Published online Sep 28, 2008. doi: 10.3748/wjg.14.5557
Revised: August 26, 2008
Accepted: September 2, 2008
Published online: September 28, 2008
AIM: To investigate the dynamic characteristics and the correlation between PCNA, Bax, nm23, E-cadherin expression and apparent diffusion coefficient (ADC) on MR diffusion-weighted imaging (DWI) after chemoembolization in rabbit liver VX-2 tumor model.
METHODS: Forty New Zealand rabbit liver VX-2 tumor models were included in the study. DWI was carried out periodically after chemoembolization. All VX-2 tumor samples in each group were examined by histopathology and Strept Avidin-Biotin Complex (SABC) immunohistochemical staining.
RESULTS: The PCNA expression index in VX-2 tumors was higher than in the normal parenchyma around the tumor (P < 0.001). Nm23, Bax or E-caderin expression index in VX-2 tumors were lower than in the normal parenchyma around the tumor (all P < 0.001). PCNA and nm23 expression in the VX-2 tumor periphery first increased and then decreased (P < 0.001 and P = 0.03, respectively), while the expression of Bax and E-cadherin before and after chemoembolization was insignificant. When b-value was 100 s/mm2, there was a linear correlation between PCNA expression and ADC in the area of VX-2 tumor periphery (P < 0.001), and PCNA expression in VX-2 tumor periphery influenced the ADC.
CONCLUSION: The potential of VX-2 tumor infiltrating and metastasizing decreases, while its ability to proliferate increases for a short time after chemoembolization. To some degree, the ADC value indirectly reflects the proliferation of VX-2 tumor cells.
- Citation: Yuan YH, Xiao EH, Liu JB, He Z, Jin K, Ma C, Xiang J, Xiao JH, Chen WJ. Gene expression and MR diffusion-weighted imaging after chemoembolization in rabbit liver VX-2 tumor model. World J Gastroenterol 2008; 14(36): 5557-5563
- URL: https://www.wjgnet.com/1007-9327/full/v14/i36/5557.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5557
| PCNA | nm23 | Bax | E-cad | |
| Normal parenchyma | 8.28 ± 5.61 | 16.53 ± 14.66 | 40.00 ± 23.72 | 78.15 ± 18.35 |
| Tumor outer layer | 65.45 ± 3.43 | 1.74 ± 4.07 | 1.91 ± 2.66 | 6.18 ± 7.55 |
| Tumor periphery area | 72.73 ± 14.23 | 0.42 ± 0.64 | 1.05 ± 1.09 | 1.96 ± 1.79 |
| Tumor center area | 58.37 ± 15.25 | 6.74 ± 10.13 | 2.16 ± 4.59 | 1.55 ± 3.65 |
| Time | Area | PCNA | nm23 | Bax | E-cad |
| A time | E | 72.73 ± 14.23 | 0.42 ± 0.64 | 1.05 ± 1.09 | 1.96 ± 1.79 |
| F | 8.28 ± 5.61 | 16.53 ± 14.66 | 40.00 ± 23.72 | 78.15 ± 18.35 | |
| B time | E | 80.04 ± 22.84 | 2.29 ± 1.58 | 0.86 ± 1.44 | 2.87 ± 2.04 |
| F | 4.04 ± 2.06 | 57.35 ± 28.36 | 81.22 ± 23.31 | 86.41 ± 12.65 | |
| C time | E | 92.60 ± 4.76 | 7.56 ± 8.88 | 0.45 ± 0.57 | 0.93 ± 1.48 |
| F | 21.87 ± 24.59 | 67.64 ± 30.54 | 85.63 ± 20.51 | 91.03 ± 9.65 | |
| D time | E | 87.63 ± 7.12 | 4.14 ± 4.71 | 0.78 ± 1.05 | 1.07 ± 0.81 |
| F | 19.38 ± 17.74 | 40.84 ± 34.90 | 87.08 ± 10.16 | 91.03 ± 5.11 |
| ADC in tumor periphery area | ADC in normal parenchyma | |||
| b = 100 | b = 300 | b = 100 | b = 300 | |
| A time | 1.71 ± 0.27 | 1.48 ± 0.23 | 2.71 ± 0.42 | 2.30 ± 0.40 |
| B time | 1.24 ± 0.22 | 1.12 ± 0.20 | 2.10 ± 0.54 | 1.65 ± 0.37 |
| C time | 1.48 ± 0.37 | 1.23 ± 0.16 | 2.10 ± 0.49 | 1.97 ± 0.29 |
| D time | 1.57 ± 0.23 | 1.40 ± 0.18 | 2.43 ± 0.33 | 2.06 ± 0.23 |
Hepatocellular carcinoma is one of the most common malignant tumors worldwide. The genetic mutation and abnormal gene expression of hepatocellular carcinoma during its origin, progression and after transcatheter arterial chemoembolization (TACE) have been investigated in several clinical and experimental studies, with respect to PCNA, Bax, E-caderin (E-cad) and nm23 gene expression
The expression of Proliferating Cell Nuclear Antigen (PCNA) is high in the S, G2 and M stages of cell division, and low in the G0 and G1 stages. The degree of PCNA expression reflects cellular reproductive activity, which correlates significantly and positively with the pathological grade, the potential for recurrence, and survival time of malignant tumors. Therefore, it is important to measure the degree of PCNA expression in evaluating the malignant potential and prognosis of tumors[1-5]. Shi et al[4] observed that the degree of PCNA expression correlated with the size, invasive potential and degree of enhancement on CT scan of hepatocellular carcinoma. As a member of the Bcl-2 family, Bax gene is an important apoptotic controlling gene. Most investigations have demonstrated that Bax is a protein-inducing apoptosis, which promotes cell apoptosis and suppresses cell proliferation through multiple mechanism[6-9]. The nm23-H1 gene expresses nucleoside diphosphate kinase (ndpk), and has a significant impact on tumor metastasis. As reported in several studies, low expression of nm23 and the absence of alleles correlates with a high rate of infiltration and metastasis in many tumors, including hepatocellular carcinoma[4-10]. The coding product of E-cad gene is a transmembrane glycoprotein that is calcium depended and is able to mediate adhesion between cells. When it is low or absent, the adhesion between cells decreases, enabling the cancer cells to separate easily from the primary tumor, resulting in local infiltration and distant metastasis[10].
Diffusion-weighted imaging (DWI) is used much less frequently in the diagnosis of hepatic tumor and for the evaluation of tumor progression, because of its poor imaging quality[11-14]. With the development of MRI software and scanning technology, especially for echoplanar imaging (EPI) in recent years, many deficiencies, such as poor imaging quality and slow scanning speed, have been resolved[15-17]. As reported by several workers (Ichikawa et al[18,19], Yamashita et al[20], Taouli et al[21], Sun et al[22] and Yang et al[23,24]), the apparent diffusion coefficient (ADC) of hepatic cysts, hemangioma, hepatocellular carcinoma, metastatic lesions, normal parenchyma and liver cirrhosis decrease gradually on DWI. The ADC of hepatic cysts is the highest, while ADC of benign tumors is higher than that of malignant tumors. Colagrande et al[25] demonstrated that coagulation necrosis had low signal compared to viable tumor. Kamel et al[26] confirmed these findings in a study on 8 patients with hepatocellular carcinoma; ADC increased with the degree of tumor necrosis, and in 6 tumors the DWI values were higher compared to the normal parenchyma. Based on these observations, DWI, and especially ADC have potential value of differentiating benign tumor from malignant tumor.
However, few studies have been carried out to investigate the dynamic characteristics of hepatocellular carcinoma gene expression after chemoembolization. Moreover, the ADC of hepatocellular carcinoma on DWI has not been investigated by image-pathology-gene expression.
The purpose of the present study was to investigate the dynamic characteristics of PCNA, Bax, nm23 and E-cad expression after chemoembolization, and to evaluate the correlation between ADC on DWI and gene expression in rabbit VX-2 tumor model. However, the signal dynamic characteristics on DWI and the pathological mechanisms will be discussed in separate report.
Animal studies were carried out under the supervision of a veterinarian according to the guidelines on the Use of Laboratory Animals of the Ministry of Public Health of China. All animals were provided by the Laboratory Animal Center of the Second Xiangya Hospital and all protocols were approved by the Animal Use and Care Committee of the Second Xiangya Hospital.
Forty New Zealand rabbits were included in the study. Twenty-two were male and eighteen were female rabbits. The weight ranged from 1.7 kg to 2.5 kg, and the ages from 5 mo to 6 mo. All New Zealand white rabbits were healthy. Forty-seven rabbit VX-2 tumor models were developed by implanting directly into the liver, after opening the abdominal cavity. The rabbit VX-2 tumor strain was provided by the Fourth Military Medical University.
Forty VX-2 tumor models were chosen randomly from forty-seven VX-2 tumor models and were divided into four groups, a control group (non-interventional group, group A) and three investigation groups (group A, 16 h after chemoembolization; group B, 32 h after chemoembolization; and group C, 48 h after chemoembolization).
After DWI was performed on the 21st day after implantation, trans-hepatic artery catheterization chemoembolization was carried out in the animal operating room of the Second Xiangya Hospital, in study groups B, C and D.
First, the rabbits were anesthetized by injecting 3% soluble pentobarbitone. The abdominal skin was then disinfected, and the abdomen was opened by incising through the skin and the musculi recti abdominis. The organs of hepatic hilar region were exposed and the different structures such as arteria coeliaca, arteria hepatica communis, arteria hepatica propria, arteria gastroduodenalis and portal vein were recognized. The distal end of arteria gastroduodenalis was occluded, and a drop of aethocaine was placed on it. The arteria gastroduodenalis was punctured and a plastic transfixion pin was introduced and advanced into arteria hepatica propria, followed by infusion of iodized oil (0.3 mL/kg) and pharmorubicin (2 mg/kg). Finally, after making certain that there was no bleeding from the liver and other structures, the abdominal membrane, musculature and skin were sutured layer by layer.
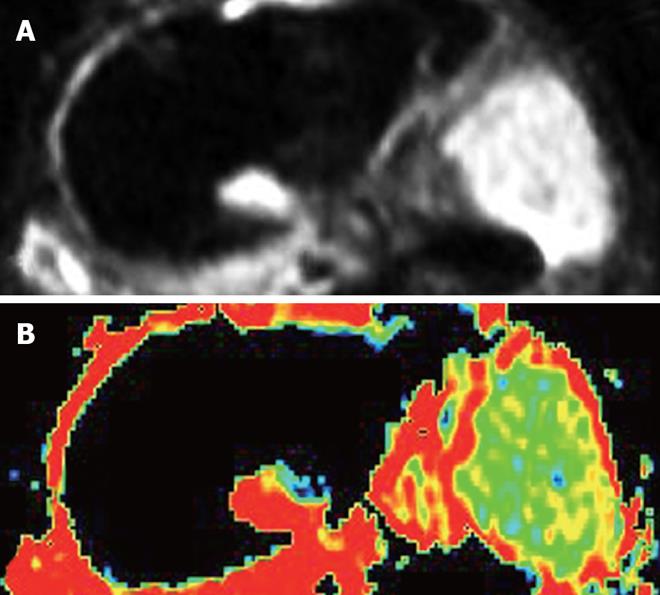
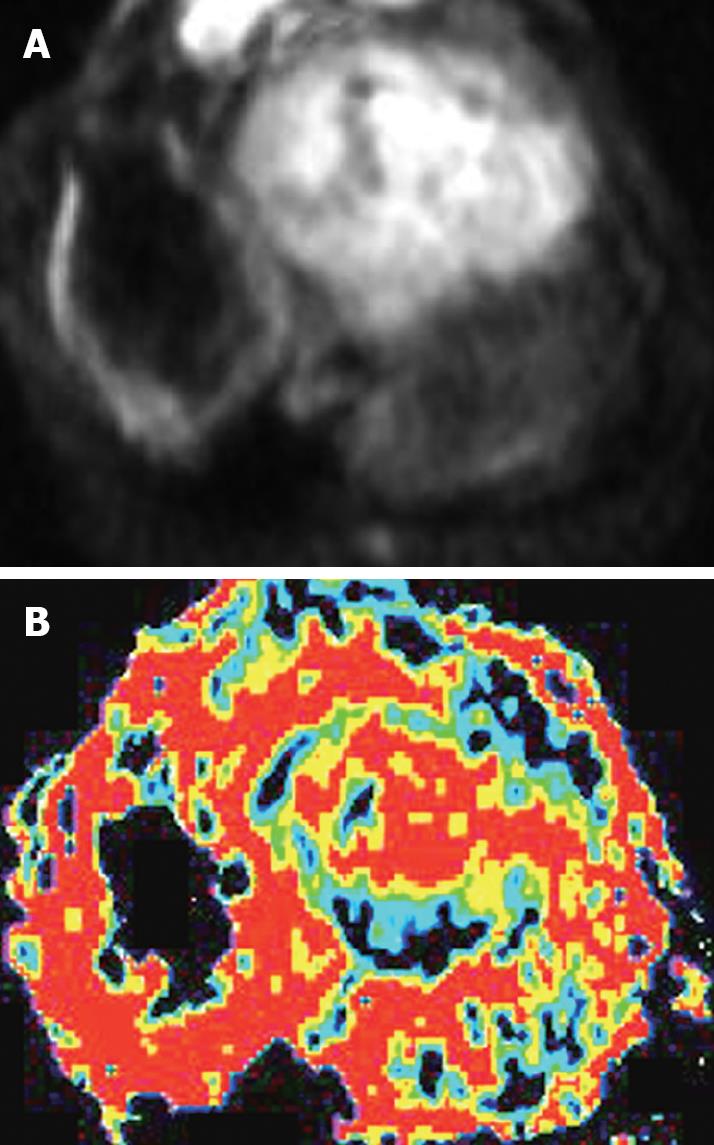
After the animals were anesthetized with 3% soluble pentobarbitone at a dose of 1 mL/kg or with different doses based on the animal status, in order to make certain that the animals had stable breathing, DWI(axial) was performed on a 1.5-Tesla Signa Twinspeed MR scanner (General Electron Medical Systems, USA), using a small diameter cylindrical brain radiofrequency coil. The scans were done before chemoembolization, and again at 6 h, 16 h, 32 h and 48 h after chemoembolization. The DWI scanning parameters included spin echo echoplanar imaging (SE-EPI) series, b-value 100 and 300 s/mm2, repetition time (TR) 6000 ms, echo time (TE) 45 ms, 20 cm × 15 cm field of view (FOV), 8 NEX, 2 mm thickness layer, and 0.5 mm Space, 128 × 128 matrix.
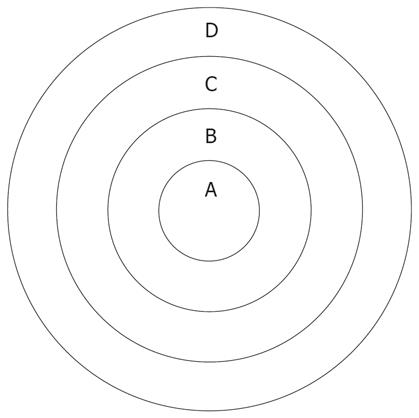
The ADC values and signal values were obtained in the VX-2 tumor periphery, VX-2 tumor center and normal liver parenchyma around the tumor (Figure 1) using Function software in GE workstation. Three different regions of interest (ROIs, 50 mm2 each area) were chosen in the normal liver parenchyma (area D in Figure 1). The average reading was considered as the ADC value or the signal value of the normal liver parenchyma around the tumor. The thickness of the area A and B in Figure 1 was respectively two fifth of the diameter of the VX-2 tumor. Similarly, the average readings of three different ROIs ADC values or signal values in area B were considered as the ADC value or the signal value of VX-2 tumor periphery. The ADC value or signal value of area A was the center of the VX-2 tumor. All measurements were made by two senior attending physicians or associate professors.
DWI was performed before chemoembolization, and 16 h, 32 h and 48 h after chemoembolization. The rabbits were then sacrificed by injecting an overdose of 3% soluble pentobarbitone. Under aseptic conditions, we obtained layer by layer VX-2 samples (Figure 1). Each VX-2 tumor was divided into the outer layer area, the periphery area and center area (Figure 1). All tissue samples were fixed in formaldehyde for 24 h before embedding in mineral wax.
Immunohistochemical staining was performed, using the SP method. Monoclonal antibody against PCNA, Bax, nucleoside diphosphate kinase (ndpk, expression product of nm23 gene) and e-cadherin endogenous avidin and biotin block ade solution, biotin-marked SP were used for immunostaining. PBS was used as a negative control, and all the results were negative. A known positive specimen of mammary adenocarcinoma was used as a positive control for PCNA, Bax and ndpk instead of PHC tissue, while pancreatic carcinoma was used for E-cad; the results were all positive.
Expression index was used to estimate the degree of gene expression, which was based on the contrast of positive cells with the total number of cells, as seen under the microscope. When the cell nucleus appeared as brown-yellow with PCNA immunohistochemical staining, the PCNA expression was considered as positive. When cytolymph appeared as brown-yellow in Bax, nm23 or E-cad immunohistochemical staining, the test was considered as positive. Ten random locations were examined under a 400 × microscope in non-necrotic areas, and the number of PCNA, Bax, nm23 and E-cad expressing cells as well as the total number of cells were counted in a double blind fashion by two investigators who were not part of our study group. The average value was considered as the expression index. When the antibody was replaced by the balanced solution, all the gene expressions were negative.
Based on the apparent diffusion coefficient (ADC) value of ROIs and expression index of PCNA, Bax, nm23 and E-cad, the differences between the different areas, the different time groups and the correlation between ADC and gene expression was assessed. The statistical significance was calculated by analysis of variance (ANOVA), non-parameter and multiple linear regression, using the SPSS 12.0 software.
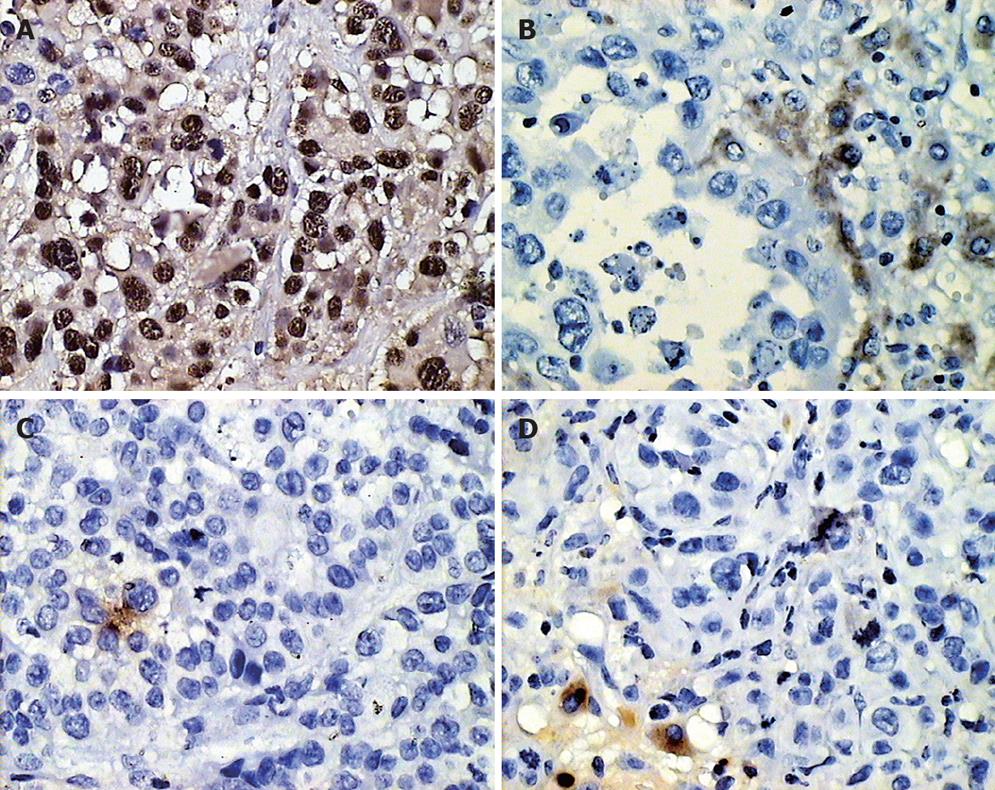
The results of PCNA, nm23, Bax and E-cad expression in VX-2 tumors and in the normal parenchyma around the tumor before chemoembolization are shown in Table 1 and Figure 2.
The differences in the PCNA, Bax, nm23 and E-cad expression index in the different areas before chemoembolization were statistically significant (χ2 = 19.08, P < 0.001; χ2 = 20.165, P < 0.001; χ2 = 12.86, P < 0.001; χ2 = 22.20, P < 0.001). The differences in the PCNA, Bax, nm23 and E-cad expression index between the tumor periphery and the normal parenchyma around the tumor were statistically significant (Z = -5.51, P < 0.001; Z = -5.39, P < 0.001; Z = -5.51, P = 0.003; Z = -5.511, P < 0.001).
The results of PCNA, nm23, Bax and E-cad expression in groups A, B, C, and D in the VX-2 tumor periphery and normal parenchyma around the tumor are shown in Table 2 and Figure 2.
The differences in the PCNA, nm23 and Bax expression in the normal parenchyma between groups A, B, C, and D were statistically significant (χ2 = 10.92, P = 0.04; χ2 = 14.83, P < 0.001; χ2 = 17.28, P = 0.03), but E-cad expression was not significant (χ2 = 3.410, P > 0.05). The differences in PCNA and nm23 expression in the tumor periphery between groups A, B, C, and D were significant (χ2 = 14.37, P < 0.001; χ2 = 8.94, P = 0.03) but that for Bax and E-cad were not significant (χ2 = 1.98, P > 0.05; χ2 = 3.88, P > 0.05).
When the b-value was 100 s/mm2, there was a significant correlation between the expression index of PCNA, nm23, Bax, E-cad and ADC values in the normal parenchyma around VX-2 tumor (r = -0.50, P < 0.001; r = -0.38, P = 0.08; r = -0.27, P = 0.04; r = -0.25; P = 0.04) . There was a linear correlation between PCNA and nm23 expression index and ADC value (F = 8.15, P < 0.001), and PCNA and nm23 expression influenced ADC. There was a linear correlation between PCNA expression index and ADC value in the VX-2 tumor periphery (F = 32.69, P < 0.001), while PCNA expression influenced ADC. However, there was no correlation between nm23, Bax and E-cad expression index, and ADC value (r = -0.20, P = 0.11; r = 0.17, P = 0.15; r = -0.10, P = 0.28).
When the b-value was 300 s/mm2, there was a correlation between PCNA, nm23, Bax and E-cad expression index, and ADC value in the normal parenchyma around VX-2 tumor (r = -0.37, P = 0.01; r = -0.37, P = 0.01; r = -0.28, P = 0.04; r = -0.31, P = 0.03), and there was a linear correlation between PCNA and nm23 expression index and ADC value (F = 5.00, P < 0.05), and PCNA and nm23 expression influenced ADC. There was a linear correlation between PCNA expression index and ADC value in the VX-2 tumor periphery (F = 29.08, P < 0.001), and PCNA expression influenced ADC, but there was no correlation between nm23, Bax and E-cad expression index and ADC value in the VX-2 tumor periphery (r = -0.15, P = 0.18; r = 0.14, P = 0.19; r = -0.04, P = 0.40).
Before and after chemoembolization, PCNA expression was significantly higher in VX-2 tumors compared with the normal parenchyma, whereas nm23, Bax and E-cad expression in the normal parenchyma was significantly higher. PCNA expression in the VX-2 tumor periphery was higher compared to the tumor outer layer and its center, while nm23 and Bax expression in the VX-2 tumor center were higher compared to the outer layer and the peripheral area of the tumor. The high level of PCNA expression indicated that the VX-2 tumor cells had powerful heteromorphism and reproductive activity. The considerable reduction in Bax expression resulted in a decrease in cell apoptosis such that the cell reproductive activity increased, while the reduction in nm23 and E-cad expression led to a marked increase in the ability of the tumor to infiltrate and metastasize. In general, the VX-2 tumor had a high malignant potential, with capacity for rapid reproduction, infiltration and metastasis.
At the same time, the ADC values of VX-2 tumor were significantly lower than the normal parenchyma, and ADC showed an inverse correlation with PCNA expression, but not with nm23, Bax and E-cad expression. These findings suggest that the ability of cell proliferation increases because gene expression increases relative to tumor proliferation such that water molecular diffusion is limited and ADC decreases. In other words, reduction in ADC indirectly increases the gene expression relative to the tumor proliferation. Similarly, Ding et al[27] reported that decrease in E-cad expression leads an increase in tumor reproductive activity and Luo et al[5] demonstrated that nm23 expression had an inverse correlation with PCNA. To a certain extent, tumor ADC can reflect its ability to infiltrate and metastasize.
After chemoembolization, PCNA expression in VX-2 tumors was slightly higher than before chemoembolization. PCNA expression increased gradually until 32 h, and then decreased slightly. PCNA expression in the normal parenchyma was significantly lower than that in VX-2 tumors, and its expression increased after chemoembolization. Soon after chemoembolization, the tumor reproductive activity may increase, but then decreases which may be related to the stimulation caused by chemoembolization. Therefore, the frequency of chemoembolization should be determined carefully because viable tumor cells may have powerful reproductive activity. In the present study, the increase in the reproductive activity of normal parenchyma cells may be related to nonselective catheterization, indicating the importance of selective catheterization. After chemoembolization, nm23 expression in the VX-2 tumor or normal parenchyma was higher than before chemoembolization, and it continued to increase until 32 h, followed by decreased expression. These findings indicate that the risk of tumor metastasis may decrease after chemoembolization. The expression of E-cad and Bax in the tumors did not show a significant change after chemoembolization, while E-cad and Bax expression increased in the normal parenchyma. On the other hand, both with b-value 100 s/mm2 and b-value 300 s/mm2, the ADC levels in the VX-2 tumor periphery decreased gradually until 16 h after chemoembolization, and then increased gradually. The ADC level in the tumor was inversely related to PCNA expression. Besides water molecules “Brownian Motion”, to a certain degree the ADC value indirectly impacts the reproductive activity of cells. With an increase in tumor cell reproduction, the diffusion motion of water cells becomes limited, such that the ADC value decreases. This may be one of the reasons why the ADC of malignant tumors is lower than that of benign tumors. Therefore, the ADC value has potential in the diagnosis and differentiation of tumors, as reported in previous studies by Ichikawa et al[18,19], Yamashita et al[20] and Kim et al[28].
Diffusion-weighted imaging (DWI) has not been extensively used in the diagnosis as well as in the evaluation of the progression and outcome of hepatic tumors, and few studies have been carried out to investigate the dynamic characteristics of hepatocellular carcinoma gene expression after chemoembolization. We believe that DWI has the potential value of differentiating benign tumors from malignant tumors after chemoembolization. In the present study, we investigated the characteristics of proliferating cell nuclear antigen (PCNA), Bax, nm23 and E-cadherin (E-cad) expression after chemoembolization, and evaluated the correlation between the apparent diffusion coefficient (ADC) on DWI and gene expression, in the rabbit VX-2 tumor model.
There are very few studies on hepatic pathological changes on DWI. The ADC value of benign lesions, such as hepatic cysts and hemangiomas was higher than that of malignant lesions such as hepatocellular carcinoma and metastasis. Several studies have shown that signals from areas of tumor coagulation necrosis are lower compared to tumor viable areas. Several studies have reported on the PCNA, Bax, nm23 and E-cad expression after chemoembolization. However, few studies have investigated the dynamic characteristics of hepatocellular carcinoma gene expression after chemoembolization, using image-pathology-gene expression
The present study clearly demonstrates that DWI has the potential application of detecting and differentiating viable tumors from necrotic tumors after chemoembolization. PCNA expression in VX-2 tumors was slightly higher compared to the level before chemoembolization. The nm23 expression in VX-2 tumor was higher, such that the potential for VX-2 tumor to infiltrate and metastasize decreases, while the ability to proliferate increases for a short period after chemoembolization. To some extent, the ADC value is able to indirectly reflect the proliferation of VX-2 tumor cells.
Physicians can apply this knowledge to evaluate hepatic tumors that are clearly progressive in nature, and accurately differentiate areas of necrotic tumor from viable tumor after chemoembolization, as well as determine to some extent the proliferation of VX-2 tumor cells on ADC.
This is an interesting, well designed and written study on the clinical significance of diffusion-weighted imaging. The manuscript contains important information on the manifestations of viable, necrotic and liquefied or cystic areas in liver tumors.
Peer reviewer: Dr. Serdar Karakose, Professor, Department of Radiology, Meram Medical Faculty, Selcuk University, Konya 42080, Turkey
S- Editor Zhong XY L- Editor Anand BS E- Editor Lin YP
| 1. | Nan KJ, Ruan ZP, Jing Z, Qin HX, Wang HY, Guo H, Xu R. Expression of fragile histidine triad in primary hepatocellular carcinoma and its relation with cell proliferation and apoptosis. World J Gastroenterol. 2005;11:228-231. |
| 2. | Nan KJ, Jing Z, Gong L. Expression and altered subcellular localization of the cyclin-dependent kinase inhibitor p27Kip1 in hepatocellular carcinoma. World J Gastroenterol. 2004;10:1425-1430. |
| 3. | Nanashima A, Yano H, Yamaguchi H, Tanaka K, Shibasaki S, Sumida Y, Sawai T, Shindou H, Nakagoe T. Immunohistochemical analysis of tumor biological factors in hepatocellular carcinoma: relationship to clinicopathological factors and prognosis after hepatic resection. J Gastroenterol. 2004;39:148-154. |
| 4. | Shi YH, Wang B, Zhu YL, Gao ZQ. Correlation of Spiral CT Signs with the Expression of p21~(WAF1), p16~(INK4) and PCNA in HCC. Linchuang Fangshexue Zazhi. 2003;22:847-851. |
| 5. | Luo HY, Wang HC, Feng DY. [Expression of proliferating cell nucleus antigen and nm23 protein and their relation with metastasis in hepatocellular carcinoma]. Hunan Yi Ke Da Xue Xue Bao. 2003;28:17-19. |
| 6. | Simile MM, Pagnan G, Pastorino F, Brignole C, De Miglio MR, Muroni MR, Asara G, Frau M, Seddaiu MA, Calvisi DF. Chemopreventive N-(4-hydroxyphenyl)retinamide (fenretinide) targets deregulated NF-{kappa}B and Mat1A genes in the early stages of rat liver carcinogenesis. Carcinogenesis. 2005;26:417-427. |
| 7. | Osada S, Saji S, Kuno T. Clinical significance of combination study of apoptotic factors and proliferating cell nuclear antigen in estimating the prognosis of hepatocellular carcinoma. J Surg Oncol. 2004;85:48-54. |
| 8. | Xiao EH, Liu DT, Shen SB, Zhou SK, Tan LH, Wang YH, Luo JG, Wu YZ, Tan CL, Liu H. Effect of preoperative transcatheter arterial chemoembolization on apoptosis of hepatocellular carcinoma cells. Zhonghua Yixue Zazhi. 2003;116:203-207. |
| 9. | Xiao EH, Hu GD, Li JQ. Relationship between apoptosis and invasive and metastatic potential of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2002;1:574-576. |
| 10. | Miyoshi A, Kitajima Y, Sumi K, Sato K, Hagiwara A, Koga Y, Miyazaki K. Snail and SIP1 increase cancer invasion by upregulating MMP family in hepatocellular carcinoma cells. Br J Cancer. 2004;90:1265-1273. |
| 11. | Chan JH, Tsui EY, Luk SH, Fung AS, Yuen MK, Szeto ML, Cheung YK, Wong KP. Diffusion-weighted MR imaging of the liver: distinguishing hepatic abscess from cystic or necrotic tumor. Abdom Imaging. 2001;26:161-165. |
| 12. | Moteki T, Horikoshi H, Oya N, Aoki J, Endo K. Evaluation of hepatic lesions and hepatic parenchyma using diffusion-weighted reordered turboFLASH magnetic resonance images. J Magn Reson Imaging. 2002;15:564-572. |
| 13. | Aube C, Racineux PX, Lebigot J, Oberti F, Croquet V, Argaud C, Cales P, Caron C. [Diagnosis and quantification of hepatic fibrosis with diffusion weighted MR imaging: preliminary results]. J Radiol. 2004;85:301-306. |
| 14. | Chow LC, Bammer R, Moseley ME, Sommer FG. Single breath-hold diffusion-weighted imaging of the abdomen. J Magn Reson Imaging. 2003;18:377-382. |
| 15. | Boulanger Y, Amara M, Lepanto L, Beaudoin G, Nguyen BN, Allaire G, Poliquin M, Nicolet V. Diffusion-weighted MR imaging of the liver of hepatitis C patients. NMR Biomed. 2003;16:132-136. |
| 16. | Hori M, Ichikawa T, Sou H, Tsukamoto T, Kitamura T, Okubo T, Araki T, Amemiya Y, Okamoto E, Obara M. [Improving diffusion-weighted imaging of liver with SENSE technique: a preliminary study]. Nippon Igaku Hoshasen Gakkai Zasshi. 2003;63:177-179. |
| 17. | Murtz P, Flacke S, Traber F, van den Brink JS, Gieseke J, Schild HH. Abdomen: diffusion-weighted MR imaging with pulse-triggered single-shot sequences. Radiology. 2002;224:258-264. |
| 18. | Ichikawa T, Haradome H, Hachiya J, Nitatori T, Araki T. Diffusion-weighted MR imaging with a single-shot echoplanar sequence: detection and characterization of focal hepatic lesions. AJR Am J Roentgenol. 1998;170:397-402. |
| 19. | Ichikawa T, Haradome H, Hachiya J, Nitatori T, Araki T. Diffusion-weighted MR imaging with single-shot echo-planar imaging in the upper abdomen: preliminary clinical experience in 61 patients. Abdom Imaging. 1999;24:456-461. |
| 20. | Yamashita Y, Tang Y, Takahashi M. Ultrafast MR imaging of the abdomen: echo planar imaging and diffusion-weighted imaging. J Magn Reson Imaging. 1998;8:367-374. |
| 21. | Taouli B, Martin AJ, Qayyum A, Merriman RB, Vigneron D, Yeh BM, Coakley FV. Parallel imaging and diffusion tensor imaging for diffusion-weighted MRI of the liver: preliminary experience in healthy volunteers. AJR Am J Roentgenol. 2004;183:677-680. |
| 22. | Sun XJ, Quan XY, Liang W, Wen ZB, Zeng S, Huang FH, Tang M. [Quantitative study of diffusion weighted imaging on magnetic resonance imaging in focal hepatic lesions less than 3 cm]. Zhonghua Zhongliu Zazhi. 2004;26:165-167. |
| 23. | Yang ZH, Xie JX, Hu BF, Zhang YW, Zhou C. Evaluation of Liver Cirrhosis with Diffusion-weighted MR Imaging: An Experimental Study. Zhongguo Yixue Yingxiang Jishu. 2002;18:849-851. |
| 24. | Yang ZH, Xie JX. Clinical application of diffusion weighted MR imaging in the liver. Zhonghua Fangshexue Zazhi. 1999;10:684-688. |
| 25. | Colagrande S, Politi LS, Messerini L, Mascalchi M, Villari N. Solitary necrotic nodule of the liver: imaging and correlation with pathologic features. Abdom Imaging. 2003;28:41-44. |
| 26. | Kamel IR, Bluemke DA, Ramsey D, Abusedera M, Torbenson M, Eng J, Szarf G, Geschwind JF. Role of diffusion-weighted imaging in estimating tumor necrosis after chemoembolization of hepatocellular carcinoma. AJR Am J Roentgenol. 2003;181:708-710. |
| 27. | Ding YL, Zhao RY, You CZ, Li ZT. Study of the relation ship between expression of E cadherin and PCNA in hepatocellular carcinoma. Zhonghua Zhongliu Fangzhi Zazhi. 2002;9:556-557. |
| 28. | Kim T, Murakami T, Takahashi S, Hori M, Tsuda K, Nakamura H. Diffusion-weighted single-shot echoplanar MR imaging for liver disease. AJR Am J Roentgenol. 1999;173:393-398. |