Published online Oct 14, 2008. doi: 10.3748/wjg.14.5823
Revised: August 20, 2008
Accepted: August 27, 2008
Published online: October 14, 2008
AIM: To examine the expression of pancreatic duodenal homeobox-1 (PDX-1) transcription factor in human colorectal cancer.
METHODS: RT-PCR, Western blotting, and immuno-histochemistry were performed to determine the expression pattern of transcription factor PDX-1 in primary colorectal tumor, hepatic metastasis, and benign colon tissue from a single patient.
RESULTS: The highest PDX-1 transcription levels were detected in the metastasis material. Lower levels of PDX-1 were found to be present in the primary tumor, while normal colon tissue failed to express detectable levels of PDX-1. Western blot data revealed a PDX-1 expression pattern identical to that of mRNA expression. Immunohistochemistry confirmed high metastasis PDX-1 expression, lower levels in the primary tumor, and the presence of only traces of PDX-1 in normal colon tissue.
CONCLUSION: These data argue for further evaluation of PDX-1 as a biomarker for colorectal cancer.
- Citation: Ballian N, Liu SH, Brunicardi FC. Transcription factor PDX-1 in human colorectal adenocarcinoma: A potential tumor marker? World J Gastroenterol 2008; 14(38): 5823-5826
- URL: https://www.wjgnet.com/1007-9327/full/v14/i38/5823.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5823
In 2008, colorectal cancer is projected to be the third leading cause of cancer-related mortality in USA with an estimated 148 000 new cases and 50 000 deaths[1]. At least 40% of patients with colorectal cancer will experience metastases at some time during their illness[2]. Early detection of disease can improve prognosis, and survival varies significantly between patients with early-stage tumors and patients with metastases[3,4]. A number of studies have shown decreased mortality in populations undergoing colorectal cancer screening[5-8]. There is an increasing demand for colon cancer tumor markers for risk assessment and early diagnosis[9,10].
Pancreatic duodenal homeobox 1 (PDX-1) is a transcription factor with a critical role in pancreatic development[11]. PDX-1 regulates pancreatic cell proliferation and differentiation, and increased expression of this transcription factor has been described in human pancreatic adenocarcinoma and cell lines[12,13]. We recently found increased PDX-1 expression in benign tissues and malignant tumors from patients with pancreas, breast, colon, prostate, and renal cancers[14]. This indicates a possible role of PDX-1 as a tumor marker in patients with these malignancies.
In this report, levels of PDX-1 expression were quantified in a primary colorectal tumor, a metastasis, and in benign tissue from a single patient. Of particular interest were the expression pattern of PDX-1 and its potential use as a tumor marker in colorectal cancer.
A 46-year-old male patient presented with a right-sided colorectal adenocarcinoma metastatic to the peritoneum and greater omentum. The patient underwent chemotherapy with capecitabine and oxaliplatin from August 2003 to February 2004 and right hemicolectomy in June 2004 for tumor-related bowel obstruction. Samples from the primary cecal tumor, omental metastases, and macroscopically normal colon mucosa from the distal end of the right hemicolectomy specimen (transverse colon) were collected during surgery (n = 16).
Tissues were fixed in 4% paraformaldehyde overnight at 4°C. Tissue processing, section preparation, and H&E staining were performed as described previously[14].
Western blotting was performed as described previously[15].
All samples were snap-frozen in liquid nitrogen. RNA was prepared according to procedures described in TRIzol Reagent manual (Cat. NO. 15596-026/-018). RT reactions were carried out according to the protocol of SuperScript III First-Strand Synthesis System (Invitrogen Cat. No. 18080-051). PCR products were loaded on a 1.5% agarose gel and visualized and quantified by ethidium bromide staining using an UVP imaging system (UVP, Upland, CA).
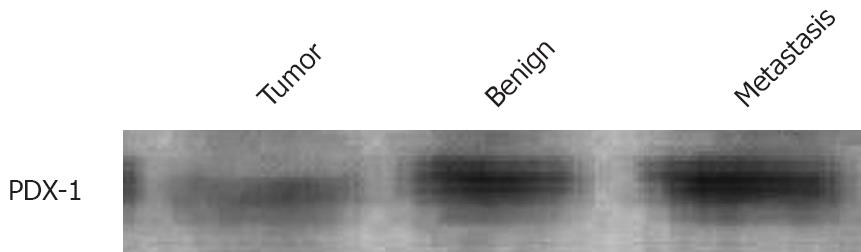
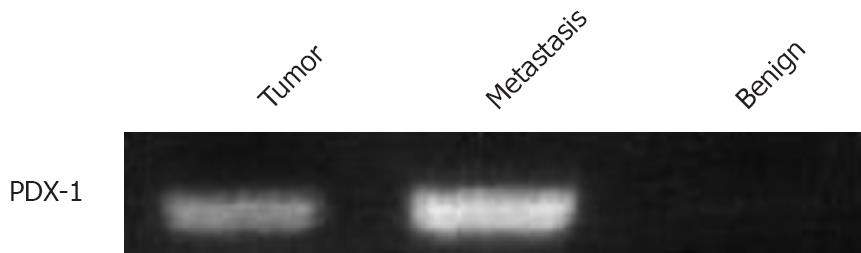
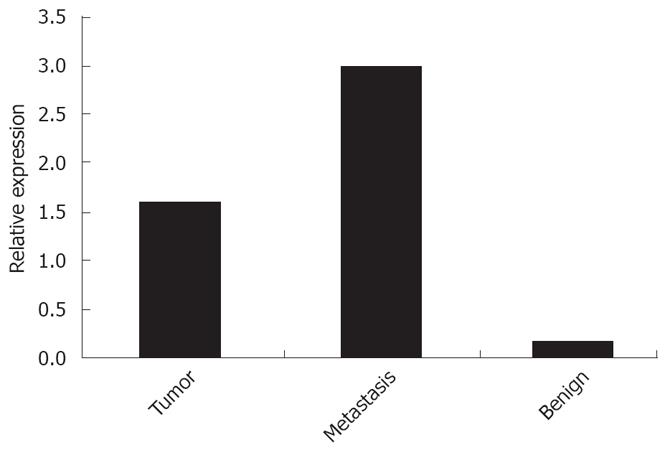
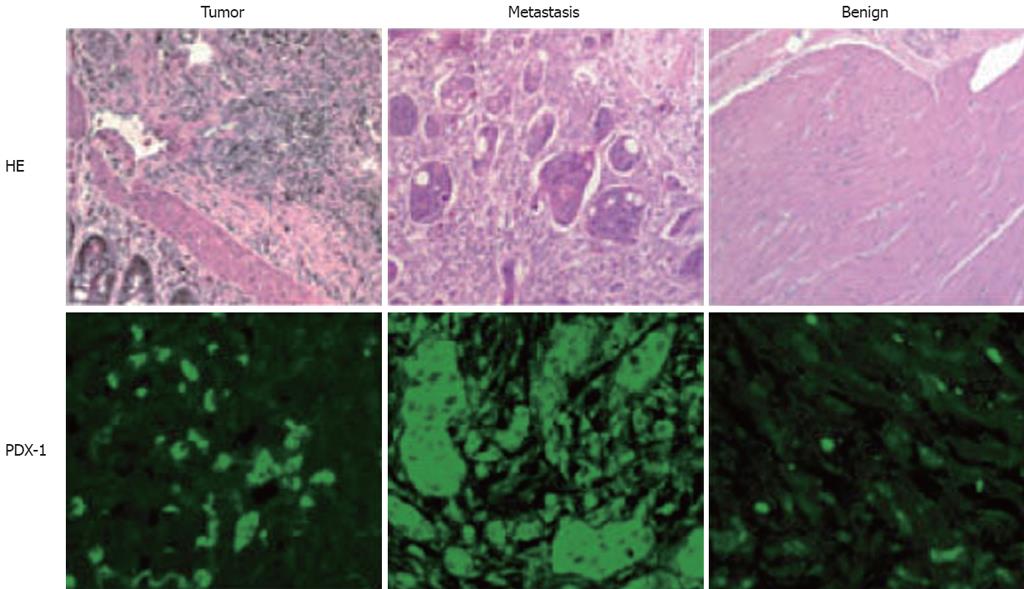
As shown in Figure 1 (Western blot), high PDX-1 protein levels were found in the metastasis and the benign colon mucosa distant from the tumor. RT-PCR results in Figures 2 and 3 show that the highest PDX-1 mRNA levels were detected in the metastasis. Significant but lower levels were present in the primary tumor, while normal colon tissue had close to undetectable levels of PDX-1 mRNA. Immunohistochemistry (Figure 4) confirmed the high PDX-1 expression in the metastasis, the lower levels in the primary tumor, and the traces of PDX-1 in normal tissue.
PDX-1 regulates pancreatic cell proliferation and differentiation[16-19]. Its aberrant expression in a number of human malignant tumors suggests a potential as a molecular marker. Among these malignancies, colorectal cancer is a common and frequently fatal disease for which screening programs are already being used in the United States[20]. Colonoscopy, the most accurate screening method, can cause significant patient discomfort and has risks of perforation and bleeding[21]. Perhaps for these reasons, participation in colorectal cancer screening programs is low[22]. Hence, safer and more acceptable screening methods are needed.
An important finding of this study is that malignant tissue was found to have significantly higher PDX-1 expression than normal colon mucosa outside the tumor. We have previously shown PDX-1 expression in 10 colon cancer specimens to be significantly elevated in both the nucleus and cytoplasm of malignant cells, compared to lower levels found in benign tissues[14]. In that study, six samples of colon tissue from colon cancer patients taken from sites outside the primary tumor were examined and found to express increased PDX-1 levels, although lower than that of tumors. In contrast, our current study showed colon mucosa distant from the primary tumor had nearly undetectable PDX-1 expression. These differences could be due to variations in the benign tissue sample distance from the primary tumor. Unfortunately, this was not recorded in our previous study and hence an accurate comparison is not possible.
Another significant observation is that, despite high levels of mRNA, PDX-1 protein levels are low in the primary tumor. This is consistent with posttranscriptional control of PDX-1 expression, which has been shown to occur in the pancreas[18]. In contrast, metastatic tissues retain high levels of both mRNA and protein expression. Although Western blotting showed lower levels of protein in the primary tumor compared to benign mucosa, immunohistochemistry did confirm high expression of PDX-1 protein in tumor cells. Hence, PDX-1 protein is present in tumor cells and normal colonocytes, which are excreted in feces. This is a prerequisite for fecal screening for colorectal cancer. Interestingly, others have shown an upregulation of PDX-1 expression in colonic hyperplastic polyps and adenomas, the latter being precursors of adenocarcinomas[23].
Although the etiology of aberrant PDX-1 expression by colorectal cancer cells is unknown, a clue for this comes from its association with caudal homeobox 2 (Cdx-2). The genes for PDX-1 and Cdx-2 are closely linked on chromosome 5[24]. Cdx-2 is expressed in colon epithelium during embryonic development and has a central role in the differentiation of midgut endoderm[24]. It encodes for a transcription factor that is expressed in the proximal colon[25]. Cdx-2 expression is significantly reduced in colorectal adenocarcinoma proximal to the splenic flexure[26] and during the later stages of colorectal carcinogenesis[27]. In vitro studies show that PDX-1 physically interacts with and inhibits transcriptional activation by Cdx-2[28].
In this report, we have found expression of PDX-1 in a colorectal adenocarcinoma, its metastases, and macroscopically normal colonic mucosa from the same patient. This indicates a potential for use of this transcription factor as a molecular marker for colorectal cancer. Levels of PDX-1 in primary tumors and metastases from a large number of patients with and without colorectal cancer would need to be measured to confirm these observations. In addition, the levels of PDX-1 in stool samples from these patients need to be determined. This case report is an initial observation that PDX-1 expression could be indicative for colorectal carcinoma. A prospective study would be required to further evaluate its impact.
Colon cancer is a major cause of cancer-related morbidity and mortality and new diagnostic markers could improve the results of screening. Pancreatic duodenal homeobox-1 (PDX-1) is a transcription factor that regulates differentiation and proliferation. Increased PDX-1 levels have been found in colorectal adenocarcinoma compared to normal colon mucosa from a single patient.
New molecular markers that could improve the accuracy of colorectal cancer screening are being sought. Development of molecular markers aims at developing non-invasive screening methods for colorectal cancer.
Detection of colorectal cancer-specific mutations in stool has been examined but is laborious and expensive. New molecular markers that will improve the efficiency and accuracy of non-invasive screening are needed.
Demonstrating overexpression of PDX-1 in the colon of patients with colorectal cancer is the first step in evaluating this molecule as a marker for colorectal cancer. If this observation is confirmed in a large sample of colorectal cancer patients, PDX-1 could prove valuable as a colorectal cancer marker.
PDX-1 is a transcription factor essential for normal pancreatic organogenesis. Aberrant PDX-1 expression by a number of malignant tumors has been described.
This study is very interesting. It suggests the possibility of PDX-1 as a biomarker for early diagnosis of colorectal cancer, so further study is needed to evaluate this potential.
Peer reviewer: Yutaka Saito, Division of Endoscopy, National Cancer Center Hospital, 5-1-1, Tsukiji, Chuo-ku, Tokyo 104-0045, Japan
S- Editor Li DL L- Editor Mihm S E- Editor Zhang WB
| 1. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96. |
| 2. | Labianca R, Pessi MA, Zamparelli G. Treatment of colorectal cancer. Current guidelines and future prospects for drug therapy. Drugs. 1997;53:593-607. |
| 3. | Meyerhardt JA, Mayer RJ. Systemic therapy for colorectal cancer. N Engl J Med. 2005;352:476-487. |
| 4. | National Center for Health Statistics, Division of Vital Statistics, Centers for disease control. Available from: URL: http://www.cdc.gov. |
| 5. | Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, Ederer F. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365-1371. |
| 6. | Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467-1471. |
| 7. | Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, James PD, Mangham CM. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472-1477. |
| 8. | Mandel JS, Church TR, Bond JH, Ederer F, Geisser MS, Mongin SJ, Snover DC, Schuman LM. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med. 2000;343:1603-1607. |
| 9. | Srivastava S, Verma M, Henson DE. Biomarkers for early detection of colon cancer. Clin Cancer Res. 2001;7:1118-1126. |
| 10. | Mandel JS. Screening for colorectal cancer. Gastroenterol Clin North Am. 2008;37:97-115, vii. |
| 12. | Koizumi M, Doi R, Toyoda E, Masui T, Tulachan SS, Kawaguchi Y, Fujimoto K, Gittes GK, Imamura M. Increased PDX-1 expression is associated with outcome in patients with pancreatic cancer. Surgery. 2003;134:260-266. |
| 13. | Liu S, Ballian N, Belaguli NS, Patel S, Li M, Templeton NS, Gingras MC, Gibbs R, Fisher W, Brunicardi FC. PDX-1 acts as a potential molecular target for treatment of human pancreatic cancer. Pancreas. 2008;37:210-220. |
| 14. | Wang XP, Li ZJ, Magnusson J, Brunicardi FC. Tissue MicroArray analyses of pancreatic duodenal homeobox-1 in human cancers. World J Surg. 2005;29:334-338. |
| 15. | Tirone TA, Wang XP, Templeton NS, Lee T, Nguyen L, Fisher W, Brunicardi FC. Cell-specific cytotoxicity of human pancreatic adenocarcinoma cells using rat insulin promoter thymidine kinase-directed gene therapy. World J Surg. 2004;28:826-833. |
| 16. | Dutta S, Gannon M, Peers B, Wright C, Bonner-Weir S, Montminy M. PDX:PBX complexes are required for normal proliferation of pancreatic cells during development. Proc Natl Acad Sci USA. 2001;98:1065-1070. |
| 17. | Beattie GM, Itkin-Ansari P, Cirulli V, Leibowitz G, Lopez AD, Bossie S, Mally MI, Levine F, Hayek A. Sustained proliferation of PDX-1+ cells derived from human islets. Diabetes. 1999;48:1013-1019. |
| 18. | Sharma A, Zangen DH, Reitz P, Taneja M, Lissauer ME, Miller CP, Weir GC, Habener JF, Bonner-Weir S. The homeodomain protein IDX-1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes. 1999;48:507-513. |
| 19. | Feanny MA, Fagan SP, Ballian N, Liu SH, Li Z, Wang X, Fisher W, Brunicardi FC, Belaguli NS. PDX-1 expression is associated with islet proliferation in vitro and in vivo. J Surg Res. 2008;144:8-16. |
| 20. | Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, Dash C, Giardiello FM, Glick S, Johnson D. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570-1595. |
| 21. | Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Risk of advanced proximal neoplasms in asymptomatic adults according to the distal colorectal findings. N Engl J Med. 2000;343:169-174. |
| 22. | Peterson NB, Murff HJ, Ness RM, Dittus RS. Colorectal cancer screening among men and women in the United States. J Womens Health (Larchmt). 2007;16:57-65. |
| 23. | Mochizuka A, Uehara T, Nakamura T, Kobayashi Y, Ota H. Hyperplastic polyps and sessile serrated 'adenomas' of the colon and rectum display gastric pyloric differentiation. Histochem Cell Biol. 2007;128:445-455. |
| 24. | Chawengsaksophak K, Beck F. Chromosomal localization of cdx2, a murine homologue of the Drosophila gene caudal, to mouse chromosome 5. Genomics. 1996;34:270-271. |
| 26. | James R, Erler T, Kazenwadel J. Structure of the murine homeobox gene cdx-2. Expression in embryonic and adult intestinal epithelium. J Biol Chem. 1994;269:15229-15237. |
| 27. | Okon K, Zazula M, Rudzki Z, Papla B, Osuch C, Stachura J. CDX-2 expression is reduced in colorectal carcinomas with solid growth pattern and proximal location, but is largely independent of MSI status. Pol J Pathol. 2004;55:9-14. |
| 28. | Ee HC, Erler T, Bhathal PS, Young GP, James RJ. Cdx-2 homeodomain protein expression in human and rat colorectal adenoma and carcinoma. Am J Pathol. 1995;147:586-592. |