Published online Oct 14, 2008. doi: 10.3748/wjg.14.5842
Revised: August 21, 2008
Accepted: August 28, 2008
Published online: October 14, 2008
AIM: To investigate the potential of bone marrow mononuclear cells (BM-MCs) in the regeneration of hepatic lesions induced by Schistosoma mansoni (S.mansoni) chronic infection.
METHODS: Female mice chronically infected with S.mansoni were treated with BM-MCs obtained from male green fluorescent protein (GFP) transgenic mice by intravenous or intralobular injections. Control mice received injections of saline in similar conditions. Enzyme-linked immunosorbent assay (ELISA) assay for transforming growth factor-beta (TGF-β), polymerase chain reaction (PCR) for GFP DNA, immunofluorescence and morphometric studies were performed.
RESULTS: Transplanted GFP+ cells migrated to granuloma areas and reduced the percentage of liver fibrosis. The presence of donor-derived cells was confirmed by Fluorescence in situ hybridization (FISH) analysis for detection of cells bearing Y chromosome and by PCR analysis for detection of GFP DNA. The levels of TGF-β, a cytokine associated with fibrosis deposition, in liver fragments of mice submitted to therapy were reduced. The number of oval cells in liver sections of S.mansoni-infected mice increased 3-4 fold after transplantation. A partial recovery in albumin expression, which is decreased upon infection with S.mansoni, was found in livers of infected mice after cellular therapy.
CONCLUSION: In conclusion, transplanted BMCs migrate to and reduce the damage of chronic fibrotic liver lesions caused by S.mansoni.
-
Citation: Oliveira SA, Souza BSF, Guimarães-Ferreira CA, Barreto ES, Souza SC, Freitas LAR, Ribeiro-dos-Santos R, Soares MBP. Therapy with bone marrow cells reduces liver alterations in mice chronically infected by
Schistosoma mansoni . World J Gastroenterol 2008; 14(38): 5842-5850 - URL: https://www.wjgnet.com/1007-9327/full/v14/i38/5842.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5842
Chronic infection by Schistosoma mansoni (S.mansoni) is one of the experimental models of hepatic fibrosis used to elucidate the mechanisms involved in the fibrogenic processes. In schistosomiasis, the main immune-inflammatory response is directed against the parasite eggs, which, when led to portal circulation, may become lodged into hepatic portal venules, eliciting a granulomatous response. In the mouse model of schistosomiasis, the persistence of the stimulus leads to the development of two pathological patterns; isolated granulomas or periportal fibrosis, the latter resembling the pipe-stem fibrosis found in the severe hepatosplenic form of human disease[1].
Liver fibrosis occurs in the setting of chronic injury caused by different etiologies constituting a serious worldwide public health problem. Whereas in acute hepatic injury nonviable cells are replaced by normal tissue, during chronic injuries a persistent repair response may lead in fibrosis and scar formation as a result of an imbalance between proliferation and degradation of the extracellular matrix components[2]. In addition to schistosomiasis, hepatopathies due to alcohol, viral hepatitis, drugs, metabolic and autoimmune diseases, and congenital abnormalities are important causes of liver fibrosis[2].
New therapeutic strategies aiming to minimize damages caused by hepatic fibrogenesis in chronic liver diseases are of great interest. Adult bone marrow contains pluripotent stem cells with the ability to differentiate into diverse cell types, including hepatocytes[3]. The regenerative potential of bone marrow stem cells has been tested in experimental models of hepatic injury, demonstrating the ability of bone marrow cells (BMCs) to generate hepatocytes under tissue stress in mice and human[4,5]. In this context, regenerative medicine has emerged as an alternative therapy to improve damaged liver function[5-7].
In this report, we used an experimental model of hepatic fibrogenesis caused by chronic infection with S.mansoni in order to evaluate the contribution of cellular therapy in hepatic diseases. We investigated the potential of syngeneic bone marrow mononuclear cells in the modulation of fibrosis, albumin expression and cellular alterations.
Female or male C57Bl/6 wild-type and enhance green fluorescence protein (EGFP) transgenic mice (4-6 wk old) were used as recipients and as donors of BMCs, respectively. All the animals weighting 20-23 g were raised and maintained at the Gonçalo Moniz Research Center/FIOCRUZ in rooms with controlled temperature (22 ± 2°C) and humidity (55% ± 10%) and continuous air renovation. Animals were housed in a 12 h light/12 h dark cycle (6 am-6 pm) and provided with rodent diet and water ad libitum. Animals were handled according to the NIH guidelines for animal experimentation. All procedures described here had prior approval from the local animal ethics committee.
C57Bl/6 mice were infected by transcutaneous route with 30 S.mansoni cercariae of the Feira de Santana strain[8]. This strain was maintained through successive passages in laboratory-raised Biomphalaria glabrata (B.glabrata). Two weeks later the animals were exposed to reinfection with 15 cercariae to increase the hepatic injury. The infection was confirmed 40 d after the primary infection by parasitological exam of feces. Only mice presenting viable eggs in the stools were used. For the retreat of the injury stimulus mice were treated with praziquantel (Farmanguinhos, Fiocruz, Rio de Janeiro, Brazil) by gavage in a single dose of 400 mg/kg per wk 4 mo after the primary infection.
BMCs were obtained from femurs and tibiae of 4-6 wk old C57Bl/6 EGFP transgenic mice. BMC were purified by centrifugation in Ficoll gradient at 1000 g for 15 min (Histopaque 1119 and 1077, 1:1; Sigma, St. Louis, MO). After two washings in RPMI medium (Sigma), the mononuclear cell fraction was suspended in saline, filtered over nylon wool and used for therapy. BMC population was analyzed by flow cytometry using the following conjugated antibodies from Becton Dickinson (San Diego, CA, USA): Sca 1-PE/Cy5, CD45-APC, CD44-PE, CD34-PE, CD11b-PE, and CD117-PE. Acquisition and analysis were performed in a FACScalibur flow cytometer (Becton Dickinson). The following percentages were obtained: 96.51 ± 1.32 for GFP+ cells; 0.11 ± 0.032 for Sca 1+ cells; 96.35 ± 3.12 for CD45+ cells; 92.72 ± 3.23 for CD44+ cells; 0.02 ± 0.05 for CD34+ cells; 60.22 ± 5.71 for CD11b+ cells; and 0.17 ± 0.04 for CD117+ cells. For therapy, one group of mice received one administration of BMC (3 × 107 cells/mouse) directly into the left hepatic lobe (intralobular). In the other experiments, mice were treated with BMC (3 × 107 cells/mouse) intravenous (iv, by retroorbital plexus), once a week, during 3 wk. Control mice received injections of saline in similar conditions to the respective experimental groups. Mice were submitted to euthanasia at different times after therapy, under anesthesia with ketamine and xylazine.
TGF-β levels were measured by enzyme-linked immunosorbent assay (ELISA) in total protein of homogenized liver tissue (50 mg) in 100 μL PBS containing 0.4 mol/L NaCl, 0.05% Tween 20 and protease inhibitors (0.1 mmol/L PMSF, 0.1 mmol/L benzethonium chloride, 10 mmol/L EDTA and 20 KI aprotinin A/100 mL). The samples were centrifuged at 10 000 r/min for 10 min at 4°C and the supernatant was frozen at -80°C for later quantification. TGF-β levels were measured using a sandwich ELISA assay following the manufacturers’ instructions (R&D Systems, Minneapolis, MN).
Mice were perfused under anesthesia through the heart with 50 mL of PBS followed by 200 mL of 16 g/L paraformaldehyde at 4°C. Liver slices were fixed in Bouin or formalin at 10% and, after paraffin embedding, 5 μm-thick sections were obtained and stained with conventional hematoxylin-eosin or with picrosirius-red for collagen[9]. Quantification of fibrosis was carried out in sections stained with picrosirius-red examined by optical microscopy, in 10 fields per liver in 5-14 mice per group. Images were digitalized using a color digital video camera (CoolSnap, Montreal, Canada) adapted to a BX41 microscope (Olympus, Tokyo, Japan) and analyzed using Image Pro program (version 6.1; Media Cybernetics, San Diego, CA). For morphometric measurements of granuloma area, a total sectional area of 723 255 μm2 per animal was evaluated. All periocular granulomas were included. Albumin granules and OV-6+ cells were quantified in five liver sections of seven or five animals per group (normal, treated and untreated), respectively, by fluorescence microscopy (BX61 Olympus) and analyzed using Image Pro program. Albumin granules were automatically quantified and the number of hepatocytes was estimated manually by nuclear staining in order to determine the average number of albumin granules/cell. All the analyses were done double-blinded.
Five micrometer frozen sections obtained at various times after transplantation were prepared and fixed in 16 g/L cold paraformaldehyde in 0.1 mol/L phosphate buffer. The presence of transplanted GFP+ BMC in the liver tissue was analyzed by direct fluorescence. Oval cells were stained using a biotinylated anti-OV-6 antibody (R&D Systems, Minneapolis, MN) followed by streptavidin Alexa 568 (Molecular Probes, Carlsbad, CA). For albumin visualization and quantification, liver sections were stained using a rabbit anti-human albumin (DAKO, Glostrup, Denmark) followed by anti-rabbit IgG conjugated with Alexa fluor 568 (Molecular Probes). For collagen I quantification, sections were stained with rabbit anti-human collagen (Novatec, Saint Martin La Garenne, France) followed by anti-rabbit IgG conjugated with Alexafluor 568 (Molecular Probes). Nuclei were stained with 4,6-diamidino-2-phenylindole (VectaShield Hard Set mounting medium with DAPI H-1500; Vector Laboratories, Burlingame, CA). The presence of fluorescent cells was determined by observation in a BX61 microscope with epifluorescence system plus grid to enhance the fluorescence resolution (Optigrid, Structured-light Imaging System, Thales Optem inc, Fairport, NY) using appropriate filters (Olympus). Images were captured using a color digital video SPOT flex camera (15.2, 64 Mp, Shifting Pixel, Diagnostic Instruments inc, Sterling Heights, MI).
Liver sections 3-6 μm thick were prepared on glass slides and dried overnight at 37°C. After dewaxing in xylene and rehydration, sections were incubated with sodium thiocyanate solution for 10 min at 80°C, washed in PBS followed by incubation with pepsin solution for 10 min at 37°C. Sections were washed in PBS, post-fixed in paraformaldehyde solution for 2 min, washed in PBS, and dehydrated through graded alcohols before air drying. Detection of Y chromosome was done using Y-paint probe kit (Cambio, Cambridge, UK), according to the manufacturers’ instructions. Slides were mounted with coverslips using Vectashield mounting set with DAPI (Vector), and analyzed by fluorescence microscopy.
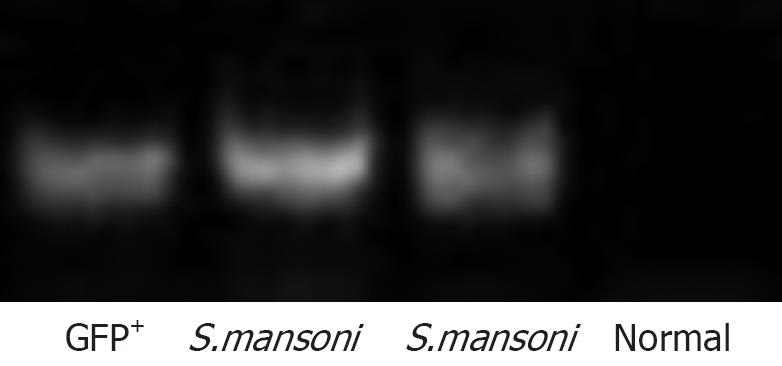
The presence of donor-derived DNA in liver tissue was analyzed 2 mo after BMC transplantation in S.mansoni-infected mice. DNA was extracted from liver tissue using a DNA extraction kit (QIAamp® DNA Mini Kit-50, Qiagen, Hilden, Germany) according to the manufacturers’ instructions. DNA concentrations were quantified in 1 μL in a spectrophotometer ND-1000 (Nanodrop Technologies, Wilmington, DE). All samples were diluted to 100-200 ng/μL. PCR amplification of GFP cDNA was performed using Taq polymerase (Invitrogen, Carlsbad, CA) using standard procedures and the following primers pair (Invitrogen): forward, 5′-CGTCGCCGTCCAGCTCGACCAG-3′, reverse, 5′-CATGGTCCTGCTGGAGTTCGTG-3′.
Data were analyzed using Student’s t-test, Mann Whitney, ANOVA and Newman-Keuls multiple comparison test with the aid of Prism Software (version 3.0, GraphPad Software, San Diego, CA). Differences were considered significant if P≤ 0.05.
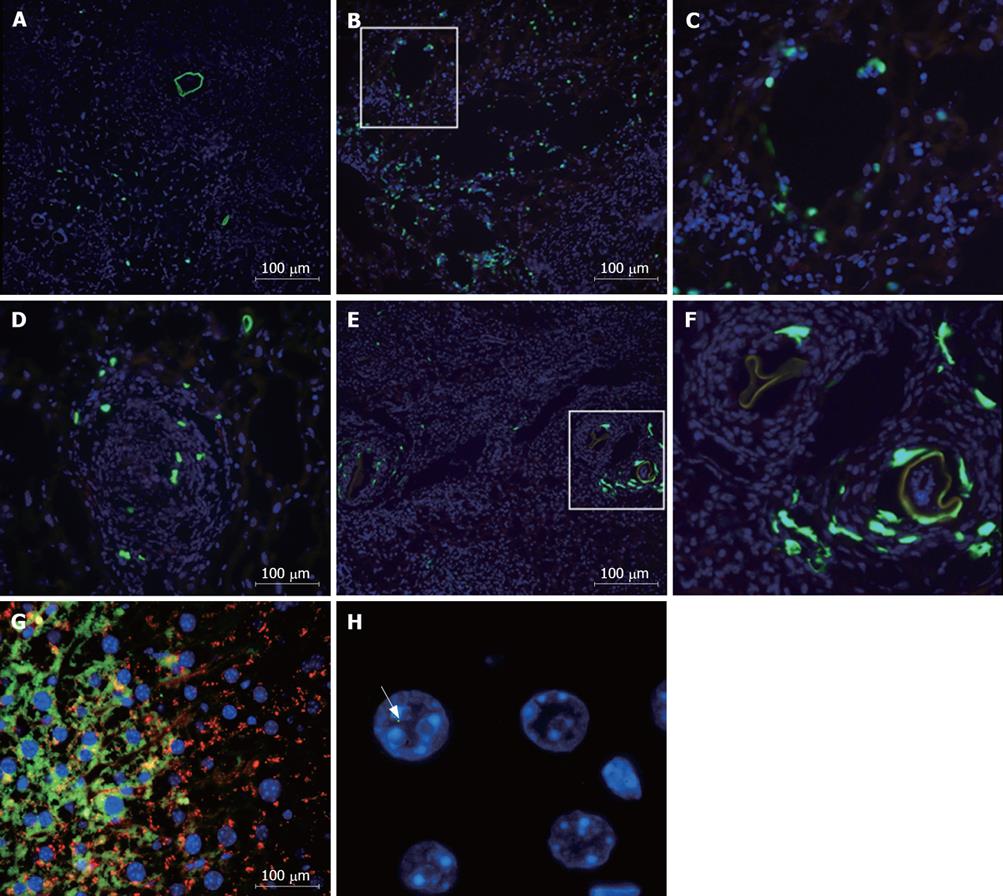
Mice chronically infected with S.mansoni were treated with GFP+ mononuclear BMC by injection into the left hepatic lobe. Liver sections were obtained at different times after transplantation. Bright GFP+ cells were found around periocular granulomas, in the injected lobe, 2 h after cell infusion (Figure 1A to C). After 24 h of injection, GFP+ cells were also found inside granulomas in sections of injected (Figure 1D) as well as in non-injected lobes (not shown). Five days after transplantation, elongated, spindle shape GFP+ cells were mainly found inside granuloma areas (Figure 1E and F). Bright GFP+ cells were not found in sections of BMC-treated mice 1 or 2 mo after transplantation by iv route, although faint fluorescent cells co-expressing albumin were found in the hepatic parenchyma at this later timepoint (Figure 1G). The presence of donor-derived hepatocyte-like cells was confirmed by detection of Y-chromosome by FISH analysis in parenchymatous cells in liver sections of BMC-treated mice (Figure 1H). Furthermore, the presence of GFP DNA was detected by PCR in liver samples of BMC-treated mice 2 mo after therapy (Figure 2).
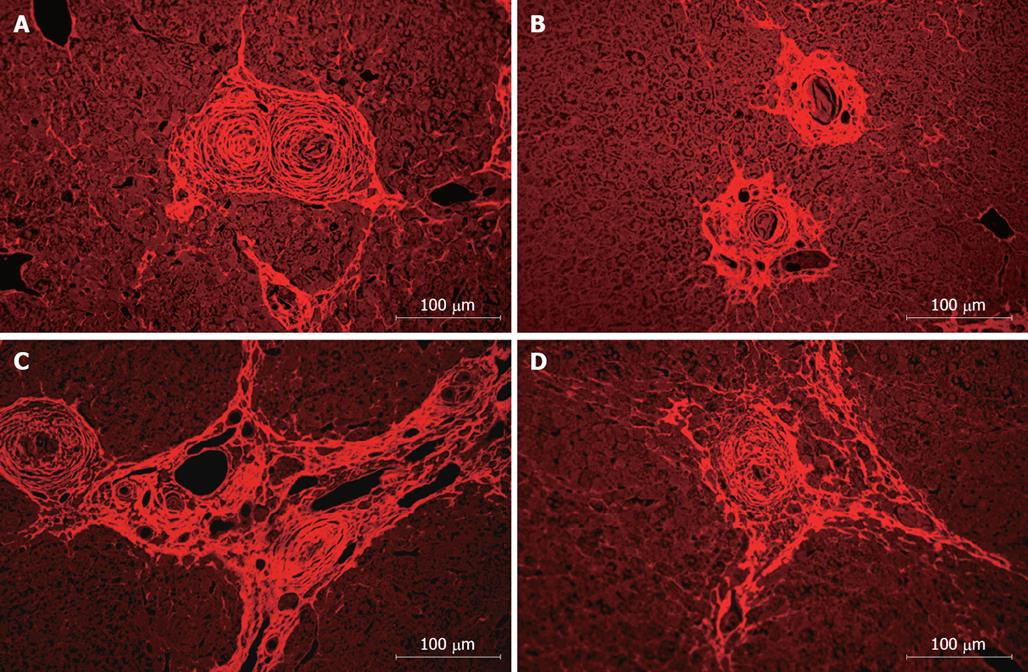
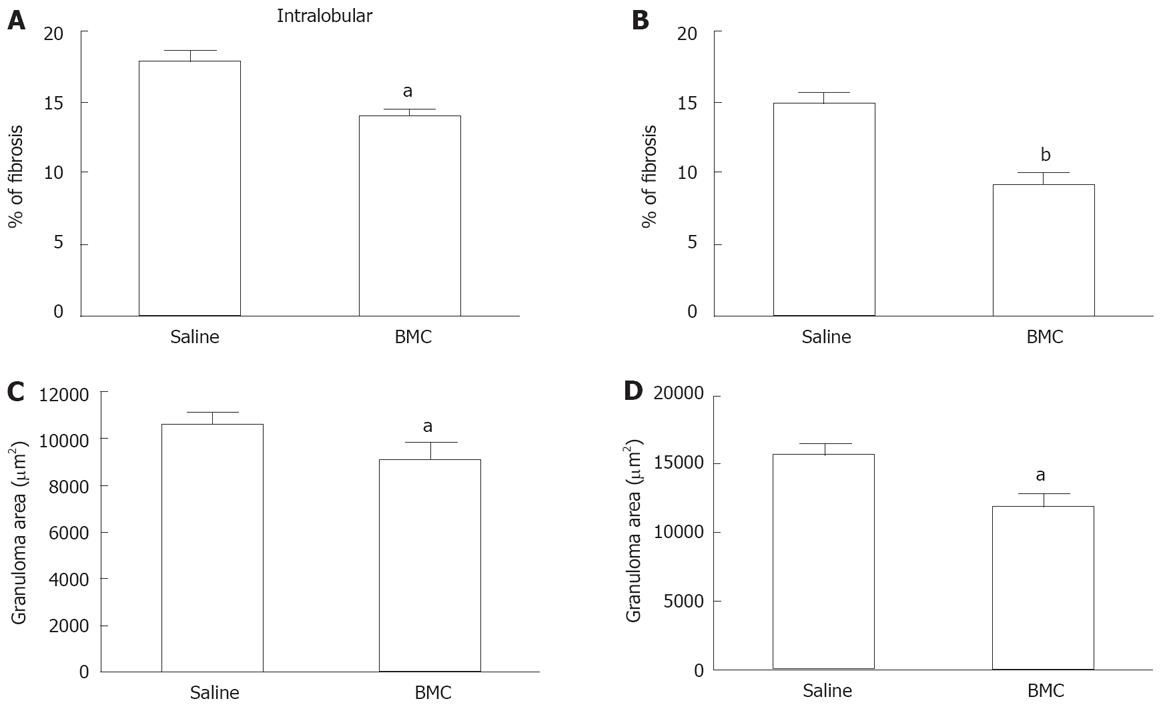
Liver sections of S.mansoni-infected mice were analyzed, showing a granulomatous inflammatory process in all groups, with periocular granulomas well delimited and homogeneous in shape and cellular infiltrate with predominance of mononuclear cells and with regular and concentric deposits of collagen fibers (Figure 3A to C). After treatment with BMC, we observed a reduction of fibrosis (Figure 3B and D), compared to saline-treated animals. In animals treated by intralobular injection of BMC, a statistically significant reduction of fibrosis was observed in treated lobes 2 mo after treatment (Figure 4A). In addition, a reduction of fibrosis was also found in sections of untreated lobes (% of fibrosis in untreated lobes: 14.1 ± 0.7 compared to livers of saline-treated mice: 17.9 ± 0.7; P < 0.001). Fibrosis reduction was also observed when BMC were injected by iv route (Figure 4B). Morphometric evaluation of granuloma area showed a significant reduction after therapy in animals treated with BMC by intralobular (Figure 4C) as well as by iv injection (Figure 4D). This reduction was confirmed in sections stained with ant-collagen I antibodies, by morphometry (saline-treated: 7.14% ± 0.45% compared to BMC-treated: 4.20% ± 0.25%; P < 0.0004).
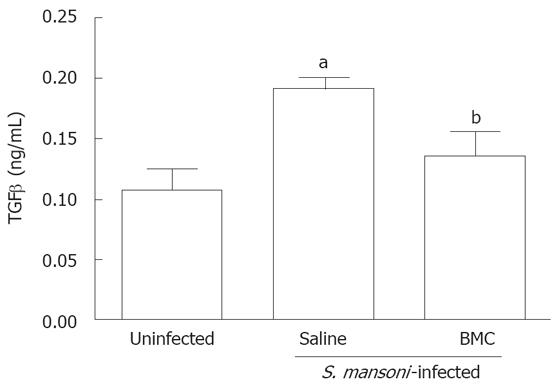
TGF-β levels in liver fragments were assessed 2 mo after BMC therapy by iv route. A significant decrease of TGF-β levels was observed in livers of BMC-treated mice compared to saline-treated S.mansoni-infected mice. The levels of TGF-β in BMC-treated mice were similar to those of normal mice (Figure 5).
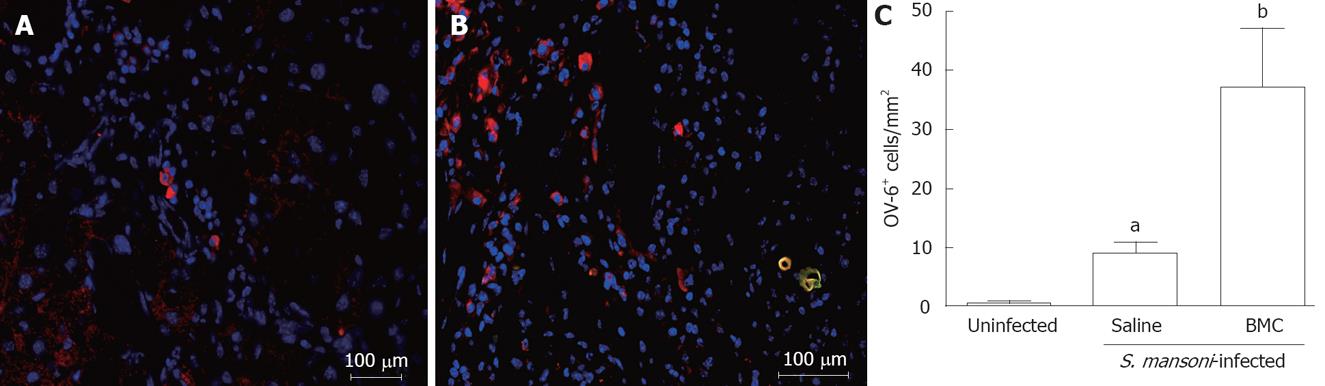
The presence of oval cells in liver sections of normal, S.mansoni-infected mice treated or not with BMC was evaluated by immunofluorescence using an anti-OV-6 antibody. Sections of normal livers had none or few OV-6+ cells (Figure 6A). Infection with S.mansoni caused an increase in the number of OV-6+ cells, which were mainly found in zone 1 areas of the hepatic lobe in saline-treated (Figure 6B), as well as in BMC-treated animals (Figure 4B) 2 mo after iv route. The number of OV-6+ cells, however, was 3-4 times higher in sections of BMC-treated mice (Figure 6C; P = 0.0027).
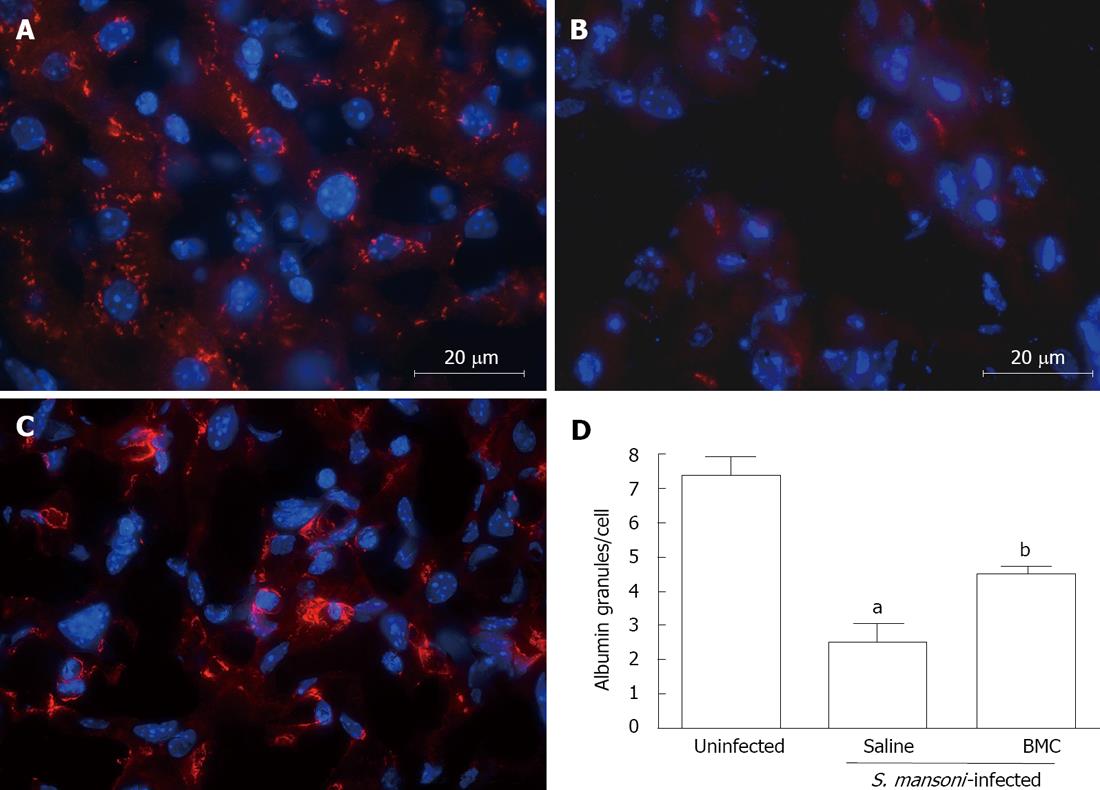
Immunostaining for albumin in liver sections of mice chronically infected with S.mansoni showed an abnormal pattern of albumin expression. A bright intracytoplasmic granular staining is found in sections of uninfected mice (Figure 7A), whereas liver sections of untreated S.mansoni-infected mice had few and abnormal albumin granules, preferentially located in the periphery of hepatocytes (Figure 7B). Liver sections of BMC-treated mice also had an altered albumin staining pattern, although more intense than untreated mice (Figure 7C). When the number of albumin+ granules was evaluated, a significant difference between uninfected and infected mice was found, showing a 3-fold higher number of granules in normal mice compared to saline-treated infected mice (Figure 7D; P < 0.001). BMC therapy caused a significant increase in the number of granules, compared to saline-treated, S.mansoni-infected mice (P < 0.05), although the levels where still below those of normal livers (Figure 7D; P < 0.001).
Cellular therapy for liver regeneration has been intensively investigated mainly in models of hepatic diseases caused by drug administration, surgical interventions and by genetic disorders[10-13]. A number of reports have demonstrated that transplantation of BMCs improves liver function, decreases fibrosis and contributes to parenchyma regeneration[11,14,15]. In this report, we describe for the first time the effects of BMC therapy in a model of chronic liver disease caused by a parasitic infection.
The recruitment of BMCs to liver lesions has been well documented in several models of liver diseases[10-12,14]. In our study, we also found that liver injury caused by S.mansoni infection elicits the migration of BMCs, which were able to reach the granuloma areas. Many of the cells observed in the liver sections are probably leukocytes (found in the mononuclear cell fraction used in the cellular therapy), that are chemoattracted by the intense inflammatory reaction around periocular granulomas. Most GFP+ cells that migrated inside the granulomas, however, are larger than leukocytes and could be spindle-shaped like fibroblasts or myofibroblasts. Both of these cells are well known to participate in the fibrogenesis associated with S.mansoni infection and also to take part in collagen degradation during fibrosis modulation[16-18]. The differentiation of BMCs into myofibroblasts and/or fibroblasts may be an important step in the modulation of fibrosis observed in the present study. Recently, it has been shown that a significant number of myofibroblasts in human hepatic fibrosis are of bone marrow origin[19].
In latter time points, cells expressing weak GFP fluorescence co-expressing albumin were observed. The presence of transplanted BMC-derived parenchymatous cells was confirmed by observation of donor DNA by PCR analysis (for detection of GFP DNA) and FISH (for detection of Y chromosomes in nuclei). These results reinforce a contribution of BMCs in the reconstitution of liver parenchyma, as described before in other models of liver diseases[20,21]. In our study, we found few hepatocyte-like cells bearing Y chromosome (1-2 cells/section). In addition, we did not find binucleated cells with Y chromosome in one nucleus. Although we cannot rule out that fusion of transplanted BMC with resident hepatocytes occurs, these findings indicate that the few hepatocyte-like cells derived from transplanted BMC observed in our model are generated by transdifferentiation. In addition to the population of stem cells, the potential of transdifferentiation into hepatocytes has been recently extended to another cell type present in the bone marrow, the monocytes[22]. Thus, it is possible that more than one cell type present in the cell preparation used in our study take part in the generation of hepatocyte-like cells. It is also likely that the transplanted cells take part in the immune regulation of the liver[23], causing the modulation of fibrosis, cytokine production and albumin synthesis observed in our study.
Fibrosis is a common feature of chronic liver diseases. The increased deposition of extracellular matrix causes structural alterations in the liver and in its function, and portal hypertension due to the obstruction of vessels and focal ischemic lesions. Although spontaneous regression of fibrosis occurs when the stimulus for hepatic liver damage is removed, in diseases such as viral hepatitis the stimulus cannot be completely removed, in addition to being a slow process[24]. Therefore, means to decrease liver fibrosis are extremely relevant to improve liver function and to decrease complications related to chronic hepatopathies. In this regard, schistosomiasis is an interesting model of chronic fibrotic liver disease. In our study, we observed a significant decrease of liver fibrosis after cellular therapy, as demonstrated by morphometrical analysis of granuloma area and total liver fibrosis. This was achieved both by intralobular administration as well as by iv injection, indicating that fibrosis does not hamper the influx of cells to the liver of S.mansoni-infected mice. In a recent report, Higashiyama et al (2007), demonstrated that bone marrow-derived cells which migrated to fibrotic livers express matrix metalloproteinases. Thus, the transplanted cells found in the hepatic parenchyma of S.mansoni-infected mice may be acting directly to increase the degradation of extracellular matrix components.
One of the main mediators involved on fibrosis deposition during hepatic injury is TGF-β[25]. This cytokine stimulates the transition of stellate cells to myofibroblasts, which secrete high amounts of extracellular matrix and inhibit its degradation[26]. TGF-β levels were lowered after BMC therapy in livers of S.mansoni-infected mice, reaching levels close to those found in normal mice. A decrease in TGF-β production was also observed by Fang et al[14] after therapy using bone marrow-derived mesenchymal stem cells in mice with liver injury caused by CCl4 administration.
Oval cells are hepatic precursors of hepatocytes and bile duct cells[27]. An increase in oval cell numbers was found in liver sections of S.mansoni-infected mice, compared to those of normal mice. Transplantation of BMC, however, caused a 3-4 fold increase in oval cell numbers in infected mice. This may be explained by the fact that oval cells can be originated from BMCs[4]. Alternatively, the effects of BMC injection on oval cell numbers may be due to their migration to and action in other organs, such as the bone marrow. It is possible that the increase in oval cells contribute to the replacement of areas where fibrosis degradation occurs with functional parenchymatous cells after BMC therapy.
Although alterations in albumin levels in S.mansoni-infected individuals are rare, this can be observed in some cases. A study by Cook et al[28] reported hypoalbuminemia in S.mansoni-infected children, which suggests that this biochemical alterations associated with undernourishment or to effects of repeated digestive hemorrhages. In contrast, chronic infection with S.mansoni causes hypoalbuminemia in mice. This phenomenon occurs simultaneously to the increase in collagen deposition and could be associated with the decrease in albumin mRNA found in S.mansoni-infected mice[29]. In our study, we also observed a marked alteration of albumin expression in hepatocytes of infected mice, compared to normal controls. BMC therapy significantly increased the expression of albumin in livers of S.mansoni-infected mice, although the pattern was still altered in comparison to that of normal livers. An increase in albumin levels was also found after cell therapy in a model of CCl4-induced lesion in rats[30].
In conclusion, transplantation of BMCs in mice with chronic liver disease caused by S.mansoni infection decreased liver fibrosis and contributed to an increase in precursor cells as well as to the generation of new hepatocytes and/or to the improvement of the function of resident hepatocytes. Although there are still many unanswered questions regarding the mechanisms of action of transplanted cells in hepatic lesions, our results reinforce the use of cell-based therapies for patients with chronic liver diseases.
Liver fibrosis occurs in the setting of chronic injury caused by different etiologies, constituting a serious worldwide public health problem. Chronic infection by S.mansoni is one of the experimental models of hepatic fibrosis used to elucidate the mechanisms involved in the fibrogenic processes.
New therapeutic strategies aimed at minimizing damage caused by hepatic fibrogenesis in chronic liver diseases are of great interest. Adult bone marrow contains pluripotent stem cells with the ability to differentiate into diverse cell types, including hepatocytes, in situations under tissue stress in mice and human.
A number of reports have demonstrated that transplantation of bone marrow cells (BMCs) improves liver function, decreases fibrosis and contributes to parenchyma regeneration. In this report, described for the first time, are the effects of BMC therapy in a model of chronic liver disease caused by a parasitic infection.
Fibrosis is a common feature of chronic liver diseases. The increased deposition of extracellular matrix causes structural alterations in the liver and causes portal hypertension due to the obstruction of vessels and focal ischemic lesions. Therefore, means to decrease liver fibrosis are extremely relevant to improve liver function and to decrease complications related to chronic hepatopathies. In this study, the authors observed a significant decrease in liver fibrosis, an increase in precursor cells, and the generation of new hepatocytes after therapy with BMCs.
Authors investigated the putative role in hepatic regenerative medicine by bone marrow mononuclear cells (BM-MC) in the experimental model of S.mansoni chronic infection. The experimental animal model used is appropriate and the study is in principle of interest.
Peer reviewers: Natalia A Osna, Liver Study Unit, Research Service (151), VA Medical Center, 4101 Woolworth Avenue, Omaha NE 68105, United States; Maurizio Parola, Professor, Department of Dip. Medicina e Oncologia Sperimentale, Universita degli Studi di torino, Corso Raffaello 30, Torino 10125, Italy
S- Editor Li DL L- Editor Rippe RA E- Editor Yin DH
| 1. | Andrade ZA, Cheever AW. Characterization of the murine model of schistosomal hepatic periportal fibrosis ('pipestem' fibrosis). Int J Exp Pathol. 1993;74:195-202. |
| 2. | Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209-218. |
| 3. | Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369-377. |
| 4. | Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168-1170. |
| 5. | Alison MR, Poulsom R, Jeffery R, Dhillon AP, Quaglia A, Jacob J, Novelli M, Prentice G, Williamson J, Wright NA. Hepatocytes from non-hepatic adult stem cells. Nature. 2000;406:257. |
| 6. | Braun KM, Sandgren EP. Cellular origin of regenerating parenchyma in a mouse model of severe hepatic injury. Am J Pathol. 2000;157:561-569. |
| 7. | Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman IL, Grompe M. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229-1234. |
| 8. | Andrade ZA, Sadigursky M. [A comparative study of the Feira de Santana (Bahia) and Porto Rico strains of Schistosoma mansoni in experimental infection of mice]. Mem Inst Oswaldo Cruz. 1985;80:37-40. |
| 9. | Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11:447-455. |
| 10. | Jang YY, Collector MI, Baylin SB, Diehl AM, Sharkis SJ. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol. 2004;6:532-539. |
| 11. | Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, Okita K. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304-1311. |
| 12. | Fujii H, Hirose T, Oe S, Yasuchika K, Azuma H, Fujikawa T, Nagao M, Yamaoka Y. Contribution of bone marrow cells to liver regeneration after partial hepatectomy in mice. J Hepatol. 2002;36:653-659. |
| 13. | Kallis YN, Alison MR, Forbes SJ. Bone marrow stem cells and liver disease. Gut. 2007;56:716-724. |
| 14. | Fang B, Shi M, Liao L, Yang S, Liu Y, Zhao RC. Systemic infusion of FLK1(+) mesenchymal stem cells ameliorate carbon tetrachloride-induced liver fibrosis in mice. Transplantation. 2004;78:83-88. |
| 15. | Zhao DC, Lei JX, Chen R, Yu WH, Zhang XM, Li SN, Xiang P. Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World J Gastroenterol. 2005;11:3431-3440. |
| 16. | de Freitas LA, Chevallier M, Louis D, Grimaud JA. Human extrahepatic biliary atresia: portal connective tissue activation related to ductular proliferation. Liver. 1986;6:253-261. |
| 17. | Grimaud JA, Borojevic R. Portal fibrosis: intrahepatic portal vein pathology in chronic human schistosomiasis mansoni. J Submicrosc Cytol. 1986;18:783-793. |
| 18. | de Freitas LA, Grimaud JA, Chevallier M, Andrade ZA. Morphological aspects of early and late collagen degradation in granulation tissue. Exp Toxicol Pathol. 1992;44:128-133. |
| 19. | Forbes SJ, Russo FP, Rey V, Burra P, Rugge M, Wright NA, Alison MR. A significant proportion of myofibroblasts are of bone marrow origin in human liver fibrosis. Gastroenterology. 2004;126:955-963. |
| 20. | Kashofer K, Siapati EK, Bonnet D. In vivo formation of unstable heterokaryons after liver damage and hematopoietic stem cell/progenitor transplantation. Stem Cells. 2006;24:1104-1112. |
| 21. | Higashiyama R, Inagaki Y, Hong YY, Kushida M, Nakao S, Niioka M, Watanabe T, Okano H, Matsuzaki Y, Shiota G. Bone marrow-derived cells express matrix metalloproteinases and contribute to regression of liver fibrosis in mice. Hepatology. 2007;45:213-222. |
| 22. | Yan L, Han Y, Wang J, Liu J, Hong L, Fan D. Peripheral blood monocytes from patients with HBV related decompensated liver cirrhosis can differentiate into functional hepatocytes. Am J Hematol. 2007;82:949-954. |
| 23. | Chen X, Armstrong MA, Li G. Mesenchymal stem cells in immunoregulation. Immunol Cell Biol. 2006;84:413-421. |
| 24. | Kumar M, Sarin SK. Is cirrhosis of the liver reversible? Indian J Pediatr. 2007;74:393-399. |
| 25. | Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fibrosis. Front Biosci. 2002;7:d793-d807. |
| 26. | Gressner AM. Transdifferentiation of hepatic stellate cells (Ito cells) to myofibroblasts: a key event in hepatic fibrogenesis. Kidney Int Suppl. 1996;54:S39-S45. |
| 27. | Fausto N, Campbell JS. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev. 2003;120:117-130. |
| 28. | Cook JA, Baker ST, Warren KS, Jordan P. A controlled study of morbidity of schistosomiasis mansoni in St. Lucian children, based on quantitative egg excretion. Am J Trop Med Hyg. 1974;23:625-633. |
| 29. | Saber MA, Shafritz DA, Zern MA. Changes in collagen and albumin mRNA in liver tissue of mice infected with Schistosoma mansoni as determined by in situ hybridization. J Cell Biol. 1983;97:986-992. |