± 0.54 | |
| Hct (%) | CPB | 41.80 ± 6.73 | 26.45 ± 4.24ac | 21.54 ± 3.71ac | 27.82 ± 3.66ac |
| Sham | 41.10 ± 1.85 | 40.20 ± 1.53 | 40.60 ± 1.46 | 39.80 ± 1.35 | |
| RT (°C) | CPB | 36.17 ± 1.85 | 33.17 ± 1.83ab | 35.12 ± 2.05 | 36.21 ± 1.24 |
| Sham | 36.25 ± 1.42 | 37.35 ± 1.65 | 36.42 ± 1.05 | 36.85 ± 1.06 |
Intestinal mucosa serves as a major anatomic and functional barrier separating potentially harmful intraluminal elements such as bacteria and endotoxins from extraintestinal tissues and the systemic circulation[1]. Some studies have shown that cardiopulmonary bypass (CPB) predisposes the intestines to inadequate perfusion, hypoxic injury, and increases in gut permeability[2,3]. During CPB, endotoxin which may originate from the gut has been found to rise in the circulation and may contribute to the cytokine and complement activation caused by the exposure of blood to artificial surfaces of the oxygenator. The resulting proinflammatory reaction can cause fever and high oxygen consumption in the direct postoperative period, i.e., the so-called post-perfusion (or “post-pump”) syndrome[4-6].
Tight junctions (TJs) are located at the apical part of lateral membranes of polar epithelial cells, forming a barrier that regulates the permeability of ions, macromolecules, and cells through the paracellular pathway[7,8]. Three groups of macromolecules are considered as bona fide integral components of the TJ: occludin(s), claudins and junction adhesion molecule[9]. Zonula occludens (ZO)-1, -2 and -3 are the major TJ-plaque proteins that bind to the intracellular domain of occludins. The interaction between occludin and ZO-1 plays a crucial role in maintaining the structure of TJ and epithelial barrier function[10]. TJ structure and function present a highly dynamic nature, subjected to regulation by a variety of intracellular and extracellular signals, both in normal and pathologic conditions[7,8]. Intracellular events that may influence TJs’ stability are related to energy depletion and extracellular events include cytokine activation, oxidative stress, attachment of enteropathogens on epithelial surface and neutrophil transmigration[7,11-14]. Most of these events have been demonstrated to play an important role during CPB[15-17].
According to our knowledge, there are no previous studies investigating whether cardiopulmonary bypass disrupts intestinal mucosal barrier by altering TJs’ formation and function. The present study was initiated to address two important questions in the field of CPB-induced gut barrier dysfunction and the molecular mechanisms involved: first, to determine any change in occludin and ZO-1 expressions in the intestinal epithelium and, second, to determine if there is any correlation between occludin or ZO-1 expressions and intestinal mucosal damage during CPB, as reflected by plasma levels of diamine oxidase (DAO) and d-lactate[18,19].
Adult male Sprague-Dawley (SD) rats (provided by the department of experimental animals, China Medical University) weighing 300-400 g were used in the present study. They were housed in stainless steel cages, three rats per cage, under controlled temperature (23°C) and humidity conditions, with 12-h dark/light cycles, and maintained on standard laboratory diet with tap water ad libitum throughout the experiment, except for an overnight fast before surgery.
The experiments were carried out according to the guidelines set forth by the Ethics Committee of China Medical University, Shenyang, China.
Experimental protocol: Thirty rats were randomly divided into two groups: Group S (n = 10) served as sham-operation and group C (n = 20) served as CPB. The rats of group C underwent 60 min of CPB and were sacrificed at the end of CPB (n = 10) or at 2 h after CPB (n = 10) randomly.
Surgical procedure for CPB[20-22]: Rats were anaesthetized with intraperitoneal administration of chloral hydrate (350 mg/kg). Once the anesthesia was achieved for the surgical level, the rats were placed in supine position. A 14G catheter was inserted into the trachea and the lungs were mechanically ventilated with a small animal ventilator (DH2800, Zhejiang University Medical Apparatus Co, China). The tidal volume was approximately 10 mL/kg, the respiratory rate was 60/min, peak airway pressure was 20-30 mmH2O and the respiration concentration of O2 was 100%. Anesthesia was maintained throughout the experiment with additional doses of chloral hydrate (100-150 mg/kg). All subsequent procedures were performed under aseptic conditions.
The left femoral artery was cannulated with a 24-gauge catheter to monitor systemic arterial pressure (SAP) and to collect arterial blood (0.2 mL) for arterial blood gas analysis before CPB, 30 min, 1 h and 2 h after CPB (blood gasanalyzer, GEM Premier 3000, USA). The homolateral femoral vein was cannulated with a 20-gauge catheter for fluid replacement (0.9% NS 0.5-1.0 mL/h). Following administration of heparin (300 U/kg), a 16-gauge catheter, modified to a multiside-orifices cannula in the forepart, was inserted into the right jugular vein and advanced to the right atrium for blood drained. A 22-gauge catheter was cannulated to the right carotid artery which served as the arterial infusion line for the CPB circuit. The electrocardiogram (ECG) was performed and rectal temperature (RT) was monitored.
The minute CPB circuit comprised a venous reservoir, a specially designed membrane oxygenator (Ke Wei Co. Ltd, Guandong, China ), a roller pump(Longer Precision Pump Co. Ltd, Baoding, China), and sterile tubing with an inner diameter of 4 mm for the venous line and of 1.6 mm for the arterial line. The main priming fluid of the circuit constituted Hespander (synthetic colloid, 7 mL), Ringer’s lactate solution (7 mL), 15% mannitol (1 mL) and 8% sodium bicarbonate (1 mL). After careful check, the clamps were released and the bypass was started. With an inspired oxygen fraction of 100%, 50 mL/min gas flow was sufficient to achieve adequate oxygenation and to maintain the PaCO2 within 35-45 mmHg. At the initiation of perfusion, the flow-rate was gradually adjusted to a level that would sustain the arterial pressure near 60 mmHg. At this point, mechanical ventilation was terminated and CPB was stably performed at 90-100 mL/kg per min throughout the experiment. Body central temperature was monitored with a rectal probe and kept at about 33°C. After 60 min, the bypass was terminated when the temperature gradually elevated to 37°C by a heat lamp placed above the animal and the infusion warmer (BIGGER, CBW68, USA). The mechanical ventilation was resumed until the rats gained steady active breath. The remaining priming solution was infused gradually after the termination of CPB and dopamine (3 μg/kg per min) was infused when the main arterial pressure was less than 60 mmHg.
The anesthetic and surgical procedure performed in the sham-group (n = 10) were the same as in the CPB group. Stable Mbp was maintained, but without CPB, for the same duration of surgery and anesthesia as in the CPB 1 h or 2 h group.
Sample collection: The rats from each group were sacrificed by decapitation. The arterial blood (0.2 mL) was sampled before CPB, at the end of CPB and 2 h after CPB for determination of serum DAO and d-lactate levels. At end of CPB and 2 h after CPB, intestinal (ileum) tissue samples were collected for immunohisto-chemical and Western blotting analysis. The animals in the sham-group were sacrificed at the end of the time period equivalent to the CPB-2 h group. All samples were determined in a blinded fashion; results were not available until completion of the study.
Plasmad-lactate measurement: Plasma d-lactate levels were measured by an enzymatic spectrophotometric assay using a centrifugal analyzer at 30°C (Hoffmann-LaRoche, Basle, Switzerland) as described earlier[23]. d-lactate, d-lactate dehydro-genase and NAD+ were purchased from Sigma Chemical Company (Milan, Italy).
Plasma DAO measurement: Plasma DAO activities were determined using an enzymatic spectrophotometry as described earlier[24]. O-dianisidine, Gadaverine Dihydrochloride and DAO were purchased from Sigma Chemical Company (Milan, Italy).
Immunohistochemistry for occludin and ZO-1: Four to 5 μm thick histologic sections, mounted on gelatinized glass slides, were dewaxed in xylene and hydrated through a series of graded concentrations of alcohol. Endogenous peroxidase activity was blocked with 1% hydrogen peroxide in methanol for 15 min. Sections were then processed in a microwave oven twice, 5 min each time at high power for antigen retrieval. Subsequently, a standard streptavidin-biotin-peroxidase technique was applied to detect occludin and ZO-1. After incubation with 1% bovine serum albumin to block nonspecific binding, sections were incubated with rabbit polyclonal antioccludin or antiZO-1 antibody (1:100, Zymed Laboratories, San Francisco, CA) for 1 h, and with biotinylated antirabbit immunoglobulin-G for 30 min. Color was developed with diaminobenzidine (Sigma Fast DAB tablets, D-4293) and counterstained with Harris hematoxylin. All procedures took place at room temperature and sections were washed in tris-buffered saline. The specimens were examined under microscope (Olympus BX51, Japan). The integrated optical density of ZO-1 or occludin in intestinal sections was analyzed using an image analysis system (UIC, MetaMorph, USA).
Western blotting analysis: After rats were killed as described above, terminal ileums were isolated, excised, flushed with ice-cold PBS to remove the fecal debris, and then opened longitudinally. Intestinal mucosa was harvested with a razor blade. Tissues were homogenized by mechanical disruption in lysis buffer (50 mmol/L Tris-Cl, pH 8.0, 150 mmmol/L NaCl, 0.1% dodecyl sodium sulfate, 1% Nonidet P-40, 0.5% sodium deoxycholate, 100 mg/mL phenylmethylsulfonyl fluoride, 1 mg/mL Aprotinin) and incubated on ice for 30 min. Homogenate was then pelleted at 6000 g for 10 min and supernatant was collected. Using Lowry method, the quantity of total protein was determined in the supernatant. The protein extracts were incubated with primary antisera (rabbit anti-occludin and anti-ZO-1, 1:500; Zymed Laboratories, San Francisco, CA) at 4°C overnight and then the mixture was incubated with secondary antibody (horseradish peroxidase conjugated goat anti-rabbit IgG, 1:1000; Beijing Zhongshan Biotech,Co.) at 4°C for 6-8 h. Immunoprecipitates were subjected to SDS-PAGE and electrotransferred to nitrocellulose membrane. After a final wash in TBST, using the enhanced chemiluminescence (ECL) kit (Shang hai Pufei Biotech, Co.), chemluminescent signals were collected on autoradiographic film. The quantity of band intensity was carried out using electrophoresis Gel Imaging Analysis System (ChemiImager 5500, ALPHA INNOTECH, USA). Results were expressed as ratio of relative intensity of target protein to that of the internal standard, β-actin.
Electron microscopy: Samples were immediately fixed in 2.5% glutaraldehyde in 0.1 mol/L sodium cacodylate buffer (pH 7.4) for 2 h at 22°C, rinsed for 18 h (4°C) with 0.05 Tris buffer (pH 7.6), and washed three times, for 5 min each time. An H-2000 transmission electron microscope was used for the examination.
Data were analyzed using a sigma stat statistical software (SPSS, Chicago, IL) and expressed as mean ± SD. Statistical significance of differences between groups were determined using one way analysis of variance (ANOVA) followed by LDS (for multiple comparisons. Linear regression analysis was used to assess associations between TJ protein relative expression levels and DAO or d-lactate levels at each period. P < 0.05 was considered as significant in difference.
All experiments progressed without incident and all animals survived from the operative process. Table 1 displays the physiologic data from the CPB and Sham rats over the study period. In the CPB group, CPB and post-CPB MAP, PaO2 values were significantly lower than baseline (P < 0.05), whereas PaCO2 remained stable. In the Sham group, there were no changes in MAP, PaO2 and PaCO2during the whole experiment. Hematocrit (HCT) had a significant decrease in the CPB group (P < 0.05) during the perfusion process but had no change in the Sham group. The pH values in the CPB group decreased and this group showed a tendency to develop a metabolic acidosis.
There were no significantly differences between CPB groups and Sham group at baseline (P > 0.05, Table 2). In the CPB group, CPB and post-CPB DAO activity was significantly higher than baseline (P < 0.05), and DAO activity at 2 h after CPB was significantly higher than that at the end of CPB (P < 0.05). In the Sham group, there were no changes in plasma DAO activity during the whole experiment.
There were no significantly differences between CPB groups and Sham group at baseline (P > 0.05, Table 2). Plasma d-lactate levels increased significantly (P < 0.05) at the end of CPB compared with baseline or control group and remained higher at 2 h after CPB. In the Sham group, there were no changes in plasma d-lactate levels during the experiment.
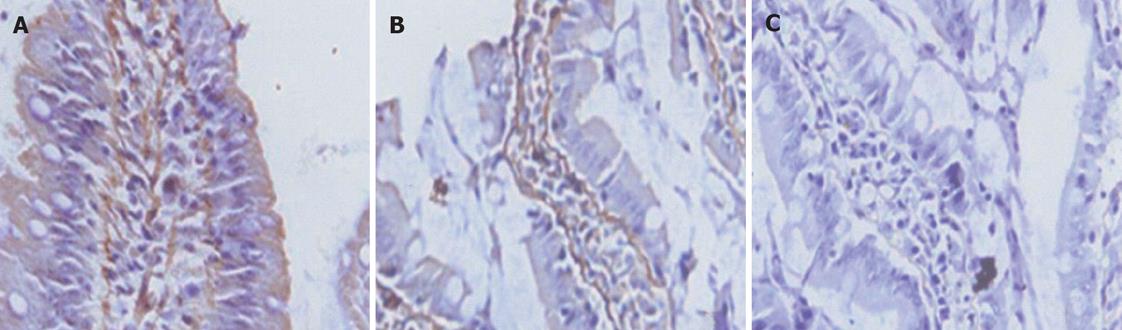
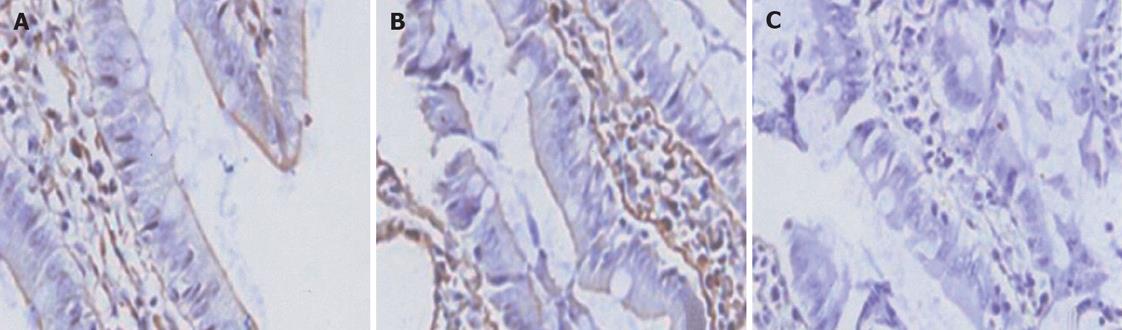
All of the epithelial cells lining villi exhibited positive immunostaining for ZO-1 and occludin in control group (Figures 1-2A). In group C, there was loss of occludin and ZO-1 expressions in about 50% enterocytes lining villi (Figures 1-2B); this effect was more profound at 2 h after CPB and loss was about 80% (Figures 1 and 2).
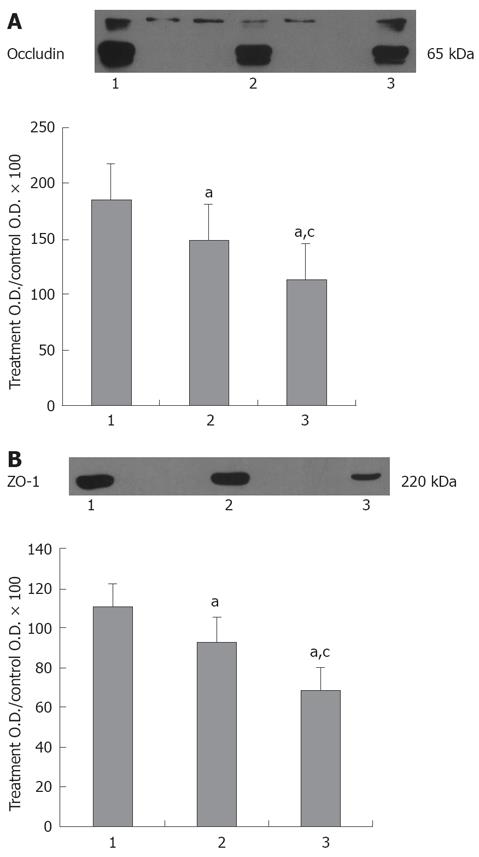
Western blot analyses demonstrated alterations in expression of tight junctional proteins after CPB. Figure 3A shows the integral protein occludin with dual bands at 65-kDa .The 65-kDa band showed a significant (P < 0.05) decrease in group C compared with control(76.4% ± 22.5% vs 96.5% ± 28.5%) and reached lower value at 2 h after CPB (50.5% ± 10.5%). Figure 3B shows that ZO-1 expression significantly (P < 0.05) decreased after CPB compared with control (62.4% ± 10.1% vs 85.5% ± 25.6%) and became lower at 2 h after CPB (45.3% ± 9.5%).
To assess the relationship between changes of occludin and ZO-1 expressions and the damages of intestinal mucosa after CPB, we used regression analysis to assess associations between TJ protein relative expression levels, DAO and d-lactate levels at each period. Significant negative correlations were observed between occludin or ZO-1 expression and DAO (r2 = 0.5629, r2 = 0.5424, P < 0.05) or d-lactate levels (r2 = 0.6512, r2 = 0.7073, P < 0.05) both at the end of CPB and at 2 h after CPB.
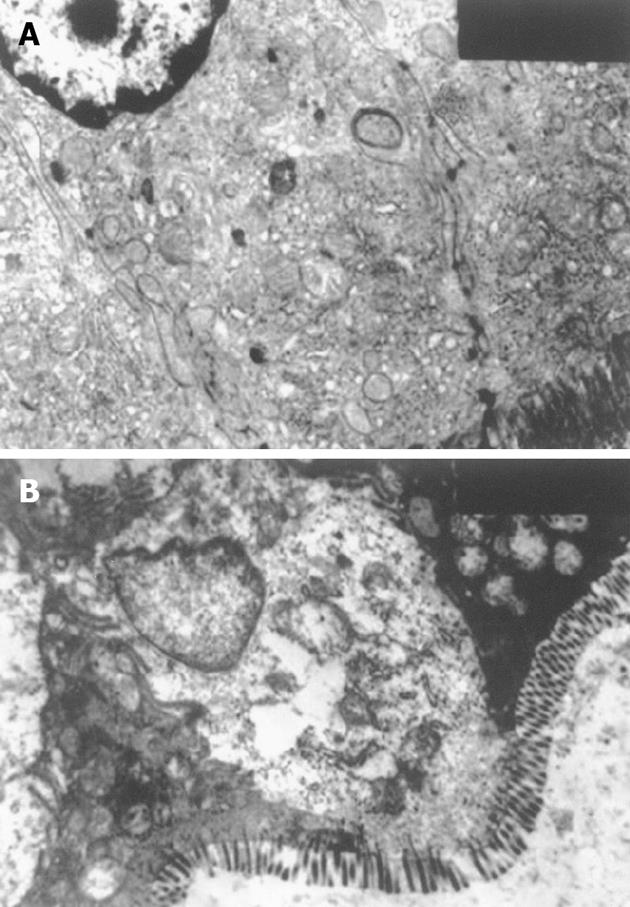
To assess the relationship between changes of occludin and ZO-1 expressions and alterations of intestinal mucosal ultrastructure after CPB, we performed a morphological analysis of TJ (Figure 4), as occludin is an important component in TJ. In control group, regularly-aligned microvilli in intestinal epithelium, integral mitochondria and rough endoplasmic reticulum (RER) and distinct junction complex were observed. In experimental group, the amounts of microvilli were decreased with irregular length and arrangement. The mitochondria were swollen with cracked and vacuolated cristae. Some structures of the RER were destroyed. Intercellular space between epithelial cells was widened. The structure of TJ became short, and the dotted crystal structures were obscured or disappeared; other structures were disrupted and even disappeared. The damage was more severe at 2 h after CPB.
Although CPB is essential for some procedures in cardiovascular surgery, it causes peripheral hypoperfusion because of nonpulsatile flow, low blood pressure, hemodilution, and other nonphysiologic conditions. Furthermore, an increase in intestinal permeability and bacterial translocation (BT) has been demonstrated not only in animal models but also in patients during CPB[3,25,26]. Therefore, gastrointestinal integrity during the perioperative period is now recognized as an important factor for outcome of cardiac surgical procedures. In general, changes in mucosal permeability and morphology during CPB are used to reflect the degree of damage of the barrier. But studies on the molecular mechanism of the increased permeability of intestinal mucosa during CPB are rare.
The purpose of this study was to investigate the TJ protein expressions of intestinal mucosa in an experimental model of CPB. We chose the rat model because of the anatomical and physiologic similarity of the digestive tract and cardiovascular systems in rats and humans[27]. Using immunoblotting and immunohistochemistry, we found that CPB markedly down-regulated the expression of occludin and ZO-1 proteins in intestinal mucosa. The result demonstrated that all of epithelial cells lining villi exhibited positive immunostaining for ZO-1 and occludin in control group. In group C there was loss of occludin and ZO-1 expressions in about 50% enterocytes lining villi; this effect was more profound at 2 h after CPB. The intensity of occludin and ZO-1 at the end of CPB was weakened, and more weakened 2 h after CPB. These changes of TJs’ proteins are positively associated with alterations of intestinal mucosal ultrastructure. After CPB, the mitochondria was swollen with cracked and vacuolated cristae. The structure of TJ became short, and the dotted crystal structures were obscured or disappeared; other structures were disrupted and even disappeared.
The TJ of epithelial cells forms the most apical component of the junction component of the junction complex, and appears to be a network of continuous and anastomosing filaments between adjacent epithelial cells, which consists of a continuous permeability barrier. It prevents both bacteria and toxin in the intestinal lumen from the paracellular space getting into deep tissues.
To elucidate the relationships between occludin or ZO-1 expression and intestinal mucosal damage during CPB, we measured the plasma levels of DAO and d-lactate used as indices of intestinal mucosal injury. DAO is localized mainly in the small intestine and reported to be elevated in intestinal ischemia[18]. Tsunooka demonstrated simultaneous increases in serum DAO activity and peptidoglycan concentration during clinical CPB, suggesting occurrence of small intestinal mucosal ischemia and BT[28,29]. In the present study, we got a similar result. During CPB, plasma DAO activity increased significantly and remained high at 2 h after CPB.
d-lactate is the dextrorotatory form of lactate commonly tested in the intensive care unit. Mammals only have one type of enzyme: l-lactate dehydrogenase. l-Lactate is a marker of cell hypoxemia, and its level correlates with survival of patients with septic shock[30-32]. Microorganisms, particularly bacteria, are equipped with d-lactate dehydrogenase and produce d-lactate during fermentation. d-lactate is therefore a marker of bacterial infection. d-lactate has also recently been proposed as a sensitive, specific, and early marker for translocation in gut ischemia[19]. In our rat model of CPB, plasma d-lactate levels increased significantly at the end of CPB compared with control group. It was significantly higher at 2 h after CPB.
We found significant negative correlations between occludin or ZO-1 expression and the plasma levels of DAO or d-lactate. These data support the hypothesis that CPB decreases occludin and ZO-1 expressions and then disruptes the intestinal integrity. Therefore, the plasma DAO activity and d-lactate level of the rats significantly increased. This further showed an increase of the intestinal permeability during CPB and remained higher 2 h after CPB. But the strong correlation obviously does not prove the cause and effect on intestinal permeability.
There are limitations to this complex model using rats subjected to CPB. The most important limitation of our study is that the CPB groups of animals (as compared with the Sham/control group) were exposed to severe hemodilution with much lower hematocrit, moderate hypotension, lower PaO2, and probably lower systemic perfusion, and that these differences (i.e., reduced oxygen delivery and tissue perfusion) may have caused or explained the abnormalities we observed in the CPB groups. Another limitation of the current study is that the model was non-transthoracic CPB not followed by deep hypothermic circulatory arrest. An additional limitation for this study may be the limited time that the animals were monitored after CPB. Although the postoperative period was only 2 h, numerous studies indicate that cytokine changes and neutrophil infiltration occur shortly after CPB in animals[33] and children[34].
To the best of our knowledge, this is the first study directly assessing CPB disrupts intestinal mucosal barrier by altering occludin and formation and function of ZO-1 in rat model of CPB. Our data imply that CPB markedly down-regulates the expression of occludin and ZO-1 proteins in intestinal mucosa, indicating a possible mechanism for the increased intestinal permeability and BT during CPB. Thus, further studies are required to elucidate the regulation of the TJ of intestinal epithelium.
Some studies have shown that cardiopulmonary bypass (CPB) predisposes the intestines to inadequate perfusion, hypoxic injury, and increases in gut permeability. Tight junctions (TJs) are located at the apical part of lateral membranes of polar epithelial cells, forming a barrier that regulates the permeability of ions, macromolecules, and cells through the paracellular pathway. There are no previous studies investigating whether CPB disrupts intestinal mucosal barrier by altering the formation and function of TJs.
In general, changes in mucosal permeability and morphology during CPB are used to reflect the degree of damage of the barrier function. But studies on the molecular mechanism of the increased permeability of intestinal mucosa during CPB are rare.
In this study, the effects of CPB on occludin and ZO-1 expressions in the intestinal epithelium of rats were investigated. Authors chose the rat model because of the anatomical and physiologic similarity of the digestive tract and cardiovascular systems in rats and humans.
The results show for the first time that CPB induced gut barrier dysfunction is associated, at the cellular level, with regional loss of occludin and ZO-1 expressions in the intestinal epithelium. These findings offer further insight in the pathophysiology of gut barrier failure during and after CPB. Understanding the pathophysiology of barrier alterations during CPB at the molecular level will help find novel and more effective treatment approaches.
This is an interesting paper and it deals with a relevant clinical problem. The study appears well performed and the hypothesis is clear.
| 1. | Deitch EA. The role of intestinal barrier failure and bacterial translocation in the development of systemic infection and multiple organ failure. Arch Surg. 1990;125:403-404. |
| 2. | Ohri SK, Bjarnason I, Pathi V, Somasundaram S, Bowles CT, Keogh BE, Khaghani A, Menzies I, Yacoub MH, Taylor KM. Cardiopulmonary bypass impairs small intestinal transport and increases gut permeability. Ann Thorac Surg. 1993;55:1080-1086. |
| 3. | Riddington DW, Venkatesh B, Boivin CM, Bonser RS, Elliott TS, Marshall T, Mountford PJ, Bion JF. Intestinal permeability, gastric intramucosal pH, and systemic endotoxemia in patients undergoing cardiopulmonary bypass. JAMA. 1996;275:1007-1012. |
| 4. | Oudemans-van Straaten HM, Jansen PG, Hoek FJ, van Deventer SJ, Sturk A, Stoutenbeek CP, Tytgat GN, Wildevuur CR, Eysman L. Intestinal permeability, circulating endotoxin, and postoperative systemic responses in cardiac surgery patients. J Cardiothorac Vasc Anesth. 1996;10:187-194. |
| 5. | Oudemans-van Straaten HM, Jansen PG, te Velthuis H, Beenakkers IC, Stoutenbeek CP, van Deventer SJ, Sturk A, Eysman L, Wildevuur CR. Increased oxygen consumption after cardiac surgery is associated with the inflammatory response to endotoxemia. Intensive Care Med. 1996;22:294-300. |
| 6. | Hamilton-Davies C, Barclay GR, Cardigan RA, McDonald SJ, Purdy G, Machin SJ, Webb AR. Relationship between preoperative endotoxin immune status, gut perfusion, and outcome from cardiac valve replacement surgery. Chest. 1997;112:1189-1196. |
| 7. | Madara JL. Warner-Lambert/Parke-Davis Award lecture. Pathobiology of the intestinal epithelial barrier. Am J Pathol. 1990;137:1273-1281. |
| 8. | Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995;269:G467-G475. |
| 9. | Fanning AS, Mitic LL, Anderson JM. Transmembrane proteins in the tight junction barrier. J Am Soc Nephrol. 1999;10:1337-1345. |
| 10. | Wittchen ES, Haskins J, Stevenson BR. Protein interactions at the tight junction. Actin has multiple binding partners, and ZO-1 forms independent complexes with ZO-2 and ZO-3. J Biol Chem. 1999;274:35179-35185. |
| 11. | Schmitz H, Fromm M, Bentzel CJ, Scholz P, Detjen K, Mankertz J, Bode H, Epple HJ, Riecken EO, Schulzke JD. Tumor necrosis factoralpha (TNF-a) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J Cell Sci. 1999;112:137-146. |
| 12. | Rao RK, Basuroy S, Rao VU, Karnaky Jr KJ, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J. 2002;368:471-481. |
| 13. | Simonovic I, Rosenberg J, Koutsouris A, Hecht G. Enteropathogenic Escherichia coli dephosphorylates and dissociates occludin from intestinal epithelial tight junctions. Cell Microbiol. 2000;2:305-315. |
| 14. | Kucharzik T, Walsh SV, Chen J, Parkos CA, Nusrat A. Neutrophil transmigration in inflammatory bowel disease is associated with differential expression of epithelial intercellular junction proteins. Am J Pathol. 2001;159:2001-2009. |
| 15. | Wehberg KE, Foster AH, Wise RM, McLaughlin JS, Brunner MJ. Nitric oxide mediates fluid accumulation during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1996;112:168-174. |
| 16. | Hayashi Y, Sawa Y, Nishimura M, Tojo SJ, Fukuyama N, Nakazawa H, Matsuda H. P-selectin participates in cardiopulmonary bypass-induced inflammatory response in association with nitric oxide and peroxynitrite production. J Thorac Cardiovasc Surg. 2000;120:558-565. |
| 17. | Hayashi Y, Sawa Y, Fukuyama N, Nakazawa H, Matsuda H. Inducible nitric oxide production is an adaptation to cardiopulmonary bypass-induced inflammatory response. Ann Thorac Surg. 2001;72:149-155. |
| 18. | Bounous G, Echave , Vobecky SJ, Navert H, Wollin A. Acute necrosis of the intestinal mucosa with high serum levels of diamine oxidase. Dig Dis Sci. 1984;29:872-874. |
| 19. | Günel E, Cağlayan O, Cağlayan F. Serum D-lactate levels as a predictor of intestinal ischemia-reperfusion injury. Pediatr Surg Int. 1998;14:59-61. |
| 20. | Gourlay T, Ballaux PK, Draper ER, Taylor KM. Early experience with a new technique and technology designed for the study of pulsatile cardiopulmonary bypass in the rat. Perfusion. 2002;17:191-198. |
| 21. | Dong GH, Xu B, Wang CT, Qian JJ, Liu H, Huang G, Jing H. A rat model of cardiopulmonary bypass with excellent survival. J Surg Res. 2005;123:171-175. |
| 22. | Ballaux PK, Gourlay T, Ratnatunga CP, Taylor KM. A literature review of cardiopulmonary bypass models for rats. Perfusion. 1999;14:411-417. |
| 23. | Fürst W, Schiesser A. Test for stereospecifity of an automated Dd-lactate assay based on selective removal of Ll-lactate. Anal Biochem. 1999;269:214-215. |
| 24. | Li JY, Yu Y, Hao J, Jin H, Xu HJ. Determination of diamine oxdase activity in intestinal tissue and blood using spectrophotometry. Amino Acids & Biotic Resources. 1996;18:28-30. |
| 25. | Aydin NB, Gercekoglu H, Aksu B, Ozkul V, Sener T, Kiygil I, Turkoglu T, Cimen S, Babacan F, Demirtas M. Endotoxemia in coronary artery bypass surgery: a comparison of the off-pump technique and conventional cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2003;125:843-848. |
| 26. | Watarida S, Mori A, Onoe M, Tabata R, Shiraishi S, Sugita T, Nojima T, Nakajima Y, Matsuno S. A clinical study on the effects of pulsatile cardiopulmonary bypass on the blood endotoxin levels. J Thorac Cardiovasc Surg. 1994;108:620-625. |
| 27. | Welsby IJ, Jones WL, Arepally G, De Lange F, Yoshitani K, Phillips-Bute B, Grocott HP, Becker R, Mackensen GB. Effect of combined anticoagulation using heparin and bivalirudin on the hemostatic and inflammatory responses to cardiopulmonary bypass in the rat. Anesthesiology. 2007;106:295-301. |
| 28. | Tsunooka N, Hamada Y, Imagawa H, Nakamura Y, Shiozaki T, Suzuki H, Kikkawa H, Miyauchi K, Watanabe Y, Kawachi K. Ischemia of the intestinal mucosa during cardiopulmonary bypass. J Artif Organs. 2003;6:149-151. |
| 29. | Tsunooka N, Maeyama K, Hamada Y, Imagawa H, Takano S, Watanabe Y, Kawachi K. Bacterial translocation secondary to small intestinal mucosal ischemia during cardiopulmonary bypass. Measurement by diamine oxidase and peptidoglycan. Eur J Cardiothorac Surg. 2004;25:275-280. |
| 30. | Bakker J, Coffernils M, Leon M, Gris P, Vincent JL. Blood lactate levels are superior to oxygen-derived variables in predicting outcome in human septic shock. Chest. 1991;99:956-962. |
| 31. | Bakker J, Gris P, Coffernils M, Kahn RJ, Vincent JL. Serial blood lactate levels can predict the development of multiple organ failure following septic shock. Am J Surg. 1996;171:221-226. |
| 32. | Bernardin G, Pradier C, Tiger F, Deloffre P, Mattei M. Blood pressure and arterial lactate level are early indicators of short-term survival in human septic shock. Intensive Care Med. 1996;22:17-25. |
| 33. | Brix-Christensen V, Petersen TK, Ravn HB, Hjortdal VE, Andersen NT, Tønnesen E. Cardiopulmonary bypass elicits a pro- and anti-inflammatory cytokine response and impaired neutrophil chemotaxis in neonatal pigs. Acta Anaesthesiol Scand. 2001;45:407-413. |
| 34. | Journois D, Israel-Biet D, Pouard P, Rolland B, Silvester W, Vouhé P, Safran D. High-volume, zero-balanced hemofiltration to reduce delayed inflammatory response to cardiopulmonary bypass in children. Anesthesiology. 1996;85:965-976. |
Peer reviewer: Jordi Camps, PhD, Centre de Recerca Biomèdica, Hospital Universitari de Sant Joan, C. Sant Joan s/n, Reus 43201, Spain
S- Editor Li DL L- Editor Ma JY E- Editor Yin DH