Published online Oct 14, 2008. doi: 10.3748/wjg.14.5851
Revised: July 5, 2008
Accepted: July 12, 2008
Published online: October 14, 2008
AIM: To investigate the effects of FR167653 on the development of dextran sulfate sodium (DSS)-induced colitis in mice.
METHODS: BALB/c mice were fed rodent chow containing 3.5% (wt/wt) DSS. The recipient mice underwent intra-peritoneal injection of vehicles or FR167653 (30 mg/kg per day). The mice were sacrificed on day 14, and the degree of colitis was assessed. Immunohistochemical analyses for CD4+ T cell and F4/80+ macrophage infiltration were also performed. Mucosal cytokine expression was analyzed by RT-PCR.
RESULTS: The body weight loss was more apparent in the FR167653-treated DSS mice than in the vehicle-treated DSS mice. The colon length was shorter in the FR167653-treated DSS mice than in the vehicle-treated DSS mice. Disease activity index and histological colitis score were significantly higher in FR167653- than in vehicle-treated DSS animals. Microscopically, mucosal edema, cellular infiltration (CD4 T cells and F4/80 macrophages), and the disruption of the epithelium were much more severe in FR167653-treated mice than in controls. Mucosal mRNA expression for interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) were found to be markedly reduced in FR167653-treated DSS mice.
CONCLUSION: Treatment with FR167653 aggravated DSS colitis in mice. This effect was accompanied by a reduction of mucosal IL-1β and TNF-α expression, suggesting a role of p38 mitogen-activated protein kinase (MAPK)-mediated proinflammatory cytokine induction in host defense mechanisms.
- Citation: Nishimura T, Andoh A, Nishida A, Shioya M, Koizumi Y, Tsujikawa T, Fujiyama Y. FR167653, a p38 mitogen-activated protein kinase inhibitor, aggravates experimental colitis in mice. World J Gastroenterol 2008; 14(38): 5851-5856
- URL: https://www.wjgnet.com/1007-9327/full/v14/i38/5851.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5851
Inflammatory bowel diseases (IBD), such as ulcerative colitis (UC) and Crohn’s disease (CD), are associated with chronic relapsing inflammation of the intestinal tract of unknown etiology. The most widely held hypothesis on the pathogenesis of IBD is that the mucosal immune system shows an aberrant response towards luminal antigens such as dietary factors and/or commensal bacteria in genetically susceptible individuals[1-3]. Histologically, mucosal accumulation of leukocytes is a characteristic feature of IBD, and the activation of T cells and monocytes/macrophages has been regarded as a crucial factor in its pathogenesis[1,2].
Previous studies have clearly demonstrated that bacterial lipopolysaccharides (LPS) and pro-inflammatory cytokines, such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), activate the p38 mitogen-activated protein kinase (MAPK) pathway[4-6] and suggested that the p38 MAPK family play important roles in pathophysiology of IBD[1,7-9]. The p38 is a member of the MAPK family, which is ubiquitously expressed. Serine-threonine kinases are playing important roles in various signal transduction pathways in mammalian cells[4]. It has been demonstrated that p38 mediates phosphorylation of transcription factors, thereby regulating gene expression and the induction of cytokine production[6]. p38 is activated by dual phosphorylation on Thr180 and Tyr182 by the upstream MAPKs MKK3 and MKK6. MKK3 is especially important for TNF-α-induced p38 activation[10] and for the p38-mediated synthesis of IL-12 and IFN-γ[11]. Thus, p38 is a promising candidate for targeted inhibition in acute and chronic inflammation.
FR167653 is a well-characterized inhibitor of p38 MAPK[12-16]. FR167653 specifically inhibits p38 via its ability to compete with adenosine triphosphate at adenosine triphosphate binding sites on p38 kinase[17,18], and such pharmacological actions resemble those of pyridinyl imidazole inhibitors of p38 MAPK such as SB 203580 and RWJ6. This compound dramatically and selectively attenuates the activity of p38, but does not significantly modulate JNK and ERK-1/2 activity[19]. FR167653 has been demonstrated to specifically inhibit p38 function and eventual IL-1β and TNF-α protein translation[13,20], but the expression of IL-6 and transforming growth factor (TGF)-β does not appear to be affected[21]. At present, FR167653 has not been studied in any animal models for IBD, and our knowledge of its action in chronic intestinal injury models is limited. In this study, we investigated the effects of FR167653 on the development of dextran sulfate sodium (DSS)-induced colitis in mice.
Six- to eight-week-old male BALB/c mice were purchased from Charles River Japan (Kanagawa, Japan). They were acclimatized for 1 wk before the experiment, and were housed individually in a room maintained at 22°C under a 12-h day/night cycle throughout the experiments. Mice were fed 3.5% (wt/wt) DSS (molecular weight 5000; Wako Pure Chemical Industries, Ltd, Osaka, Japan) mixed with normal chow (MF; Oriental Yeast Co., Ltd, Tokyo, Japan) and water ad libitum. The study protocol was approved by the Animal Care and Use Committee of the Shiga University of Medical Science (Otsu, Japan).
FR167653 was kindly provided by Fujisawa Pharmaceutical (Osaka, Japan). The recipient mice underwent intra-peritoneal injection of vehicles or FR167653 at a dose of 30 mg/kg per day dissolved in 200 μL phosphate-buffered saline (PBS) every day, starting on the first day of DSS administration. The dose of FR167653 was chosen with reference to publications showing in vivo effects of the drug[12,19].
Daily clinical assessment of DSS-induced colitis was performed, including measurements of food intake and body weight, an evaluation of stool consistency, and the presence of blood in the stools by a guaiac paper test. A validated clinical disease activity index ranging from 0 to 4 was calculated using the following parameters: stool consistency, presence of fecal blood, and changes in body weight[22]. The mice were sacrificed at day 14, and the length and weight of the colons were measured.
A histological examination was performed on three samples of the distal colon from each animal. The samples were fixed in 10% buffered formalin, dehydrated in ethanol, and then embedded in paraffin. Four micron-thick sections were then prepared and stained with hematoxylin and eosin. All histologic evaluation was performed in a blinded fashion using a validated scoring system[23].
For immunohistochemical staining, freshly isolated tissue from the distal portion of the colon was frozen in dry ice using OCT compound (Sakura Finetek, Tokyo, Japan). Acetone-fixed frozen sections (6 μm) were blocked with DAKO blocking reagent (#X0909, DAKO Japan, Kyoto, Japan) followed by incubation with the primary antibodies [anti-mouse CD4 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and anti-mouse F4/80 (BD Pharmingen, San Diego, CA)], diluted 1:100 in PBS containing 5% skim milk overnight at 4°C in a humidified chamber. After incubation with the primary antibody, the sections were reacted with 0.1% H2O2 in 0.1 mol/L PBS, and treated with biotin-conjugated second antibodies (diluted 1:50 in PBS containing 1% skim milk; Vector, Burlingame, CA) for 60 min at room temperature and followed by avidin-biotin-peroxidase complexes (ABC, Vector). The peroxidase activity was visualized using diaminobenzidine.
Cytokine mRNA expression in the mucosa was evaluated by RT-PCR. Total cellular RNA was isolated by the acid guanidium thiocyanate-phenol-chloroform (AGPC) method[24]. For each sample, the first-strand cDNA was synthesized using 0.5 μg of total cellular RNA with oligo (dT) primer and Superscript reverse transcriptase (GIBCO BRL, Rockville, MD). One μL of the cDNA sample was amplified in a 25 μL reaction containing 10 × Taq buffer (Perkin Elmer Cetus Corp., Norwalk, CT), 1.5 mmol/L MgCl2, 0.1 μmol/L of each 5' and 3' primers, and 1 U of TaqGold polymerase (Perkin-Elmer Cetus). The PCR was performed in a thermal cycler (GeneAmp Model 2400; Perkin-Elmer Cetus) for 25 cycles (94°C for 30 s, 55°C for 30 s, and 72°C for 40 s), followed by a 8 min extension at 72°C. Five μL of the PCR products were subjected to electrophoresis on 1.5% agarose gels and stained with 0.5 μg/mL ethidium bromide. A 100-bp DNA ladder (GIBCO BRL) was used as marker. Primers specific for the mouse IL-1β were purchased from BioSorce International, Inc. (Camarillo, CA). Specific primers for mouse TNF-α were constructed according to published sequence data[25] (5'-GCGACGTGGAACTGGCAGAAG-3', and 5'-GGTACAACCCATCGGCTGGCA-3').
Statistical analysis was performed using one-way ANOVA with Scheffe’s post hoc test or the Kruskal-Wallis test when appropriate. Two-way ANOVA for repeated measures was used to test for group and time effects on clinical data (e.g. disease activity index) over 7 successive days of clinical observation. P < 0.05 was considered to be statistically significant.
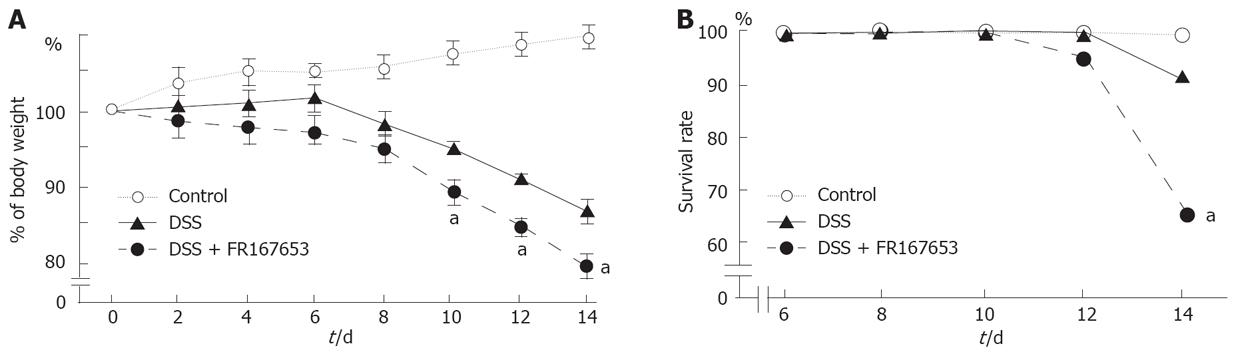
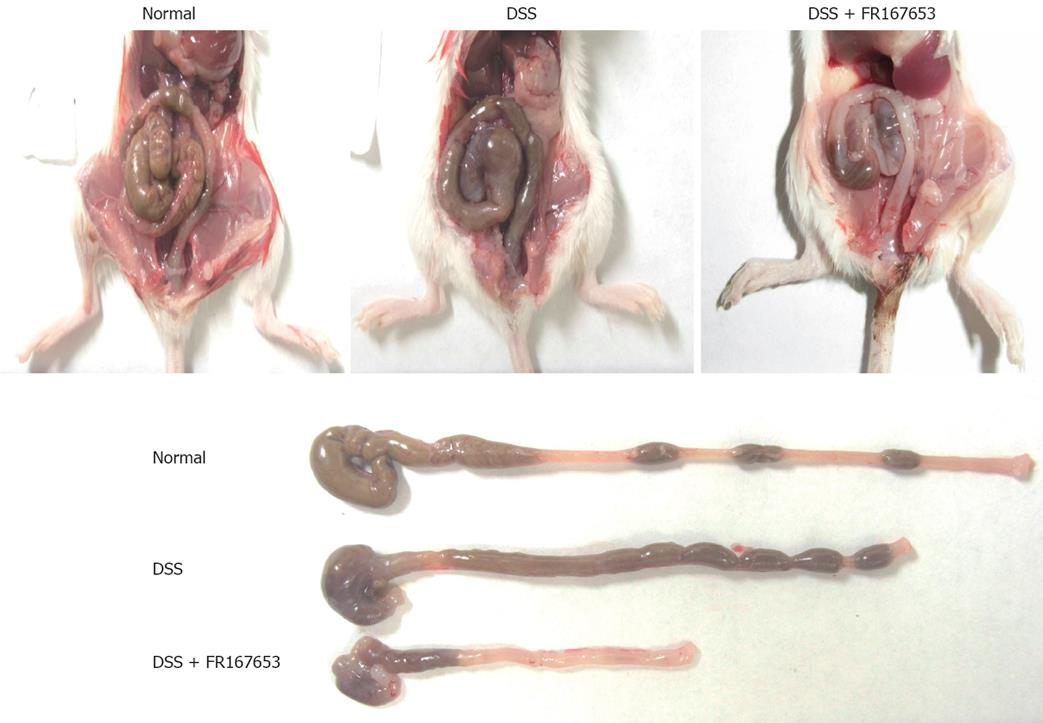
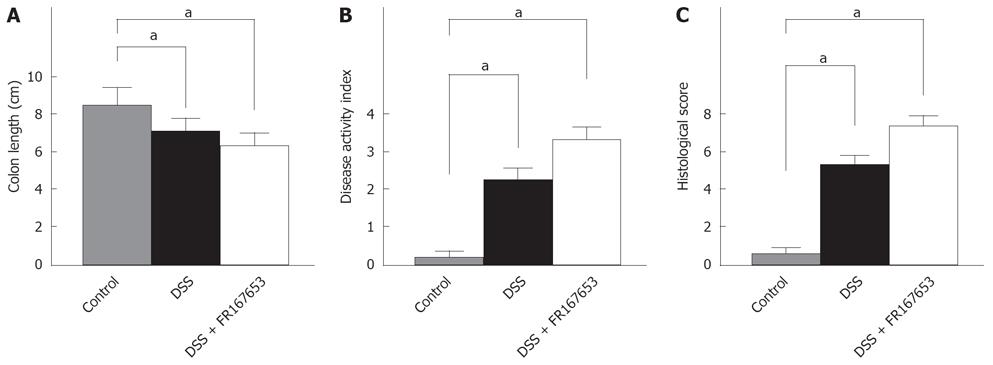
To evaluate the effect of FR167653 on DSS colitis, the administration of FR167653 was started at the time of DSS exposure. The administration of FR167653 and vehicle (PBS) was repeated every 24 h. As shown in Figure 1A, on day 10, day 12, and day 14 after the initiation of DSS-induced colitis, the body weight was significantly lower in the FR167653-treated than in the PBS-treated mice. On day 14, survival rate was significantly lower in FR167653- than in the PBS-treated DSS animals (Figure 1B). As shown in Figure 2, colon shortening and stool softening were apparent in the FR167653-treated DSS mice (Figure 2). The total colon length was significantly smaller in the FR167653-treated than in the PBS-treated DSS mice (Figure 3A).
A macroscopic examination of the colon revealed that hyperemia, erosions, and occasional tiny blood coagula occurred mainly in the rectum in both DSS-treated groups. Disease parameters such as the fecal blood score, diarrhea score, and disease activity index were significantly higher in the FR167653-treated than the PBS-treated DSS mice (Figure 3B).
DSS colitis is characterized by histological findings such as edema, infiltration of inflammatory cells into the mucosa and submucosa, ulceration, and mucosal thickening. Our histological analysis indicated that administration of FR167653 markedly enhanced the severity of the colitis as compared to the PBS-treated mice (Figure 3C).
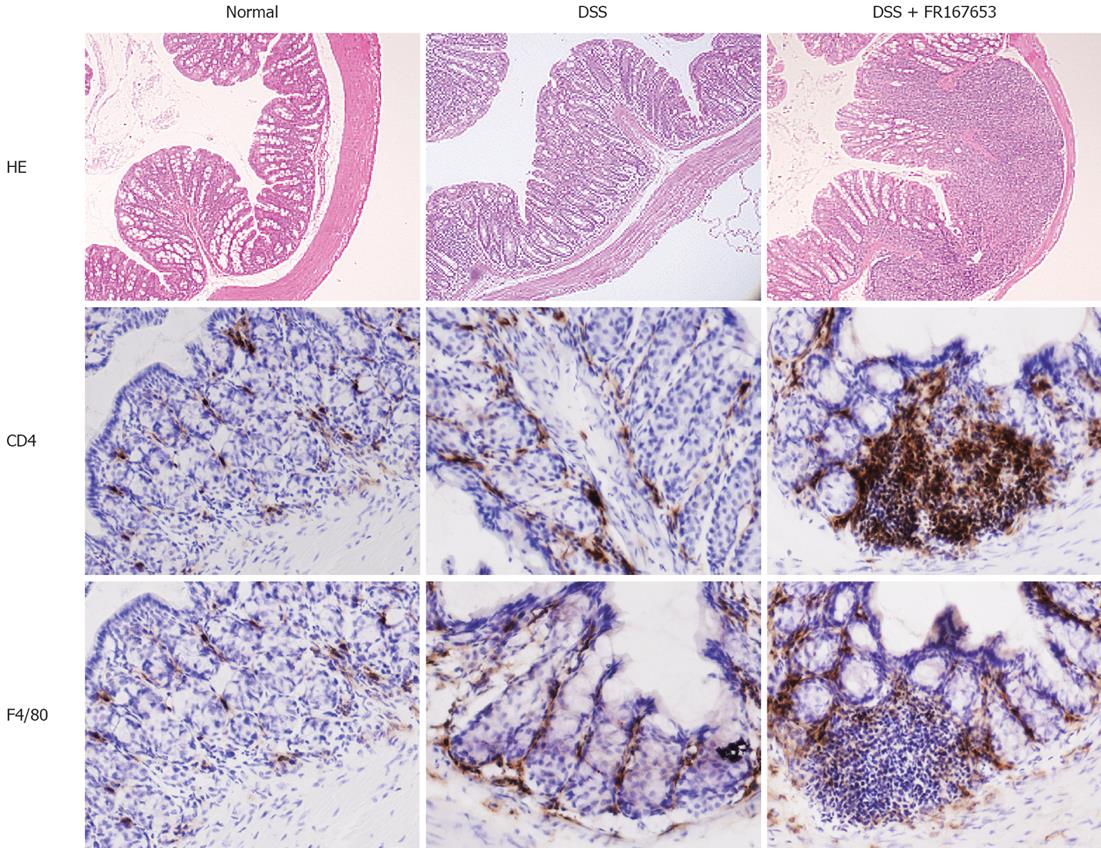
An increase in the number of infiltrating cells and an enhancement of mucosal injury were observed in the PBS-treated DSS mice (Figure 4 upper part). These changes were enhanced in the FR167653-treated DSS mice. The immunohistochemical analysis indicated that the mucosal infiltration of CD4-positive T cells and F4/80-positive monocytes/macrophages was increased in the FR167643-treated DSS mice, compared to the PBS-treated DSS mice (Figure 4 middle and lower parts).
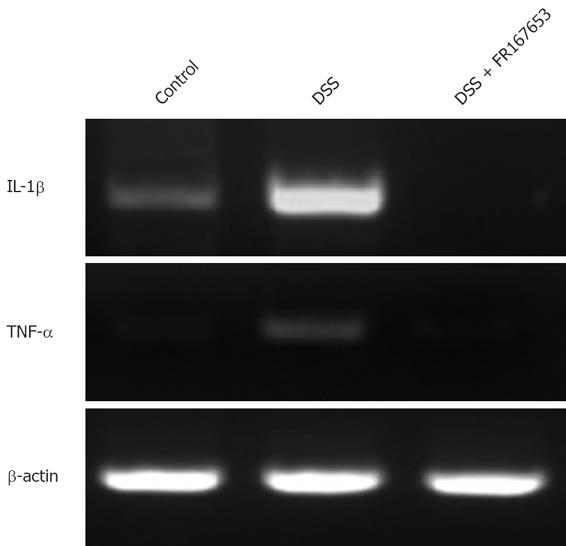
Inhibitory effects of FR167653 on IL-1β and TNF-α expression have been reported[26,27]. To confirm these responses in our model, mucosal IL-1β and TNF-α mRNA expression was evaluated by semi-quantitative RT-PCR. Representative results are shown in Figure 5. DSS mice showed increased expression of IL-1β and TNF-α. The administration of FR167653 markedly reduced the expression of IL-1β and TNF-α mRNAs, although severity of colitis was enhanced. Similar results were observed in 5 different experiments.
The aim of this study was to analyze the impact of FR167653 upon inflammation in a mouse model of IBD. DSS-induced colitis in BALB/c mice is used as a model system for the respective human IBD. FR167653 was first discovered to be a potent inhibitor of IL-1 and TNF-α production in LPS-stimulated human monocytes and activated lymphocytes[17]. Recent studies suggest that FR167653 inhibits IL-1β and TNF-α production via specific inhibition of p38 MAPK activity[14,19,21]. While we initially expected that inhibition of p38 by FR167653 would block the development of DSS colitis, administration of FR167653 aggravated DSS colitis. Because mucosal IL-1β and TNF-α expression was markedly reduced in the FR167653-treated mice, aggravation of colitis might not be mediated by enhanced proinflammatory responses.
Previous findings of p38 activation in the pathophysiology of IBD are controversial. Waetzig et al[7] demonstrated that p38 and JNK1/2 were significantly activated in the inflamed colonic mucosa of IBD patients while the protein and mRNA expression of p38 and JNK were not significantly different between patients and controls. They suggested the role of p38 in the TNF-α signaling regulation loop in active CD patients. Hommes et al[8] showed an enhancement of phospho-p38 and phospho-JNK expression in neutrophils, epithelial cells, and lamina propria mononuclear cells in six patients with active CD compared to healthy controls. In contrast, Malamut et al[28] could not confirm p38 activation in patients with IBD, by measuring the protein levels and activity of p38. Moreover, a significant decrease in p38 activity and phosphorylated p38 levels was actually observed in the trinitrobenzene sulphonic acid (TNBS) colitis model.
There are two controversial reports of p38-inhibitor effects on experimental colitis. Ten Hove et al[29] observed a dichotomous effect of a specific p38 MAPK inhibitor, SB 203580, in TNBS-induced colitis in mice. In SB 203580-treated TNBS mice, weight loss was significantly worse and colon weight was significantly increased. However, activated lymph node cells of SB 203580 treated mice showed decreased IFN-γ but an increased TNF-α production. On the other hand, Hollenbach et al[30] showed that SB 203580 improved DSS colitis as reflected in the disease activity and histological disease score. Improvement of colitis was associated with down-regulation of NF-κB signaling and cytokine production.
The precise mechanisms underlying FR167653-induced aggravation of DSS colitis are unclear. Despite of aggravation of proinflammatory cell infiltration, FR167653 strongly reduced mucosal IL-1β and TNF-α expression. It may be that activation of p38 MAPK occurs downstream of the disease perpetuating signal transduction elements and that blockade at this level does not affect disease severity. Alternatively, cells playing roles in mucosal repair or anti-inflammatory process may be dependent on p38 MAPK. For example, it has been reported that p38 MAPK play an important role in epithelial restitution, a process aimed at re-epithelializing the wounded areas[31]. A recent study showed that production of IL-10, a representative anti-inflammatory cytokine, is dependent on p38 MAPK activation[32]. However, observed effects may be specific for models used in this study, and further experiments with other colitis models are needed.
In conclusion, treatment with the p38 MAPK inhibitor FR167653 induced an aggravation of DSS colitis although it reduced mucosal IL-1β and TNF-α production. This indicates that p38 MAPK is not only responsible for the production of proinflammatory cytokines but may also be involved in critical responses in host defense.
FR167653 is a well-characterized inhibitor of p38 mitogen-activated protein kinase (MAPK). At present, FR167653 has not been studied in any animal models for inflammatory bowel disease (IBD), and knowledge of its action in chronic colitis models is limited.
Demonstration of the effects of a novel p38 MAPK inhibitor on dextran sulfate sodium (DSS) colitis.
Previous studies suggested that p38 MAPK acts as an enhancer of experimental colitis. Our study demonstrated that FR167653, a p38 MAPK inhibitor, aggravated DSS colitis, suggesting a protective role of p38 MAPK.
Clinical application of FR167653 to IBD may be possible.
FR167653 is a well-characterized inhibitor of p38 MAPK. p38 MAPK mediates a major signal transduction pathway in pro- and anti-inflammatory responses.
This is a carefully performed study detailing the effect of co-administration of a parenteral p38 MAPK inhibitor (FR167653) on the induction of DSS colitis in BALB/c mice. The use of this agent in various inflammatory conditions is not novel, nor is targeting p38 in IBD. What is novel is the finding that inhibiting p38 results in more severe DSS colitis.
| 1. | Mizoguchi A, Mizoguchi E. Inflammatory bowel disease, past, present and future: lessons from animal models. J Gastroenterol. 2008;43:1-17. |
| 2. | Sands BE. Inflammatory bowel disease: past, present, and future. J Gastroenterol. 2007;42:16-25. |
| 3. | Sartor RB, Muehlbauer M. Microbial host interactions in IBD: implications for pathogenesis and therapy. Curr Gastroenterol Rep. 2007;9:497-507. |
| 5. | Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta. 2007;1773:1358-1375. |
| 6. | Cook R, Wu CC, Kang YJ, Han J. The role of the p38 pathway in adaptive immunity. Cell Mol Immunol. 2007;4:253-259. |
| 7. | Waetzig GH, Seegert D, Rosenstiel P, Nikolaus S, Schreiber S. p38 mitogen-activated protein kinase is activated and linked to TNF-alpha signaling in inflammatory bowel disease. J Immunol. 2002;168:5342-5351. |
| 8. | Hommes D, van den Blink B, Plasse T, Bartelsman J, Xu C, Macpherson B, Tytgat G, Peppelenbosch M, Van Deventer S. Inhibition of stress-activated MAP kinases induces clinical improvement in moderate to severe Crohn's disease. Gastroenterology. 2002;122:7-14. |
| 9. | Waetzig GH, Schreiber S. Review article: mitogen-activated protein kinases in chronic intestinal inflammation - targeting ancient pathways to treat modern diseases. Aliment Pharmacol Ther. 2003;18:17-32. |
| 10. | Wysk M, Yang DD, Lu HT, Flavell RA, Davis RJ. Requirement of mitogen-activated protein kinase kinase 3 (MKK3) for tumor necrosis factor-induced cytokine expression. Proc Natl Acad Sci USA. 1999;96:3763-3768. |
| 11. | Lu HT, Yang DD, Wysk M, Gatti E, Mellman I, Davis RJ, Flavell RA. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J. 1999;18:1845-1857. |
| 12. | Yoshino O, Osuga Y, Koga K, Hirota Y, Hirata T, Ruimeng X, Na L, Yano T, Tsutsumi O, Taketani Y. FR 167653, a p38 mitogen-activated protein kinase inhibitor, suppresses the development of endometriosis in a murine model. J Reprod Immunol. 2006;72:85-93. |
| 13. | Chan KL, Guo WH, Chan KW, Tam PK. FR167653 ameliorates intestinal allograft ischemic injury. Transplant Proc. 2000;32:1643-1644. |
| 14. | Aleshin A, Sawa Y, Ono M, Funatsu T, Miyagawa S, Matsuda H. Myocardial protective effect of FR167653; a novel cytokine inhibitor in ischemic-reperfused rat heart. Eur J Cardiothorac Surg. 2004;26:974-980. |
| 15. | Yao HW, Li J, Chen JQ. FR167653 attenuates murine immunological liver injury. World J Gastroenterol. 2004;10:2267-2271. |
| 16. | Wei YH, Li Y, Qiang CJ. Effects and mechanisms of FR167653, a dual inhibitor of interleukin-1 and tumor necrosis factor, on adjuvant arthritis in rats. Int Immunopharmacol. 2004;4:1625-1632. |
| 17. | Yamamoto N, Sakai F, Yamazaki H, Sato N, Nakahara K, Okuhara M. FR167653, a dual inhibitor of interleukin-1 and tumor necrosis factor-alpha, ameliorates endotoxin-induced shock. Eur J Pharmacol. 1997;327:169-174. |
| 18. | Yamamoto N, Sakai F, Yamazaki H, Nakahara K, Okuhara M. Effect of FR167653, a cytokine suppressive agent, on endotoxin-induced disseminated intravascular coagulation. Eur J Pharmacol. 1996;314:137-142. |
| 19. | Takahashi S, Keto Y, Fujita T, Uchiyama T, Yamamoto A. FR167653, a p38 mitogen-activated protein kinase inhibitor, prevents Helicobacter pylori-induced gastritis in Mongolian gerbils. J Pharmacol Exp Ther. 2001;296:48-56. |
| 20. | Hato S, Urakami A, Yamano T, Uemura T, Ota T, Hirai R, Shimizu N. Attenuation of liver and lung injury after hepatic ischemia and reperfusion by a cytokine-suppressive agent, FR167653. Eur Surg Res. 2001;33:202-209. |
| 21. | Nishikori T, Irie K, Suganuma T, Ozaki M, Yoshioka T. Anti-inflammatory potency of FR167653, a p38 mitogen-activated protein kinase inhibitor, in mouse models of acute inflammation. Eur J Pharmacol. 2002;451:327-333. |
| 22. | Cooper HS, Murthy SN, Shah RS, Sedergran DJ. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238-249. |
| 23. | Rath HC, Herfarth HH, Ikeda JS, Grenther WB, Hamm TE Jr, Balish E, Taurog JD, Hammer RE, Wilson KH, Sartor RB. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest. 1996;98:945-953. |
| 24. | Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156-159. |
| 25. | Murphy E, Hieny S, Sher A, O'Garra A. Detection of in vivo expression of interleukin-10 using a semi-quantitative polymerase chain reaction method in Schistosoma mansoni infected mice. J Immunol Methods. 1993;162:211-223. |
| 26. | Yao HW, Yue L. Effect and mechanisms of FR167653, a dual inhibitor of TNF-alpha and IL-1, on BCG plus LPS induced-liver injury. Inflamm Res. 2005;54:471-477. |
| 27. | Takeyoshi I, Yoshinari D, Kobayashi M, Kurabayashi M, Morishita Y. A dual inhibitor of TNF-alpha and IL-1 mitigates liver and kidney dysfunction and improves survival in rat endotoxemia. Hepatogastroenterology. 2005;52:1507-1510. |
| 28. | Malamut G, Cabane C, Dubuquoy L, Malapel M, Derijard B, Gay J, Tamboli C, Colombel JF, Desreumaux P. No evidence for an involvement of the p38 and JNK mitogen-activated protein in inflammatory bowel diseases. Dig Dis Sci. 2006;51:1443-1453. |
| 29. | ten Hove T, van den Blink B, Pronk I, Drillenburg P, Peppelenbosch MP, van Deventer SJ. Dichotomal role of inhibition of p38 MAPK with SB 203580 in experimental colitis. Gut. 2002;50:507-512. |
| 30. | Hollenbach E, Neumann M, Vieth M, Roessner A, Malfertheiner P, Naumann M. Inhibition of p38 MAP kinase- and RICK/NF-kappaB-signaling suppresses inflammatory bowel disease. FASEB J. 2004;18:1550-1552. |
| 31. | Karrasch T, Steinbrecher KA, Allard B, Baldwin AS, Jobin C. Wound-induced p38MAPK-dependent histone H3 phosphorylation correlates with increased COX-2 expression in enterocytes. J Cell Physiol. 2006;207:809-815. |
| 32. | Leghmari K, Bennasser Y, Tkaczuk J, Bahraoui E. HIV-1 Tat protein induces IL-10 production by an alternative TNF-alpha-independent pathway in monocytes: role of PKC-delta and p38 MAP kinase. Cell Immunol. 2008;253:45-53. |
Peer reviewer: Peter Mannon, MD, NIH, 10-CRC/6-3742, 9000 Rockville Pike, Bethesda 20892, United States
S- Editor Li DL L- Editor Mihm S E- Editor Zhang WB