INTRODUCTION
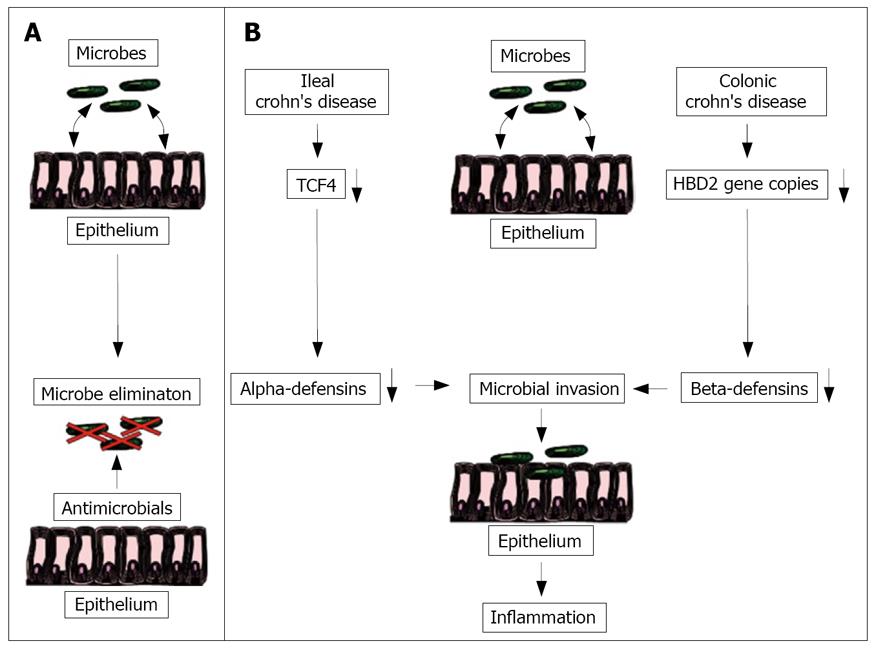
Figure 1 Proposed model for the pathogenesis of ileal and colonic Crohn's disease.
In the healthy ileal and colonic mucosa, luminal microbes are sufficiently eliminated by an adequate secretion of antimicrobials peptides (A). In ileal Crohn's disease, a reduction of the Wnt transcription factor TCF4 leads to a decreased alpha-defensin secretion. In colonic Crohn´s disease a reduced HBD2 copy number causes a weakened antimicrobial barrier mediated by the beta-defensins. Both changes may allow the luminal microbes to attach and invade in the mucosa triggering the inflammation (B).
Crohn’s disease is characterized by a chronic inflam-mation of the whole intestine most commonly occurring in the distal ileum and/or in the colon[1]. Since both disease locations contain high concentrations of intestinal bacteria (107-108 organisms/gram luminal content in the distal ileum and 1011-1012 in the colon as compared to 0-103 organisms/gram luminal content in the stomach, duodenum, jejunum and proximal ileum)[2] it is conceivable that luminal microbes are suggested to play an important role in the pathogenesis of Crohn’s disease[3]. For example, it was demonstrated that surgical diversion of the fecal stream effectively resolves Crohn’s disease inflammation distal to the surgical site[4]. Moreover, an abnormal microbial composition was found in intestinal bowel disease[5,6], especially with an enhanced E.coli virulence in Crohn’s disease[7]. The most convincing evidence for the involvement of enteric microbiota in the pathogenesis of Crohn´s disease was demonstrated by Swidsinsky et al by fluorescent in situ hybridization studies (FISH) showing a drastic increase of mucosa-associated and invasive bacteria in active Crohn’s disease, whereas these bacteria are absent from the normal small and large bowel epithelium[8].
In the past few years, several investigations implicated a central role for the adaptive immunity mediated by defensins and other antimicrobial peptides in Crohn’s disease[9]. A relative deficiency of secreted defensins in Crohn’s disease was shown in several human and animal studies shifting the balance between antimicrobial peptides and luminal bacteria towards the microbial flora[9]. As a result, an abnormal microbial composition in Crohn’s disease allows the intestinal microbes to adhere and invade into the mucosa triggering the inflammation[10].
Since Crohn’s disease is characterized by different disease locations, such as small intestinal versus colonic disease, we discuss in this review not only the concept of a defective antimicrobial shield in both phenotypes of Crohn’s disease, but also focus on the mechanisms behind ileal and colonic defensin deficiency beginning with the intestinal stem cell.
INSTESTINAL STEM CELLS
The whole intestinal tract undergoes a rapid epithelial cell turnover maintained by a population of intestinal stem cells. These enigmatic cells are characterized by a relatively undifferentiated, immature phenotype, which makes them difficult to be clearly identified even if recent studies found leucine-rich-repeat-containing G-protein-coupled receptor 5 (Lgr5) as a marker for these cells[11,12]. The stem cell compartment is located at the base of the crypts, in the small intestine intermingled with the Paneth cells[12]. They are surrounded by epithelial and mesenchymal cells, which regulate stem cell behaviour by paracrine secretion of extra-cellular substrates, such as growth factors and cytokines[13].
In this optimal microenvironment, the intestinal stem cells can give rise to four epithelial cell types[13]: The most abundant cells in the epithelium are the columnar cells, which are specialised for absorption by the presence of apical microvilli. Goblet cells are mucin producing and secreting cells forming the protective luminal mucosal layer. Neuroendocrine cells release hormones in an endo- and paracrine fashion, and finally the Paneth cells, which are found in the crypt base of the small intestine and ascending colon. Their main function is to secrete defensins and other antimicrobial molecules to keep the crypts sterile[14].
Stem cell behaviour is regulated by several cell signalling pathways, such as Wnt, Notch, Hedgehog and BMP[15,16]. Derangement of these pathways plays a crucial role in the development of malignancy within the intestinal tract[13,16,17]. The role of these pathways in inflammatory diseases, such as inflammatory bowel disease, is a new finding. In our recent work we focused on the differentiation from intestinal stem cells towards Paneth cells in ileal Crohn’s disease as a potential pathogenetic mechanism of this disease[18].
PANETH CELLS
Paneth cells are located at the crypt base adjacent to the stem cells. Their main function is to protect the intestinal mucosa against microbes by the secretion of several antimicrobial molecules into the lumen of the crypt. The principal Paneth cell products are the alpha-defensins HD5 and HD6, but also other antimicrobials like lysozyme and phospholipase A2[19]. These secretory molecules enable the Paneth cells to defend the small intestine against a broad spectrum of agents, including gram-positive and gram-negative bacteria, fungi and even some viruses[20].
The differentiation from the intestinal stem cell to the Paneth cell is mainly controlled by the Wnt signaling pathway[21]. As shown by embryonic mouse intestine studies, the Wnt transcription factor TCF4 seems to be essential for the Paneth cell gene program[21]. The activation of Wnt signaling leads to the formation of an intracellular β-catenin-TCF4-complex. This complex translocates into the nucleus, where TCF4 acts as a transcription factor to control the expression of several downstream target genes, such as Paneth cell alpha-defensins[21]. In TCF4 null mice, a direct link exists between TCF4 and cryptidins (the mouse homolog to human alpha-defensins)[21], whereas in humans, especially in Crohn´s disease evidence was missing. Therefore, we looked at the expression of Paneth cell defensins and the Wnt signaling transcription factor TCF4 in ileal Crohn’s disease.
THE ALPHA-DEFENSIN DEFICIENCY IN ILEAL CROHN’S DISEASE IS LINKED TO A REDUCED EXPRESSION OF THE TRANSCRIPTION FACTOR TCF4
In ileal Crohn’s disease, we demonstrated in two independent cohorts a decreased expression of the alpha-defensins HD5 and HD6, whereas the other Paneth cell products were unchanged or even increased when compared with controls[19,22]. Remarkably, this observation was independent of the degree of inflammation and not detected in colonic biopsies from patients with Crohn’s disease and ulcerative colitis, as well as in pouchitis samples, which were used as inflammatory controls[19,22]. Since the antibacterial activity in intestinal mucosal extracts from patients with ileal Crohn’s disease was also reduced, the decreased expression of HD5 and HD6 results in a defective antimicrobial shield in humans[19]. Transgenic mice with human HD5 expression levels (reflecting the antibacterial conditions in Crohn’s disease) had an abnormal composition of the luminal microbial flora, which also supports the relevance of alpha-defensins in regulating the bacterial flora[19]. Mutations in the NOD2 (nucleotide-binding oligomerization domain containing 2) gene, an intracellular receptor for bacterial muramyl dipeptide, are clearly associated with ileal Crohn’s disease[23,24]. It is noteworthy that Paneth cells, which are responsible for a good effective antibacterial shield in the small intestine, also express NOD2[25]. Interestingly, we found an even more pronounced decrease of alpha-defensin transcripts in patients with a NOD2 mutation as compared to the wild types[19,22]. The low defensin synthesis in those patients has been confirmed by Elphick et al[26]. Another study confirmed the low defensin formation in Crohn’s disease but linked it to inflammation and loss of Paneth cells[27]. However, this study was flawed by using different tissue samples such as biopsies versus segments from controls versus diseased patients as well as other methodological problems in normalizing their data.
In summary, these studies above implicate that the specific deficiency of Paneth cell defensins in ileal Crohn’s disease, which is even more pronounced in case of NOD2 mutations, results in a dysfunction of the mucosal barrier. The defect in innate immune defense of the ileal mucosa appears to be primary and could cause and/or maintain this inflammatory disease.
Wnt signaling is one of the crucial pathways in the differentiation from the intestinal stem cell towards the Paneth cell. In particular the Wnt transcription factor TCF4 is a known regulator of Paneth cell differentiation and also alpha-defensin expression[21]. We were able to show a reduced expression of TCF4 in patients with ileal Crohn’s disease as compared to colonic Crohn´s disease and ulcerative colitis[18]. Remarkably, this decrease of TCF4 in ileal Crohn’s disease was independent of the degree of inflammation and independent of the NOD2 genotype[18]. In accordance to prior animal models[21], TCF4 expression correlated also in human samples significantly with HD5 and HD6[18]. Moreover, we were able to demonstrate the functional relevance of this TCF4 decrease in heterozygous TCF4-knockout mice. These mice were characterized by a reduced TCF4 expression leading to a significant decrease of Paneth cell alpha-defensins and also bacterial killing[18]. We were also able to detect a high-affinity binding site in the HD5 and HD6 promotor for TCF4, which showed a significantly reduced activity in ileal Crohn’s disease[18]. Overall, a defect in intestinal stem cell differentiation towards Paneth cells mediated via the Wnt signaling transcription factor TCF4 could results in a defective antibacterial shield in ileal Crohn’s diseases by the absence of a sufficient expression of the protective Paneth cell defensins HD5 and HD6 (Figure 1).
THE BETA-DEFENSIN DEFICIENCY IN COLONIC CROHN’S DISEASE IS RELATED TO FEWER HBD2 GENE COPY NUMBERS
Since Paneth cells are rare in the colon, the contribution of the alpha-defensins to the antimicrobial defence in the large intestine is only limited. Therefore, it is not surprising that colonic HD5 and HD6 expression was weak and almost equal in Crohn´s disease and ulcerative colitis[22,28]. In contrast, the beta-defensins HBD1-3 are preferentially expressed in the colon. HBD1 is constitutively expressed in Crohn’s disease independent of the grade of inflammation[29,30], whereas HBD2 and HBD3 are inducible in intestinal bowel disease [30-32]. As shown in two separate cohorts, the induction of both beta-defensins, HBD2 and HBD3, was less pronounced in colonic mucosa of Crohn’s disease patients as compared to ulcerative colitis[31,32]. This deficiency of HBD2 expression in Crohn’s colitis was also confirmed on the protein level by immunohistochemistry[30]. HBD2 and HBD3 are strongly correlated to each other, especially in case of inflammation[31,32]. Similar to these two beta-defensins, other antimicrobial peptides like elafin, SLPI and LL37 also followed the pattern of diminished induction in Crohn’s disease compared to ulcerative colitis[33,34]. This observed colonic defect of the antimicrobial barrier caused by a diminished expression of beta-defensins and other antimicrobials was supported by a recent study, where we found a significantly lower bacterial killing using biopsy extracts from Crohn’s disease patients compared to ulcerative colitis and healthy controls[35].
In humans, the beta-defensins HBD2 and HBD3 are neighbouring genes on chromosome 8p23.1. The DNA copy number of this beta-defensin gene cluster is highly polymorphic within the healthy population[36]. A genome wide copy number profiling using a DNA microarray showed that the defective induction of HBD2 and HBD3 in colonic CD may be due to a lower gene copy number in this gene area[37]: The median number of HBD2 gene copies in the control group and in patients with ulcerative colitis was 4. In contrast, two different cohorts (an exploratory surgical cohort with ileal or colonic CD and a large confirmatory cohort with inflammatory bowel diseases those with ileal resections/disease) showed a median of only 3 copies per genome in colonic CD. Taken together, the number of HBD2 gene copies was shifted to lower numbers in colonic CD as compared to controls (3 vs 4)[37]. Since patients with 3 or less HBD2 gene copies were shown to have a significantly higher risk of developing colonic CD as compared to individuals with four or more copies (OR: 3.06, 95% CI: 1.46-6.45), the number of HBD2 gene copies seems to be functionally relevant. It is not surprising that HBD2 gene copy numbers of < 4 are associated with a diminished mucosal HBD2 mRNA expression[37]. In conclusion, a lower HBD2 gene copy number in the beta-defensin locus predisposes to colonic CD, which is most likely mediated through a diminished beta-defensin expression (Figure 1).
WHAT ABOUT ULCERATIVE COLITIS?
In active ulcerative colitis the epithelial expression of the inducible beta-defensins HBD2 (about 1000-fold), HBD3 (about 300-fold) and other antimicrobials is enhanced[9,34]. Accordingly, we were able to demonstrate an increase of the antibacterial activity toward various bacteria of the normal gut flora in cationic biopsy extracts of patients with ulcerative colitis suggesting an intact antibacterial shield in ulcerative colitis[35]. Interestingly, psoriasis, which is a non-bacterial inflammatory disease of the skin, is linked to an increased gene copy number in the beta-defensin locus[38]. It could be possible that the tremendous induction of defensins and other antimicrobials in ulcerative colitis may lead to a better antibacterial protection at the expense of enhancing the inflammatory process, since many antibacterials have proinflammatory and sometimes chemokine like effects.
CONCLUSION
The exact mechanisms behind both phenotypes of Crohn’s disease are certainly complex but the presented concept has already opened new avenues of thinking. The long nurtured alterations in adaptive immunity may be secondary to this defect in innate mucosal immunity allowing mucosal invasion of bacteria. In addition to the defect in Paneth cell differentiation in ileal disease, and a lower HBD2 gene copy number in colonic disease other mechanisms are likely to exist which may require a more systematic study of the antibacterial mucosal peptide system. Nevertheless, in the future new therapeutic strategies in the treatment of Crohn’s disease should focus on a stimulation of the protective innate immune system rather than broadly suppressing the adequate immunological response at the expense of opportunistic infections.
S- Editor Xiao LL E- Editor Yin DH