Published online Oct 14, 2008. doi: 10.3748/wjg.14.5857
Revised: September 20, 2008
Accepted: September 27, 2008
Published online: October 14, 2008
AIM: To study the role of mitochondrial energy disorder in the pathogenesis of ethanol-induced gastric mucosa injury.
METHODS: Wistar rats were used in this study. A gastric mucosal injury model was established by giving the rats alcohol. Gross and microscopic appearance of gastric mucosa and ultrastructure of mitochondria were evaluated. Malondiadehyde (MDA) in gastric mucosa was measured with thiobarbituric acid. Expression of ATP synthase (ATPase) subunits 6 and 8 in mitochondrial DNA (mtDNA) was determined by reverse transcription polymerase chain reaction (RT-PCR).
RESULTS: The gastric mucosal lesion index was correlated with the MDA content in gastric mucosa. As the concentration of ethanol was elevated and the exposure time to ethanol was extended, the content of MDA in gastric mucosa increased and the extent of damage aggravated. The ultrastructure of mitochondria was positively related to the ethanol concentration and exposure time. The expression of mtDNA ATPase subunits 6 and 8 mRNA declined with the increasing MDA content in gastric mucosa after gavage with ethanol.
CONCLUSION: Ethanol-induced gastric mucosa injury is related to oxidative stress, which disturbs energy metabolism of mitochondria and plays a critical role in the pathogenesis of ethanol-induced gastric mucosa injury.
- Citation: Pan JS, He SZ, Xu HZ, Zhan XJ, Yang XN, Xiao HM, Shi HX, Ren JL. Oxidative stress disturbs energy metabolism of mitochondria in ethanol-induced gastric mucosa injury. World J Gastroenterol 2008; 14(38): 5857-5867
- URL: https://www.wjgnet.com/1007-9327/full/v14/i38/5857.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5857
| Component | Amount |
| 25 mmol/L MgCl2 | 4.00 μL |
| Reverse transcription 10 × buffer | 2.00 μL |
| 10 mmol/L dNTP | 2.00 μL |
| Recombinant ribonuclease inhibitor | 0.50 μL |
| AMV reverse transcriptase | 15.00 U |
| Oligo (dT) 15 primer | 0.50 μg |
| Total RNA | 1.00 μg |
| Nuclease-rree water | Proper volume |
| Reacting volume | 20.00 μL |
| Component | Amount |
| cDNA | 4.00 μL |
| 10 × PCR buffer solution | 1.96 μL |
| MgCl2 (25 mmol/L) | 2.40 μL |
| Upper primer (20 pmol/L) | 1.00 μL |
| Down primer (20 pmol/L) | 1.00 μL |
| dNTP (10 mmol/L) | 0.36 μL |
| Taq DNA polymerase (5 IU/μL) | 0.10 μL |
| Deionized water | 10.08 μL |
| Reacting volume | 20.00 μL |
| Primers | GenBank (No.) | Sequence |
| β-actin | NM_031144 (nt 1-1296) | Up: 5′-TCACCCACACTGTGCCCATCTATGA-3′ |
| Down: 5′-CATCGGAACCGCTCATTGCCGATAG-3′ | ||
| ATPase subunit6 | X14848 (nt 7904-8584) | Up: 5′-ACA CCAAAAGGA CGAACCTG-3′ |
| Down: 5′-CGGTGAGAAGTGGGCTAAAG-3′ | ||
| ATPase subunit8 | X14848 (nt 7743-7946) | Up: 5′-TGCCACAACTAGACACATCCA-3′ |
| Down: 5′-TGTGGGGGTAATGAAAGAGG-3′ |
| Target sequence | Amplification conditions |
| β-actin | 94.0°C 30 s, 59.0°C 30 s, 72.0°C 30 s, 35cycles |
| ATPase subunit 6 | 94.0°C 30 s, 53.0°C 30 s, 72.0°C 30 s, 33cycles |
| ATPase subunit 8 | 94.0°C 30 s, 52.0°C 30 s, 72.0°C 30 s, 32cycles |
| Group | 3 wk | 6 wk | 9 wk | |||
| Integral indices | Pathological integrals | Integral indices | Pathological integrals | Integral indices | Pathological integrals | |
| Normal sodium | 0.000 ± 0.000 | 0.200 ± 0.422 | 0.000 ± 0.000 | 0.500 ± 0.707 | 0.000 ± 0.000 | 0.600 ± 0.699 |
| 25% (W/W) alcohol | 0.200 ± 0.422 | 0.700 ± 0.675 | 1.100 ± 0.568 | 2.400 ± 0.516 | 1.700 ± 0.483 | 4.500 ± 1.080 |
| 45% (W/W) alcohol | 0.600 ± 0.699 | 1.500 ± 0.527 | 3.300 ± 1.059 | 6.000 ± 0.817 | 8.900 ± 0.994 | 11.400 ± 0.966 |
| Group | Mitochondria (n) | ATPase subunit 6 mRNA | ATPase subunit 8 mRNA |
| Normal sodium | 74.800 ± 15.399 | 0.935 ± 0.162 | 0.768 ± 0.145 |
| 40% (W/W) ethanol | 69.200 ± 16.262 | 1.113 ± 0.135a | 0.945 ± 0.139a |
| 70% (W/W) ethanol | 63.500 ± 14.246 | 0.868 ± 0.089b | 0.677 ± 0.106b |
| 100% (W/W) ethanol | 51.500 ± 18.739 | 0.691 ± 0.053abc | 0.433 ± 0.055abc |
| Group | 3 wk | 6 wk | 9 wk | |||
| Mitochondria (n) | MDA content | Mitochondria (n) | MDA content | Mitochondria (n) | MDA content | |
| Normal sodium | 123.000 ± 23.613 | 0.717 ± 0.057 | 109.400 ± 30.303 | 0.757 ± 0.064 | 104.600 ± 20.571 | 0.728 ± 0.044 |
| 25% (W/W) alcohol | 111.000 ± 18.514 | 0.820 ± 0.051 | 106.500 ± 17.031 | 1.029 ± 0.102 | 86.300 ± 26.833 | 1.156 ± 0.058 |
| 45% (W/W) alcohol | 101.100 ± 13.186 | 0.969 ± 0.126 | 75.700 ± 17.639 | 1.225 ± 0.053 | 59.400 ± 21.767 | 1.477 ± 0.072 |
| Group | 3 wk | 6 wk | 9 wk | |||
| mtDNA ATPase 6 mRNA | mtDNA ATPase 8 mRNA | mtDNA ATPase 6 mRNA | mtDNA ATPase 8 mRNA | mtDNA ATPase 6 mRNA | mtDNA ATPase 8 mRNA | |
| Normal sodium | 0.907 ± 0.079 | 0.678 ± 0.033 | 0.876 ± 0.045 | 0.671 ± 0.038 | 0.894 ± 0.059 | 0.659 ± 0.037 |
| 25% (W/W) alcohol | 0.857 ± 0.062 | 0.655 ± 0.029 | 0.737 ± 0.060 | 0.557 ± 0.028 | 0.664 ± 0.039 | 0.512 ± 0.043 |
| 45% (W/W) alcohol | 0.756 ± 0.051 | 0.617 ± 0.026 | 0.607 ± 0.039 | 0.474 ± 0.031 | 0.501 ± 0.043 | 0.366 ± 0.028 |
Many people all over the world indulge themselves in drinking, which is correlated to a wide spectrum of medical, psychological, behavioral, and social problems. Ethanol is the major component of drinkable wine and alcoholic beverages. After drinking, alcohol is absorbed rapidly into the blood stream from the stomach and intestinal tract. High-concentration ethanol erodes directly the gastric mucosa and causes acute gastritis, leading to hyperemia, edema, hemorrhage, ulcer, etc. It is well known that chronic alcohol abuse may induce gastrointestinal dysfunction, chronic atrophic gastritis and is closely related with gastric carcinoma. However, the detailed mechanism by which ethanol affects the gastrointestinal mucosa remains to be elucidated. Thorough research on how ethanol affects gastric mucosa will benefit the protection of gastric mucosa.
The effect of ethanol on gastric mucosa is a complicated and multifaceted process. It may be associated with disturbance of the balance between gastric mucosal defense and offensive factors. Gastric mucosa contains gastric acid, pepsin, stimulant, etc, while the offensive factors contain gastric slime layer, mucosal blood flow, HCO3-, prostaglandins (PGs), epidermal growth factor (EGF) and epithelial cell renewing. Ethanol induces vascular endothelium injury of gastric mucosa, disorder of microcirculation and ischemia as a result of more production of oxygen free radicals (OFR). In vivo, ethanol metabolism also produces OFR. Alarcon et al[1] found that perfusion of OFR induces gastric mucosal injury in rats. However, perfusion of superoxide dismutase (SOD) reduces gastric mucosal injury. Itoh and Guth[2] reported that SOD and catalase (CAT) significantly diminish gastric mucosal injury after ischemia reperfusion. OFR play an crucial role in acute gastric mucosal injury. Similar to other tissue inflammation, numerous inflammatory factors which cause infiltration of neutrophils are released when gastric mucosa is attacked by various insult factors. These neutrophils produce a lot of OFR by respiratory burst. OFR, mostly the superoxide radical anion (O2·-), are metabolized through two endocellular pathways. One is peroxidation of cytomembrane lipid which produces lipoperoxide, malondiadehyde (MDA) and 4-hydroxynonena (4-HNE) after β-oxidation. MDA, a reaction product between OFR and cells, indicates the intracellular OFR. In the other path, OFR reacting with OFR scavengers (e.g. SOD) generate hydrogen peroxide (H2O2), which will be further oxidized by glutathione peroxidase (GPx) or CAT to H2O and CO2. Both of which are closely related to enzyme complexes of respiratory chain locating on the mitochondrial membrane.
Gastric parietal cells are rich in mitochondria[3-5]. Mitochondria provide energy for cells by oxidative phosphorylation (OXPHOS), which is critical to maintain the proper morphology and function of gastric mucosa. F0F1-ATP synthase, also known as cytochrome C oxidase (complex IV), is critical to oxidative phosphorylation and mediates generation of ATP[6]. The complex IV phosphorylates ADP to ATP at the advantage of transmembrane electrochemical proton gradient generated by respiratory electron-transport chain[7]. Mitochondrial ATP synthase (ATPase) subunits 6 and 8, the components of the F0 part of this enzyme, which is encoded by mitochondrial DNA (mtDNA) independently, control the synthesis of ATP by mediating the proton transfer. Mitochondrion is an easily injured organelle and mtDNA is the major target of intracellular oxidative stress associated with ethanol[8]. However, the relation between OFR and alternation of mitochondrial ultra microstructure as well as how OFR interferes with gastric mucosal energy metabolism are obscure. This study was focused on the ultra microstructure alteration of mitochondria in response to the injury induced by and the role of disturbance of mitochondrial energy metabolism in ethanol-related gastropathy.
In this research, we established an acute and chronic animal model of gastric mucosa injury by ethanol gavage. The related parameters include index of gastric mucosa injury, pathological damage, alteration of mitochondria ultra microstructure, content of MDA and expression of mtDNA of ATPase subunits 6 and 8 in rat gastric mucosa. According to the above parameters, we aimed to find the relation between OFR and mediating factors of energy metabolism as well as the role of ethanol in ethanol-induced gastric mucosal injury.
Healthy male Wistar rats of clean grade, purchased from Shanghai Slac Laboratory Animal Company, were bred in Anticancer Research Center of Xiamen University. All animal experiments were performed under the animal experimental rules of PRC.
Medical anhydrous ethanol, purchased from the Shanghai Chemical Reagent Corporation, was diluted to the desired concentration with distilled water before experiment. Ten percents (W/W) formaldehyde fixatives were provided by Pathology Department of Zhongshan Hospital, Xiamen University. MDA kits were purchased from the General Hospital of People’s Liberation Army. TRIzol reagent (Cat No. 15596-026) came from Invitrogen Corporation. RT-PCR kits (Cat No. A3500), Taq DNA polymerase, and 100 bp DNA ladder were bought from the Promega Corporation.
Gavage needles and a set of animal anatomic equipments were provided by Anticancer Research Center and Zhongshan Hospital of Xiamen University, respectively. PCR was performed on Gene Amp2700 (Applied Biosystems, USA). Gel analysis was performed by GAS7100X (USA), and transmission electron microscope was made in Japan (Hitachi-Hu-12A, Hitachi, Japan).
Thirty-two healthy male Wistar rats of clean grade, weighing 200-220 g, were randomly divided into normal control group (n = 8) and experimental group (n = 24). The rats were abstained from any food but water 24 h before experiment with free movement. Two hours before experiment, the rats were deprived of water. Rats in the control group were gavaged with 1 mL normal saline. Rats in the experimental group were divided into three subgroups (n = 8). Rats in each subgroup were gavaged respectively with 1 mL of anhydrous ethanol, 70% (W/W) ethanol and 40% (W/W) ethanol, respectively. Two hours later, all the animals were anesthetized with ether, and their abdominal cavity was dissected along the inferior border of xiphoid and their stomach was mobilized, then the gastric cavity was dissected along the greater curvature and the gastric mucosa was spread. After the mucosa was poached three times with physiologic saline, degree of the gastric mucosal injury was determined according to the Guth law and biopsy was performed.
Ninety healthy male Wistar rats of clean grade, weighing 160-180 g, were randomly divided into 3 normal control groups and 6 experimental groups (n = 10) after feeding for a week. The 3 normal control groups were gavaged with normal saline, while the 6 experimental groups were gavaged two times with 25% (W/W) ethanol and 45% (W/W) ethanol daily. The volume of gavage solution was calculated according to 9 mL/kg per day. Rats in the control and experimental groups were sacrificed on days 3, 6, and 9, respectively, and dissected in the same way as described in establishing animal model of ethanol-induced acute gastric mucosal injury.
Gastric mucosal injury indices were calculated according to the Guth standard[9]: 1 point for the punctate hemorrhage, 2 points for the injury focus with its length shorter than 1 mm, 3 points for the injury focus with its length ranging 1-2 mm, 4 points for the injury focus with its length ranging 2-4 mm, 5 points for the injury focus with its length longer than 4 mm, respectively, for the streak-like hemorrhage. The score was doubled if the streak was wider than 2 mm. Injury index of the whole gastric mucosa for each rat was the summation of injury indices for each lesion.
A 0.5 cm × 1.0 cm tissue was taken from gastric gland, fixed with a 10% (W/W) formaldehyde solution and made into a paraffin block. The tissue was cut into 5-μm thick sections which were stained with hematoxylin and eosin. Pathological changes in the gastric mucosa were observed under optical microscope. Integrals were calculated according to the scoring standard reported by Masuda et al[10], namely, 0 point for normal tissue, 1 point for epithelial damage of surface layer, 2 points for hyperemia or edema of the upper stratum, 3 points for hyperemia, edema or even hemorrhage of stratum medium or under layer. Upper mucosal glands with structural disorders or necrosis had a score of 4 points, and the tissue with deep ulcer or necrosis had a score of 5 points. Integral of each section should not exceed 15 points.
All specimens were fixed with 3% (W/W) glutaraldehyde and 1.5% (W/W) paraformaldehyde at 4°C for several days or hours, then postfixed for 1.5 h in 1% (W/W) osmium tetroxide and 1.5% (W/W) potassium ferrocyanide, and finally rinsed with PBS. The paraffin tissue block was stained in a solution buffer containing 70% (W/W) ethnal and uranyl acetate, then gradually dehydrated with alcohol-acetone and embedded in Epon 618. The tissue block was cut into ultra-thin (80 nm) sections, which were double stained with uranyl acetate and lead citrate for 5 min and examined under a Hitachi Hu-12A electron microscope.
Mucosal specimens were taken from gastric glands and weighed, then immersed into 0.02 mmol/L Tris-HCl (pH 7.4) at the ratio of 1:10 (mg/μL). Tissue homogenate was centrifuged at 13 000 r/min for 15 min at 4°C and precipitates were discarded. Supernatant (0.15 mL) was transferred to a testing tube. A standard solution (0.15 mL) was added to each standard testing tube, and 0.15 mL of distilled water was added to another blank testing tube, 2.5 mL thiobarbituric acid (TBA) was added into each of these two tubes, which were agitated several times, and then incubated at 100°C for 1 h. After that, each specimen was cooled to room temperature and centrifuged at 3000 r/min for 15 min. Finally, supernatant in each tube underwent colorimetric assay at 532 nm. MDA content (nmol/mL) was calculated according to the following formula: (absorbance of testing tube/absorbance of standard tube) × 2.5.
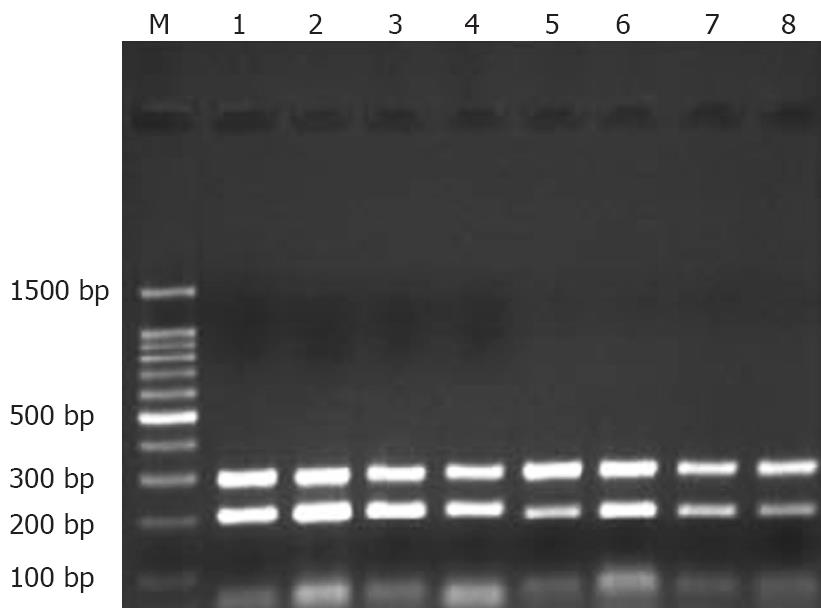
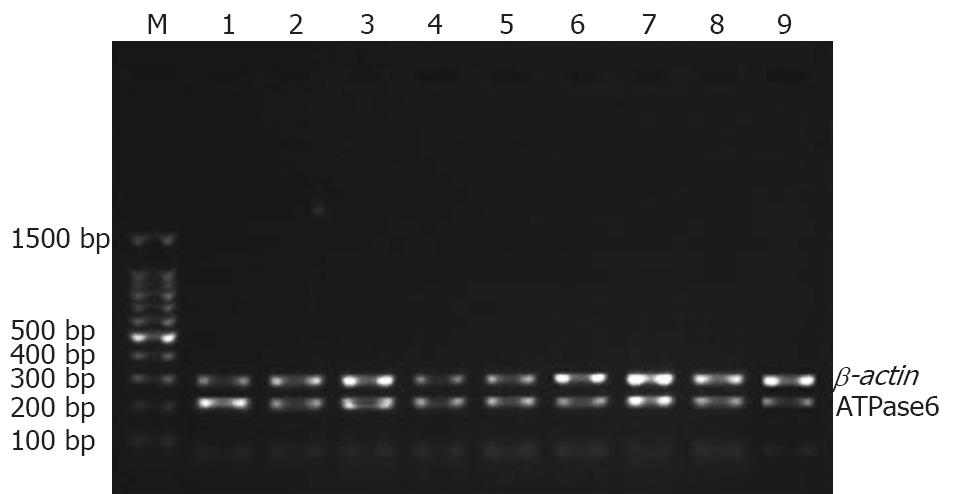
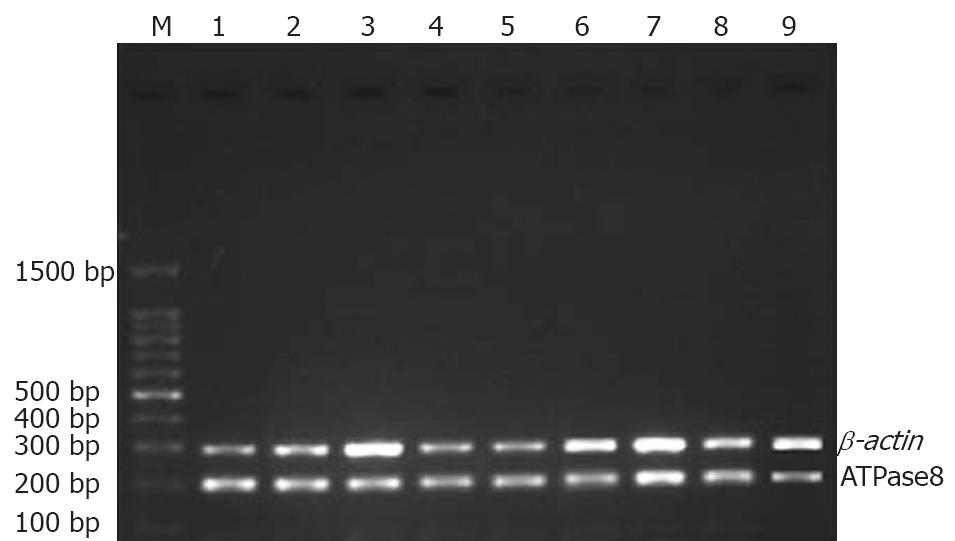
Stomach of each rat was split completely and washed at least three times with 0.1% (W/W) diethyl-pyrocarbonate (DEPC). Gastric mucosa was peeled carefully, and put into a frozen-storage pipe. Finally, each specimen was kept in liquid nitrogen. The anatomic equipments were degermed at a high-pressure and toasted at 200°C for no less than 5 h beforehand. Meanwhile, frozen pipes were soaked in 0.1% DEPC water overnight and degermed at a high-pressure to remove all vestigial DEPC. Total RNA was extracted with TrizolTM (Invitrogen) according to its manufacturer’s instructions. Reverse transcription (RT) of mRNA was performed with an AMV reverse transcription kit (Promega), following the manufacturer’s instructions. RT reaction conditions were as follows: at 42°C for 60 min, at 95°C for 5 min, at 4°C for 5 min. Reacting systems are listed in Table 1. Four uL of RT products (cDNA) was used as the template for PCR amplification. Primers were designed according to the sequences of mitochondrial ATPase subunits 6 and 8 for amplifying. For comparison, negative and positive controls were set up. β-actin gene was taken as a positive control. PCR primers were synthesized by Invitrogen Corporation. PCR products were assayed by 1.35% (W/W) agarose gel electrophoresis and stained with ethidium bromide to confirm the expected product size. Expression of ATPase subunits 6 and 8 was normalized to that of the corresponding β-actin. Analysis of PCR products was performed on GAS7100X. PCR reacting system, information about primers, amplifying conditions were listed in Tables 2, 3 and 4.
Statistical analysis was performed using SPSS 13.0 for Windows. P < 0.05 was considered statistically significant.
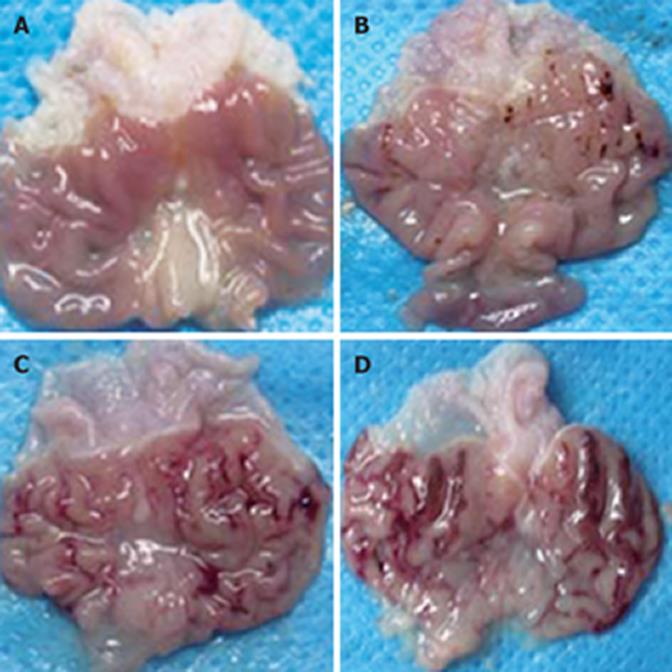
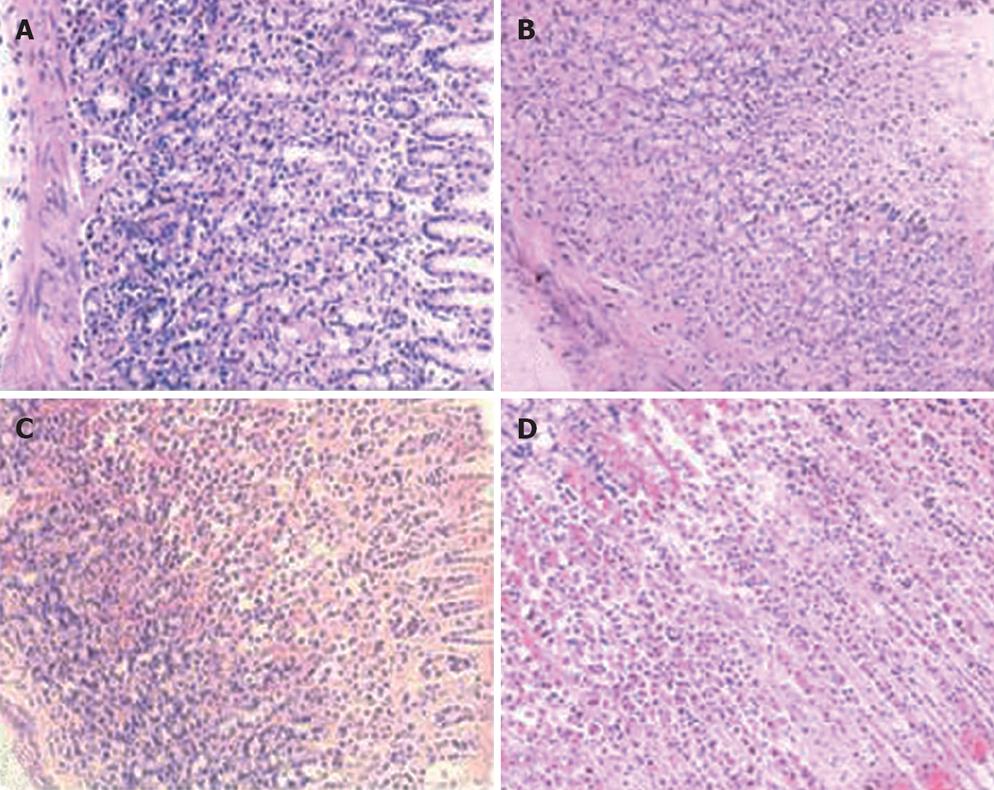
Gastric mucosa was injured in all experimental groups. A positive correlation was observed between injury and ethanol concentrations. Gastric mucosa from rats in the control group that received saline was smooth, complete, and pink, without congestion, edema, and erosion or bleeding. The mucosa was normal under microscope. With the increasing ethanol concentration, edema and congestion became obvious. In the rats that administrated 40% (W/W) ethanol, gastric mucosa became edematous and congestive. Furthermore, point and scattered bleeding lesions, focal hemorrhage, necrosis occurred. The necrosis was limited to the superficial 1/3 layer of mucosa. In the rats that administrated 70% (W/W) ethanol, congestion and edema aggravated. Superficial vessels of gastric mucosa became obviously dilated. Linear ulcer and bleeding lesions occurred. The common length of lesions was more than 4 mm, and there was significant infiltration of neutrophils and eosinophile granulocytes. Mucosa necrosis expanded to more than half of the whole layer. Rare normal mucosa was observed in the rats that administrated 100% (W/W) ethanol. Giant and deep ulcers were visible (Figures 1 and 2).
After administration of different concentrations of ethanol, the integral index was different for rats in different groups. The injury index was positively correlated with the ethanol concentration (P < 0.01, Table 5).
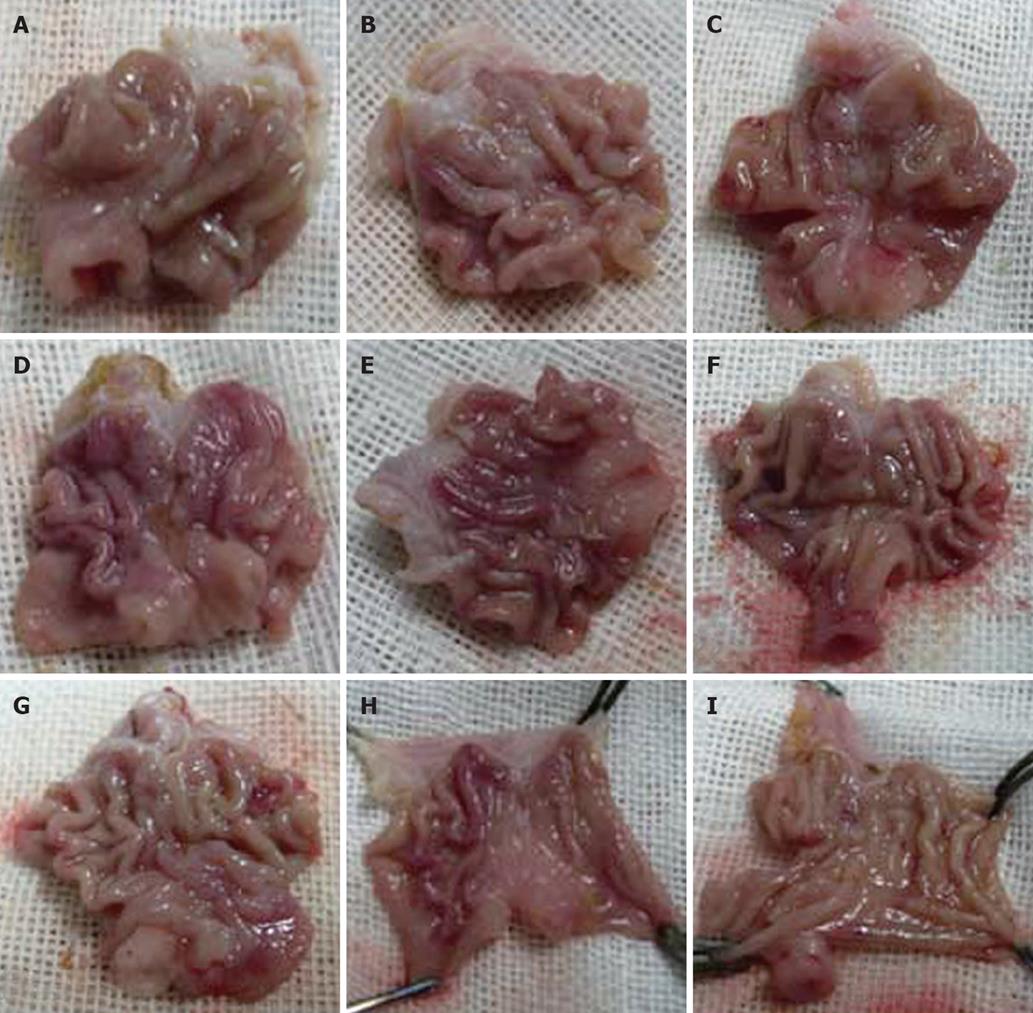
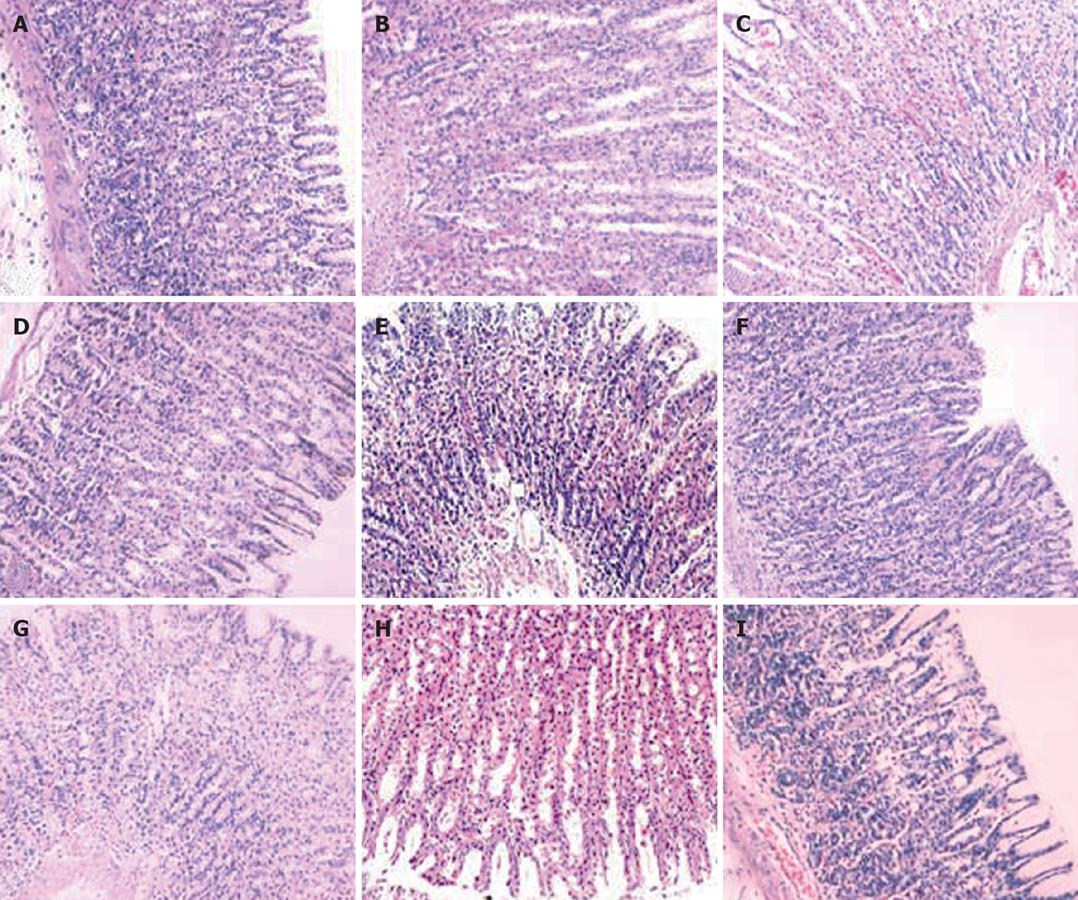
Gastric mucosa from the control group was smooth and intact. In the rats that administrated with 25% (W/W) or 45% (W/W) ethanol, injuries exacerbated with the increasing gavage time. No significant change was observed in gastric mucosa of rats that received 25% (W/W) ethanol after three weeks. Occasionally, congestion and edema could be observed under microscope. After six weeks, congestion and edema became obvious but no obvious bleeding was found. After nine weeks, congestion and edema exaggerated and some superficial bleeding lesions were noted. Disorder of glands could be observed under microscope. Gastric mucosa of rats that received 45% (W/W) ethanol became dark red after three weeks. Congestion, edema and even point erosion could be observed. There was infiltration of neutrophils and eosinophile granulocytes but no disturbed glandular structure. After six weeks, the number of scattered hemorrhage lesions increased. There was infiltration of neutrophils, lymphocytes and eosinophile granulocytes. Principal cells and parietal cells became swollen. The upper structure of glandular mucosa was damaged. After nine weeks, gastric mucosa mainly displayed a dark red or red and white performance. Congestion and edema became obviously. Infiltration of neutrophils, lymphocytes and eosinophile granulocytes exacerbated. Principal cells and parietal cells became swollen and diminished. The upper glandular structure was disturbed and the nuclei of glandular epithelium in the lower part were deeply stained. Atypical hyperplasia occurred (Figures 3 and 4).
Integral indices were different for all groups and increased with the increasing ethanol concentration (P < 0.01) and the gavage time (P < 0.05, Table 6).
Pathological integrals of all groups were different. Integral index increased with the increasing ethanol concentration (P < 0.01) and the gavage time (P < 0.01, Table 6).
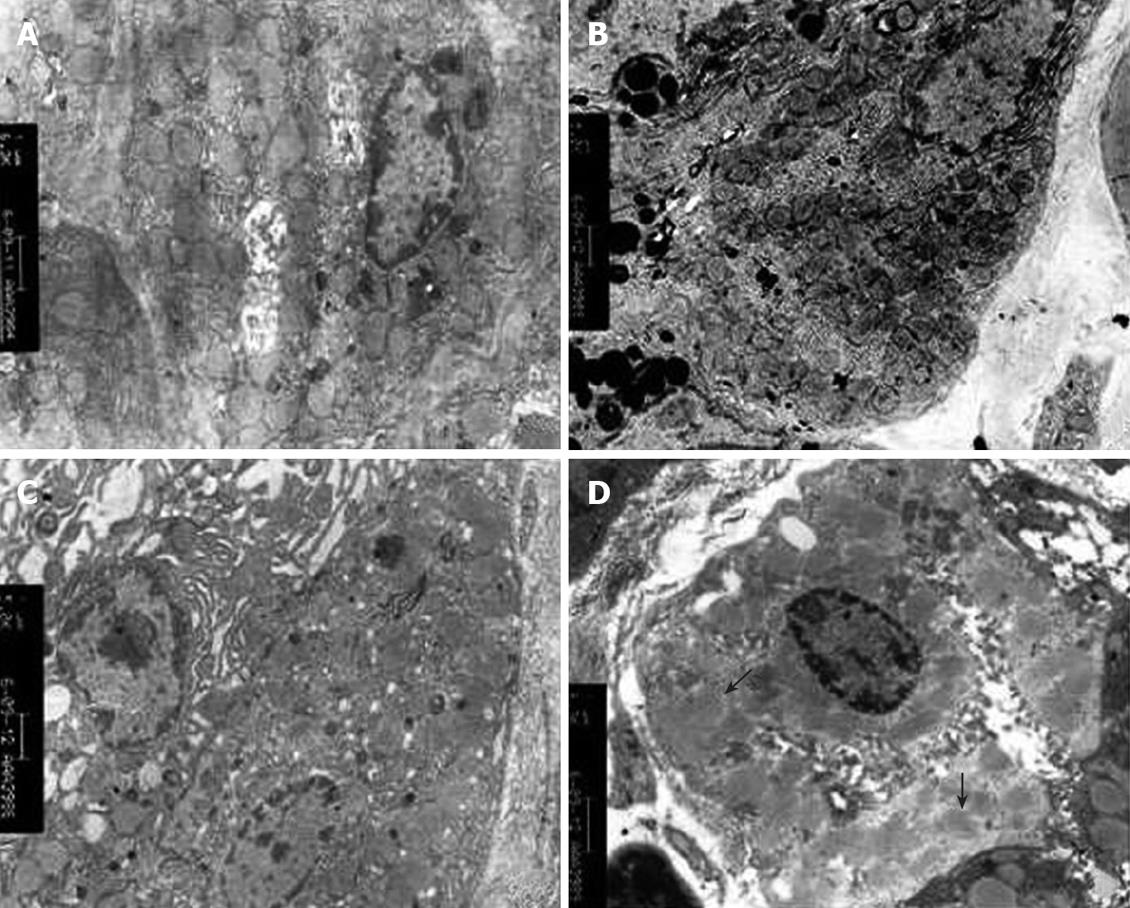
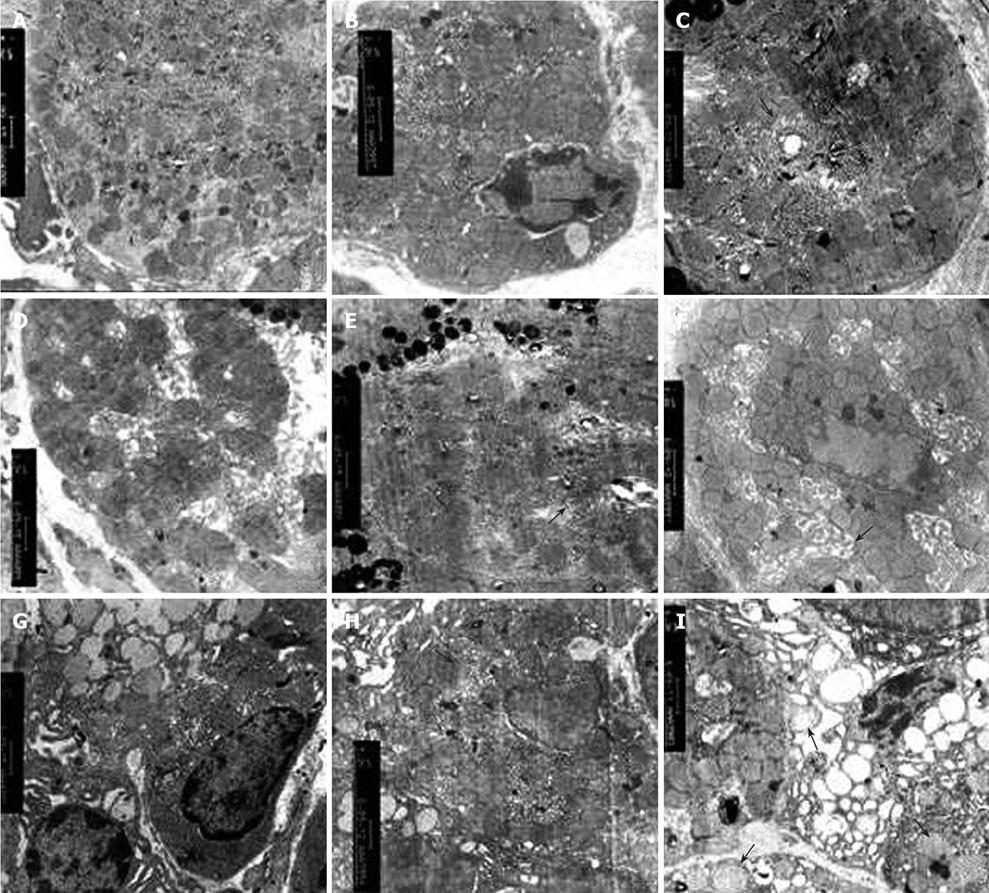
Swollen mitochondria were observed in parietal cells under transmission electron microscope and collapsed 2 h after 100% (W/W) ethanol gavage. Mitochondria became homogeneous and vacuolar. Mitochondrial cristae diminished. However, no significant change occurred in mitochondria 2 h after 40% (W/W) ethanol or 70% (W/W) ethanol gavage. The structure of mitochondria was intact and mitochondrial cristae were clear. The number of mitochondria decreased with the increasing ethanol concentration. This was especially true in rats that received 100% (W/W) ethanol. In chronic alcohol damage experiment, abnormal mitochondrial changes were observed only in rats that received 40% (W/W) ethanol. Mitochondria became sparse and swollen. The structure was blurred (Tables 7 and 8, Figures 5 and 6).
MDA content was higher in rats with ethanol-induced acute injury than in those of the control group. MDA content was positively related with ethanol concentrations (P < 0.01, Table 5).
MDA content was higher in rates with ethanol-induced chronic injury than in those of the control group. MDA content increased with the increasing ethanol concentration and gavage time (P < 0.01, Table 8).
The expression of mtDNA ATPase subunits 6 and 8 mRNA in ethanol-induced acute injury decreased with the increasing ethanol concentration. Compared with the control group, the expression of mtDNA ATPase subunits 6 and 8 mRNA increased in rats that received 40% (W/W) ethanol. However, the expression of mtDNA ATPase subunits 6 and 8 mRNA decreased in rats that received 100% (W/W) ethanol compared with rats in the control group. A same tendency was observed in rats that received 70% (W/W) ethanol (Table 7, Figure 7).
The expression of mtDNA ATPase subunits 6 and 8 mRNA was significantly different in rats with alcohol-induced chronic injury (P < 0.01). However, it was negatively related with both of ethanol concentration and gavage time (Table 9, Figures 8 and 9).
MDA and LI were positively correlated with ethanol-induced acute and chronic injury. However, ATPase subunits 6 and 8 were negatively correlated with L1 and MDA.
We successfully established an acute and chronic gastric mucosa injury model by oral administration of ethanol. MDA, a product of interaction between OFR and cell membrane, indirectly represents the OFR content in cells. The MDA content was significantly higher in rats with ethanol-induced acute and chronic injury than in those of the normal control group. Furthermore, MDA increased with the increasing ethanol concentrations indicating that MDA is positively correlated with ethanol concentration and that ethanol metabolism in the stomach produces a large quantity of OFR in a dose-dependent manner. In the acute and chronic gastric mucosa injury model, the gastric mucosal damage aggravated with the increasing content of gastric MDA. OFR tended to react with unsaturated fatty acids in cell membrane, indicating that lipid peroxidation chain reaction decreases unsaturated fatty acid content and finally affects the fluidity and permeability of cell membrane[11,12]. Gastric mucosa is rich in protein sulfhydryl groups, which may be the target of OFR. Oxidized protein sulfhydryl groups lead to protein denaturation or enzyme inactivation, and receptor damage or modification on cell membrane, thus contributing to mucosal injury[13,14]. Gastric parietal cells are abundant in mitochondria, which are critical for maintaining the normal morphology and function of gastric mucosa since they afford usable energy through oxidative phosphorylation. Two hours after administration of 100% (W/W) ethanol, mitochondrial structure was significantly damaged in the acute gastric mucosal injury model, mitochondria became swollen and disaggregated, and mitochondrial cristae were dissolved and disappeared. Nine weeks after administration of 45% (W/W) ethanol, obscuring mitochondrial structure and swollen mitochondrial cristae were found in the chronic gastric mucosal injury model. However, no significant changes occurred in other experimental groups, which may be due to the following factors, such as a small sample size. The number of mitochondria in the gastric parietal cells reduced with the increasing ethanol concentration and time, and the number of mitochondria was negatively correlated with MDA in the acute and chronic gastric mucosal injury model, suggesting that OFR can damage mitochondria.
mtDNA and expression of encoding genes have direct effects on mitochondrial respiration and ATP synthesis. mtDNA ATPase subunits 6 and 8, which are independently encoded by mtDNA, are critical parts of F0 in the respiratory enzyme, F0F1-ATPase and involved in cellular energy metabolism, acting as a proton channel in the synthesis of ATP and controlling ATP synthesis by regulating the proton transfer. mtDNA is susceptible to the attack of OFR[15], which leads to the alteration of gene expression[16]. The following characteristics of mtDNA are believed to be the main cause of this phenomenon[17-19]. mtDNA lacks the protection of histone and effective repair systems against DNA damage and is near to the inner mitochondrial membrane where OFR, a highly mutagenic product of oxidative phosphorylation, generates. mtDNA has tightly aligned genes containing few intron sequences so that random mutation may significantly change the encoding sequence of mtDNA. In the field of ethanol-induced gastric injury, few reports are available on whether OFR affects the expression of mtDNA ATPase. In the present study, the mRNA expression of mtDNA ATPase subunits 6 and 8 in acute and chronic gastric injury model decreased with the increasing ethanol concentration and was negatively correlated with the increasing MDA content, showing that OFR is negatively related with the expression of ATPase subunits 6 and 8 and that ethanol-induced gastric mucosa injury may be achieved through the detrimental effect of OFR on cellular energy metabolism. Decreasing the mRNA expression of ATPase subunits 6 and 8 can reduce the synthesis of ATPase subunit 6 and 8, the encumbrance of proton channel F0 and the activity of ATPase. Thus, the synthesis of ATP may be hindered and bring about the scarcity of provided energy. However, injured cells have an urgent demand of energy to fulfill the repair process. The contradiction between energy supply and demand eventually aggravates gastric mucosa injury, falling into a vicious cycle. The reduction in or lack of ATP synthesis may damage the gastric mucosa by inducing apoptosis and hampering the reconstruction of gastric epithelial injury. One important reaction to the epithelial damage is the rapid repair or epithelia rebuilding to recover the continuity and barrier function of mucosal epithelia. As reconstruction of gastric epithelial injury is a highly ATP-dependent process, lack of ATP may hinder this process and oxidative phosphorylation[20,21]. Lack of ATP may lead to metabolic acidosis, cellular edema, intracellular calcium overload, and further damage to gastric mucosa cells[22]. Therefore, it sounds reasonable that OFR plays an important role in ethanol-induced gastric mucosa injury. Damage may eventually affect, at least in part, cellular energy metabolism. The expression of ATPase subunits 6 and 8 in rats that received 40% (W/W) ethanol was higher than that in those of the control group, suggesting that injured gastric mucosa can protect itself against a certain extent of oxidative stress.
Our study systematically described gastric mucosa injury from three levels, namely gross, microscopic appearance and ultrastructure. We also discussed the relationship between disturbed mitochondrial structure and oxidative stress caused by ethanol. We conclude that disorder of mitochondrial energy metabolism may play an important, role in the pathogenesis of ethanol-related gastropathy. However, changes in ATPase subunits 6 and 8 at protein level and alteration of mitochondrial membrane potential remain to be elucidated.
Peer reviewer: Cintia Siqueira, Professor, Institute of Molecular Medicine, Center of Gastroenterology, Av. Egas Moniz, isboa, 1649-028, Portugal
S- Editor Xiao LL L- Editor Wang XL E- Editor Lin YP
| 1. | Alarcon JM, Quevedo L, Reyes P. Inhibitory action of hydrogen peroxide on a high-resistance epithelium. Pharmacology. 1995;50:111-118. |
| 2. | Itoh M, Guth PH. Role of oxygen-derived free radicals in hemorrhagic shock-induced gastric lesions in the rat. Gastroenterology. 1985;88:1162-1167. |
| 3. | Duman JG, Pathak NJ, Ladinsky MS, McDonald KL, Forte JG. Three-dimensional reconstruction of cytoplasmic membrane networks in parietal cells. J Cell Sci. 2002;115:1251-1258. |
| 4. | Shimokawa T, Yamagiwa D, Hondo E, Nishiwaki S, Kiso Y, Makita T. Histological observation of the proper gastric gland in Minke whale, Balaenoptera acutorostrata. J Vet Med Sci. 2003;65:423-426. |
| 5. | Yin GY, Zhang WN, Shen XJ, Chen Y, He XF. Ultrastructure and molecular biological changes of chronic gastritis, gastric cancer and gastric precancerous lesions: a comparative study. World J Gastroenterol. 2003;9:851-857. |
| 6. | Baracca A, Barogi S, Carelli V, Lenaz G, Solaini G. Catalytic activities of mitochondrial ATP synthase in patients with mitochondrial DNA T8993G mutation in the ATPase 6 gene encoding subunit a. J Biol Chem. 2000;275:4177-4182. |
| 7. | Li HS, Zhang JY, Thompson BS, Deng XY, Ford ME, Wood PG, Stolz DB, Eagon PK, Whitcomb DC. Rat mitochondrial ATP synthase ATP5G3: cloning and upregulation in pancreas after chronic ethanol feeding. Physiol Genomics. 2001;6:91-98. |
| 8. | Hoek JB, Cahill A, Pastorino JG. Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology. 2002;122:2049-2063. |
| 9. | Guth PH, Aures D, Paulsen G. Topical aspirin plus HCl gastric lesions in the rat. Cytoprotective effect of prostaglandin, cimetidine, and probanthine. Gastroenterology. 1979;76:88-93. |
| 10. | Masuda E, Kawano S, Nagano K, Tsuji S, Takei Y, Hayashi N, Tsujii M, Oshita M, Michida T, Kobayashi I. Role of endogenous endothelin in pathogenesis of ethanol-induced gastric mucosal injury in rats. Am J Physiol. 1993;265:G474-G481. |
| 11. | Bagchi D, Carryl OR, Tran MX, Krohn RL, Bagchi DJ, Garg A, Bagchi M, Mitra S, Stohs SJ. Stress, diet and alcohol-induced oxidative gastrointestinal mucosal injury in rats and protection by bismuth subsalicylate. J Appl Toxicol. 1998;18:3-13. |
| 12. | Repetto M, Maria A, Guzman J, Giordano O, Llesuy S. Protective effect of Artemisia douglasiana Besser extracts in gastric mucosal injury. J Pharm Pharmacol. 2003;55:551-557. |
| 13. | Bilici D, Suleyman H, Banoglu ZN, Kiziltunc A, Avci B, Ciftcioglu A, Bilici S. Melatonin prevents ethanol-induced gastric mucosal damage possibly due to its antioxidant effect. Dig Dis Sci. 2002;47:856-861. |
| 14. | La Casa C, Villegas I, Alarcon de la Lastra C, Motilva V, Martin Calero MJ. Evidence for protective and antioxidant properties of rutin, a natural flavone, against ethanol induced gastric lesions. J Ethnopharmacol. 2000;71:45-53. |
| 15. | Penta JS, Johnson FM, Wachsman JT, Copeland WC. Mitochondrial DNA in human malignancy. Mutat Res. 2001;488:119-133. |
| 16. | Ballinger SW, Patterson C, Yan CN, Doan R, Burow DL, Young CG, Yakes FM, Van Houten B, Ballinger CA, Freeman BA. Hydrogen peroxide- and peroxynitrite-induced mitochondrial DNA damage and dysfunction in vascular endothelial and smooth muscle cells. Circ Res. 2000;86:960-966. |
| 17. | Bai RK, Wong LJ. Detection and quantification of heteroplasmic mutant mitochondrial DNA by real-time amplification refractory mutation system quantitative PCR analysis: a single-step approach. Clin Chem. 2004;50:996-1001. |
| 18. | Zeviani M, Antozzi C. Mitochondrial disorders. Mol Hum Reprod. 1997;3:133-148. |
| 19. | Shukla A, Jung M, Stern M, Fukagawa NK, Taatjes DJ, Sawyer D, Van Houten B, Mossman BT. Asbestos induces mitochondrial DNA damage and dysfunction linked to the development of apoptosis. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1018-L1025. |
| 20. | Scholte HR. The biochemical basis of mitochondrial diseases. J Bioenerg Biomembr. 1988;20:161-191. |
| 21. | Scholte HR, Busch HF, Luyt-Houwen IE, Vaandrager-Verduin MH, Przyrembel H, Arts WF. Defects in oxidative phosphorylation. Biochemical investigations in skeletal muscle and expression of the lesion in other cells. J Inherit Metab Dis. 1987;10 Suppl 1:81-97. |
| 22. | Rong Q, Utevskaya O, Ramilo M, Chow DC, Forte JG. Nucleotide metabolism by gastric glands and H(+)-K(+)-ATPase-enriched membranes. Am J Physiol. 1998;274:G103-G110. |