Published online Sep 21, 2008. doi: 10.3748/wjg.14.5436
Revised: July 28, 2008
Accepted: August 4, 2008
Published online: September 21, 2008
AIM: To investigate the effectiveness of direct hemoperfusion with polymyxin B-immobilized fibers (DHP-PMX therapy) on warm ischemia-reperfusion (I/R) injury of the small intestine.
METHODS: The proximal jejunum and distal ileum of mongrel dogs were resected. Warm ischemia was performed by clamping the superior mesenteric artery (SMA) and vein (SMV) for 2 h. Blood flow to the proximal small intestine was restored 1 h after reperfusion, and the distal small intestine was used as a stoma. The experiment was discontinued 6 h after reperfusion. The dogs were divided into two groups: the DHP-PMX group (n = 6, DHP-PMX was performed for 180 min; from 10 min prior to reperfusion to 170 min after reperfusion) and the control group (n = 5). The rate pressure product (RPP), SMA blood flow, mucosal tissue blood flow, and intramucosal pH (pHi) were compared between the two groups. The serum interleukin (IL)-10 levels measured 170 min after reperfusion were also compared.
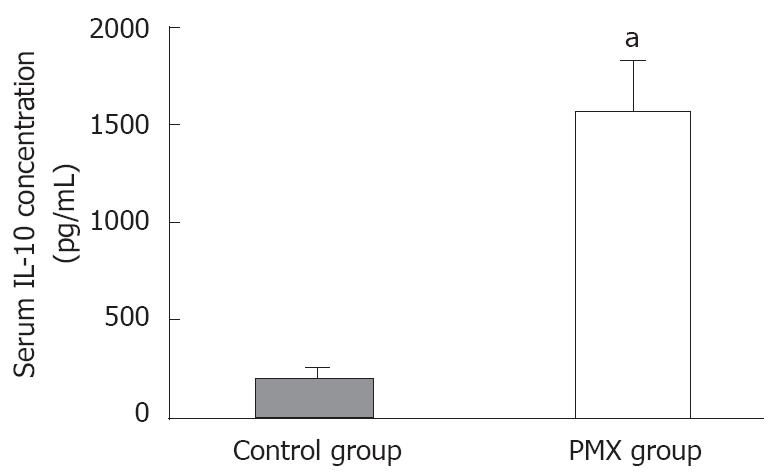
RESULTS: The RPP at 6 h after reperfusion was significantly higher in the PMX group than in the control group (12 174 ± 1832 mmHg/min vs 8929 ± 1797 mmHg/min, P < 0.05). The recovery rates of the SMA blood flow at 1 and 6 h after reperfusion were significantly better in the PMX group than in the control group (61% ± 7% vs 44% ± 4%, P < 0.05, and 59% ± 5% vs 35% ± 5%, P < 0.05, respectively). The recovery rate of the mucosal tissue blood flow and the pHi levels at 6 h after reperfusion were significantly higher in the PMX group (61% ± 8% vs 31% ± 3%, P < 0.05 and 7.91 ± 0.06 vs 7.69 ± 0.08, P < 0.05, respectively). In addition, the serum IL-10 levels just before DHP-PMX removal were significantly higher in the PMX group than in the control group (1 569 ± 253 pg/mL vs 211 ± 40 pg/mL, P < 0.05).
CONCLUSION: DHP-PMX therapy reduced warm I/R injury of the small intestine. IL-10 may play a role in inhibiting I/R injury during DHP-PMX therapy.
- Citation: Sato H, Oshima K, Arakawa K, Kobayashi K, Yamazaki H, Suto Y, Takeyoshi I. Direct hemoperfusion with a polymyxin B-immobilized cartridge in intestinal warm ischemia reperfusion. World J Gastroenterol 2008; 14(35): 5436-5441
- URL: https://www.wjgnet.com/1007-9327/full/v14/i35/5436.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5436
The small intestinal villi are extremely sensitive to ischemia-reperfusion (I/R) injury, and many microcirculatory disturbances contribute to structural and functional changes[1]. I/R injury of the small intestine is consequently a critical problem that is important in situations such as the interruption of blood flow to the intestines due to abdominal aortic aneurysm surgery, small intestinal transplantation, surgery involving cardiopulmonary bypass, strangulated hernias, and neonatal necrotizing enterocolitis[2]. Intestinal I/R injury produces injury in both the intestines and distant organs including the lungs, kidneys, and liver[3]; therefore, it is associated with high rates of morbidity and mortality in both surgical and trauma patients[4].
A polymyxin B-immobilized fiber column (PMX cartridge, Toraymyxin; Toray Industries, Tokyo, Japan), which was developed in Japan in 1994, is an extracorporeal hemoperfusion device that uses polymyxin-B fixed to α-chloroacetamide-methyl polystyrene-derived fibers packed in the cartridge. Direct hemoperfusion with PMX (DHP-PMX) therapy can remove circulating endotoxins and reduce various cytokines, even in patients with high levels of plasma cytokines[5]. DHP-PMX has been used for the treatment of endotoxemia[6] and reported to lower inflammatory cytokine and plasminogen activator inhibitor-1 (PAI-1) levels immediately[7]. DHP-PMX therapy has also been attempted for severe sepsis secondary to intra-abdominal infection[8], acute lung injury, and acute respiratory distress syndrome caused by sepsis[9], and its effectiveness has been reported. Recently, we hypothesized that DHP-PMX therapy could reduce I/R injury and demonstrated the usefulness of this therapy on pulmonary warm I/R injury in a canine model[10].
In this study, we evaluated the effectiveness of DHP-PMX on warm I/R injury of the small intestine using a canine model.
Eleven adult mongrel dogs of both sexes, weighing 7.5-15.5 kg, were used in this study. The dogs were fasted but had free access to water for 24 h prior to the experiment. All of the animals were cared for in accordance with the guidelines set forth in the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication 85-23; revised 1985). This study was also approved by the Animal Care and Experimentation Committee, Gunma University, Showa Campus, Japan.
After intramuscular administration of ketamine hydrochloride (2 mg/kg), the animals were anesthetized. Endotracheal intubation was performed, and the animals were ventilated with a respirator (Servo Ventilator 900C; Siemens-Elema, Solna, Sweden). The inspired O2 concentration (FIO2) was set at 1.0 during the experiment. Mechanical ventilation was performed with a tidal volume of 20 mL/kg and a rate of 10 breaths/min. General anesthesia was maintained with the inhalation of 1% to 2% isoflurane, and muscular relaxation was obtained with additional pancuronium bromide (0.1 mg/kg) every 20 min. The surgery was performed under sterile conditions. A polyethylene catheter was positioned in the abdominal aorta through the right femoral artery and connected to a pressure transducer to record arterial pressure. Another polyethylene catheter was inserted into the right femoral vein to use as a venous infusion line. The infusion rate for the lactated Ringer’s solution was set at 30 mL/kg per h for 6 h after reperfusion. Laparotomy was performed via a midline incision after the blood pressure and respiration parameters stabilized. The small intestine was isolated with the vascular pedicle, and both the superior mesenteric artery (SMA) and the superior mesenteric vein (SMV) were dissected from the surrounding lymph nodes, plexuses, and tissues. The proximal jejunum and distal ileum were resected to interrupt the intramural blood flow. Warm ischemia was induced by clamping the SMA and SMV for 2 h. Proximal intestinal continuity was restored with an end-to-end anastomosis 1 h after the reperfusion. The resected distal intestine was used as a stoma to measure the tissue blood flow and pHi. During the 6-h experimental period, the abdominal cavity was closed and opened temporarily for each measurement. The experiment was discontinued 6 h after the reperfusion. Catecholamines were not used at any point during the experiment.
The experimental study was composed of two groups: the DHP-PMX group (n = 6) and the control group (n = 5). The animals were randomly assigned to either the DHP-PMX group or the control group. In the DHP-PMX group, a double-lumen catheter was inserted into the portal vein through the left gastric vein, and DHP with PMX was performed with a flow rate of 80 mL/min for 180 min (from 10 min prior to reperfusion to 170 min after reperfusion) using that catheter. DHP was not performed in the control group.
The arterial blood pressure and HR were directly monitored through a catheter connected to a transducer (Spectramed TA 1017; San-ei Co., Tokyo, Japan). The rate pressure product (HR × systolic pressure, RPP) was also calculated.
The SMA blood flow was measured prior to ischemia and at 1, 3, and 6 h after reperfusion using an electromagnetic blood flow meter (Model MFV-3100; Nihonkohden Co., Ltd., Tokyo, Japan). The SMA blood flow was expressed as the percentage of the level that was determined prior to ischemia.
The tissue blood flow was measured in the small bowel mucosa using a laser Doppler flow meter (ALF 21; Advance Co., Ltd., Tokyo, Japan) prior to ischemia and at 1, 3, and 6 h after reperfusion. Each measurement was made at three points by inserting a probe through the stoma and placing it against the antimesenteric side of the bowel lumen. The laser probe reading reflects tissue blood flow within about 1.0 mm of the surface of the bowel wall. Tissue blood flow was calculated as the mean of the three measurements and expressed as the percentage of the level determined prior to ischemia.
pHi was measured prior to ischemia and at 1, 3, and 6 h after reperfusion. This method has been described previously[11]. In brief, a tonometer (Trip; Tonometrics, Helsinki, Finland) was inserted into the small bowel lumen through the stoma. Within 40 min, the PCO2 of the saline in the balloon placed at the tip of the tonometer had equilibrated with the intraluminal PCO2, which reflects the mucosal PCO2 of the bowel. The HCO3- concentration in the bowel wall was assumed to be the same as the HCO3- concentration in the arterial blood. The saline PCO2 and HCO3- concentration in the artery were determined with a blood gas analyzer (Stat Profile M; Nova Biomedical Co., Waltham, MA) and were used to calculate the pHi using the Henderson-Hasselbach equation: pHi = 6.1 + log[arterial HCO3-/(0.03 × saline PCO2)].
Arterial blood samples were collected 170 min after reperfusion (that is just before DHP-PMX removal) for measuring serum IL-10 levels using a commercial sandwich ELISA (Predicta ELISA kit; Genzyme Corp., Cambridge, MA) according to the manufacturer’s instructions. In each experiment, a standard curve was obtained with serial dilutions using linear regression analysis of specific samples versus expected concentrations. Interleukin measurements were done in duplicate.
All results are expressed as the mean ± SEM. The significance of the differences was determined using analysis of variance (ANOVA) or Mann-Whitney U-test. P < 0.05 was considered to be statistically significant.
All 11 mongrel dogs were successfully observed for 6 h after reperfusion without any complications.
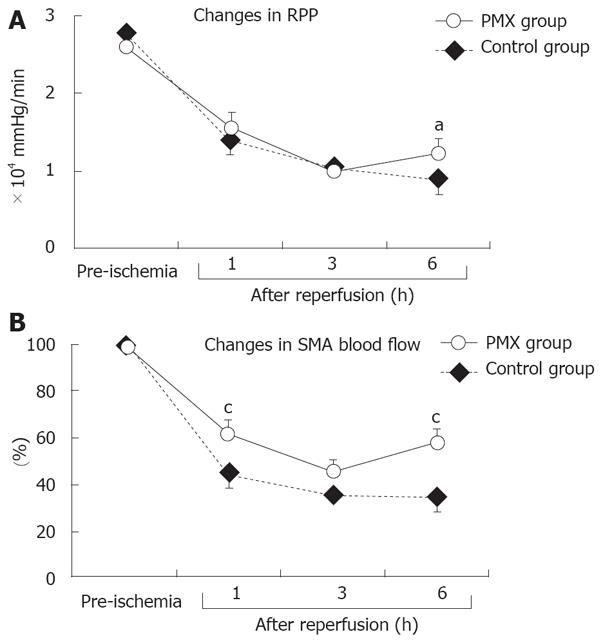
The changes in the RPP are shown in Figure 1A. The RPPs gradually decreased with time in the control group. Those in the PMX group also decreased gradually; however, it had improved at 6 h after reperfusion and was significantly different compared to the control group (P < 0.05).
The recovery rates of the SMA blood flow expressed as a percentage of the baseline control value obtained before I/R injury are shown in Figure 1B. The SMA blood flow remarkably decreased 1 h after reperfusion in both groups. Additionally, the SMA blood flow gradually decreased until 6 h after reperfusion in the control group. The changes in SMA blood flow in the PMX group, however, were consistently higher after reperfusion than those in the control group, especially with significant (P < 0.05) differences at 1 and 6 h after reperfusion (Figure 1B).
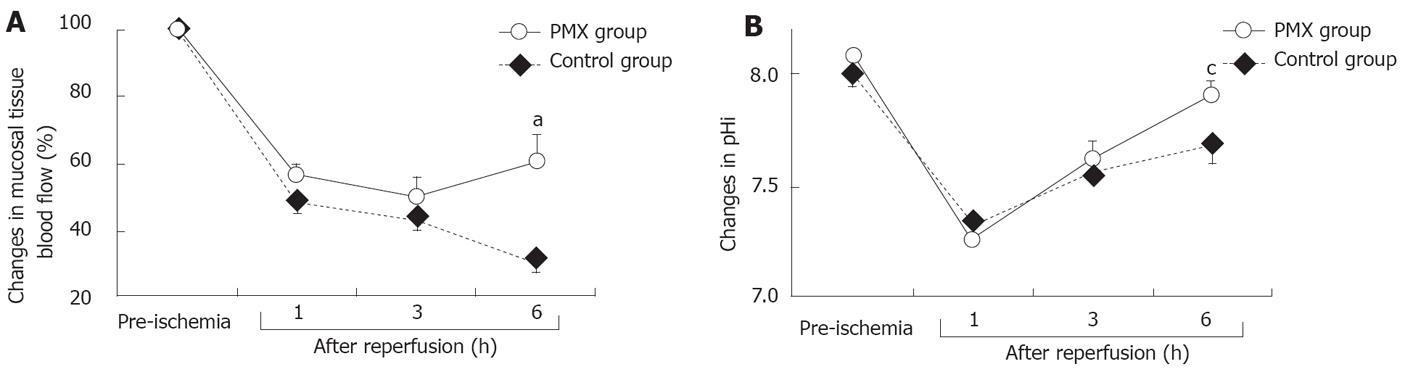
The recovery rates of the mucosal tissue blood flow expressed as a percentage of the baseline control value obtained before I/R injury are shown in Figure 2A. In both groups, the mucosal tissue blood flow decreased gradually with time except at 6 h after reperfusion in the PMX group. The decreases in the mucosal tissue blood flow after reperfusion were smaller in the PMX group than in the control group. At 6 h after reperfusion, the mucosal tissue blood flow rate was significantly higher in the PMX group than in the control group after reperfusion.
As shown in Figure 2B, the changes in pHi also decreased remarkably in both groups. No significant differences in pHi levels were observed at 1 and 3 h after reperfusion in both groups; however, the pHi level in the PMX group was significantly higher (P < 0.05) than in the control group at 6 h after reperfusion.
Polymyxin B binds to endotoxin, an outer membrane component of gram-negative bacteria that is thought to be an important pathogenic trigger for the production of inflammatory mediators. Several preclinical studies have demonstrated that hemoperfusion or plasmapheresis over immobilized polymyxin B can remove endotoxin from the blood[12-14]. Recently, some studies reported improved hemodynamic status[15] and improved survival[16] in patients with sepsis treated with PMX, and DHP-PMX therapy is effective for patients with septic shock who are infected not only by gram-negative bacteria but also by gram-positive bacteria without endotoxin release[17]. Therefore, DHP-PMX therapy has been conventionally used in patients with severe sepsis or septic shock, and its clinical effect has been confirmed. In addition, some authors have discussed the mechanism of DHP-PMX action. Kushi et al reported that the adsorption of pathogenic bacteria prevented the release of inflammatory cytokines and lessened the stimulation of vascular endothelial cells to lower PAI-1 level rather than directly inhibiting PAI-1 production by DHP-PMX therapy[18]. Tani et al speculated that a reduction in plasma endotoxins by endotoxin adsorption contributed to the cessation of cytokine gene expression and the excretion of cytokines[7]. Additionally, improvement in the PaO2/FiO2 ratio in patients with acute lung injury or acute respiratory distress syndrome caused by sepsis has been related to DHP-PMX therapy and decreases in blood neutrophil elastase (NE) and IL-8 levels[9]. We hypothesized that DHP-PMX therapy might be valuable in various inflammatory situations. Consequently, we evaluated the usefulness of DHP-PMX therapy on normothermic cardiopulmonary bypass in a pig model[19] and on pulmonary warm I/R injury in a canine model[10], and obtained satisfactory results. In the present study, we investigated the effectiveness of DHP-PMX therapy on small intestinal warm I/R injury in a canine model because intestinal I/R injury produces injury in both the intestines and distant organs including the lungs, kidneys, and liver[3] and is therefore associated with high rates of morbidity and mortality in both surgical and trauma patients[4].
As a result, the RPP was significantly (P < 0.05) better in the PMX group than in the control group at 6 h after reperfusion. The SMA and the mucosal tissue blood flow in the control group gradually decreased after reperfusion. Those parameters in the PMX group, however, had improved at 6 h after reperfusion and were significantly (P < 0.05) different compared to those parameters in the control group. The pHi level had also improved at 6 h after reperfusion and was significantly (P < 0.05) different from that in the control group. Our results showed the effectiveness of DHP-PMX therapy on small intestinal warm I/R injury.
IL-10 is a 35-kDa cytokine that regulates immune and inflammatory responses[20]. Systemic inflammatory response syndrome (SIRS) following major abdominal surgery is characterized by complex alterations in cytokine concentrations, and the balance between tumor necrosis factor (TNF)-α and IL-10 may be related to the occurrence of postoperative complications[21]. Wu et al reported that small intestinal ischemia reperfusion increased mucosal inflammatory modulator IL-6 concentration and inhibited anti-inflammatory cytokine IL-10 synthesis[22]. In addition, endogenous IL-10 exerts an anti-inflammatory role during reperfusion injury, possibly by regulating early stress-related genetic response, adhesion molecule expression, neutrophil recruitment, and subsequent cytokine and oxidant generation[23]. Malleo et al demonstrated that the absence of endogenous IL-10 enhanced organ dysfunction and mortality associated with multiple organ dysfunction syndrome in mice[24]. In this study, we focused on the serum IL-10 levels after reperfusion, and those in both groups were measured and compared. As a result, the serum IL-10 level measured 170 min (that is just before DHP-PMX removal) in the PMX group was significantly higher than in the control group. In addition, RPP, the recovery rates of SMA blood flow, the mucosal tissue blood flow, and the pHi levels after reperfusion were significantly better in the PMX group than in the control group. Therefore, we suggest that the IL-10 level is associated with the inhibition of small intestinal I/R injury using DHP-PMX therapy.
The authors of a recent study suggested that the absorption of anandamide by PMX might abolish the diverse negative effects of anandamide such as hypotension, immunosuppression, and cytotoxicity[25]. Taking these results into consideration, the possibility exists that treatment with PMX not only removes endotoxin, but also reduces inflammatory reactions through the inhibition of various inflammatory cascades and has an effective role on I/R injury. Further studies that include the role of endotoxin and alterations of inflammatory factors such as cytokines and chemokines with DHP-PMX therapy are necessary.
In conclusion, DHP-PMX therapy may reduce warm I/R injury in the small intestine, and IL-10 could play an important role in this mechanism.
Ischemia-reperfusion (I/R) injury of the small intestine is consequently a critical problem that is important in situations such as the interruption of blood flow to the intestines due to abdominal aortic aneurysm surgery, small intestinal transplantation, surgery involving cardiopulmonary bypass, strangulated hernias, and neonatal necrotizing enterocolitis. Intestinal I/R injury produces injury in both the intestines and distant organs including the lungs, kidneys, and liver, therefore, it is associated with high rates of morbidity and mortality in both surgical and trauma patients.
A polymyxin B-immobilized fiber column (PMX cartridge, Toraymyxin), which was developed in Japan in 1994, is an extracorporeal hemoperfusion device that uses polymyxin-B fixed to α-chloroacetamide-methyl polystyrene-derived fibers packed in the cartridge. Direct hemoperfusion with PMX (DHP-PMX) therapy can remove circulating endotoxins and reduce various cytokines, even in patients with high levels of plasma cytokines.
DHP-PMX has been used for the treatment of endotoxemia and reported to lower inflammatory cytokine and plasminogen activator inhibitor-1 levels immediately. DHP-PMX therapy has also been attempted for severe sepsis secondary to intra-abdominal infection, acute lung injury, and acute respiratory distress syndrome caused by sepsis, and its effectiveness has been reported. Recently, we hypothesized that DHP-PMX therapy could reduce I/R injury and demonstrated the usefulness of this therapy on pulmonary warm I/R injury in a canine model.
DHP-PMX therapy may reduce warm I/R injury in the small intestine, and IL-10 could play an important role in this mechanism.
The possibility exists that treatment with PMX not only removes endotoxin, but also reduces inflammatory reactions through the inhibition of various inflammatory cascades and has an effective role on I/R injury.
The authors demonstrated that DHP with a polymyxin B-immobilized cartridge reduced reperfusion injury in the small intestine. This study was well designed and well investigated.
| 1. | Tjärnström J, Wikström T, Bagge U, Risberg B, Braide M. Effects of hyperbaric oxygen treatment on neutrophil activation and pulmonary sequestration in intestinal ischemia-reperfusion in rats. Eur Surg Res. 1999;31:147-154. |
| 2. | Collard CD, Gelman S. Pathophysiology, clinical manifestations, and prevention of ischemia-reperfusion injury. Anesthesiology. 2001;94:1133-1138. |
| 3. | Köksoy C, Kuzu MA, Kuzu I, Ergün H, Gürhan I, Ergun H, Gurhan I. Role of tumour necrosis factor in lung injury caused by intestinal ischaemia-reperfusion. Br J Surg. 2001;88:464-468. |
| 4. | Koike K, Moore FA, Moore EE, Read RA, Carl VS, Banerjee A. Gut ischemia mediates lung injury by a xanthine oxidase-dependent neutrophil mechanism. J Surg Res. 1993;54:469-473. |
| 5. | Tsuzuki H, Tani T, Ueyama H, Kodama M. Lipopoly-saccharide: neutralization by polymyxin B shuts down the signaling pathway of nuclear factor kappaB in peripheral blood mononuclear cells, even during activation. J Surg Res. 2001;100:127-134. |
| 6. | Shoji H. Extracorporeal endotoxin removal for the treatment of sepsis: endotoxin adsorption cartridge (Toraymyxin). Ther Apher Dial. 2003;7:108-114. |
| 7. | Tani T, Hanasawa K, Kodama M, Imaizumi H, Yonekawa M, Saito M, Ikeda T, Yagi Y, Takayama K, Amano I. Correlation between plasma endotoxin, plasma cytokines, and plasminogen activator inhibitor-1 activities in septic patients. World J Surg. 2001;25:660-668. |
| 8. | Vincent JL, Laterre PF, Cohen J, Burchardi H, Bruining H, Lerma FA, Wittebole X, De Backer D, Brett S, Marzo D. A pilot-controlled study of a polymyxin B-immobilized hemoperfusion cartridge in patients with severe sepsis secondary to intra-abdominal infection. Shock. 2005;23:400-405. |
| 9. | Kushi H, Miki T, Okamaoto K, Nakahara J, Saito T, Tanjoh K. Early hemoperfusion with an immobilized polymyxin B fiber column eliminates humoral mediators and improves pulmonary oxygenation. Crit Care. 2005;9:R653-R661. |
| 10. | Oshima K, Akao T, Kobayashi K, Muraoka M, Matsumoto K, Takeyoshi I. The effect of direct hemoperfusion with a polymyxin B-immobilized fiber column (DHP-PMX therapy) on pulmonary ischemia-reperfusion injury in a canine model. J Invest Surg. 2008;21:127-132. |
| 11. | Iwanami K, Takeyoshi I, Ohwada S, Kobayashi J, Kawata K, Matsumoto K, Morishita Y. Intramucosal pH and intestinal mucosal damage in ischemia-reperfusion injury. Transpl Int. 1998;11:401-407. |
| 12. | King RC, Binns OA, Rodriguez F, Kanithanon RC, Daniel TM, Spotnitz WD, Tribble CG, Kron IL. Reperfusion injury significantly impacts clinical outcome after pulmonary transplantation. Ann Thorac Surg. 2000;69:1681-1685. |
| 13. | Cohen J, Aslam M, Pusey CD, Ryan CJ. Protection from endotoxemia: a rat model of plasmapheresis and specific adsorption with polymyxin B. J Infect Dis. 1987;155:690-695. |
| 14. | Aoki H, Kodama M, Tani T, Hanasawa K. Treatment of sepsis by extracorporeal elimination of endotoxin using polymyxin B-immobilized fiber. Am J Surg. 1994;167:412-417. |
| 15. | Tetta C, Gianotti L, Cavaillon JM, Wratten ML, Fini M, Braga M, Bisagni P, Giavaresi G, Bolzani R, Giardino R. Coupled plasma filtration-adsorption in a rabbit model of endotoxic shock. Crit Care Med. 2000;28:1526-1533. |
| 16. | Uriu K, Osajima A, Hiroshige K, Watanabe H, Aibara K, Inada Y, Segawa K, Anai H, Takagi I, Ito A. Endotoxin removal by direct hemoperfusion with an adsorbent column using polymyxin B-immobilized fiber ameliorates systemic circulatory disturbance in patients with septic shock. Am J Kidney Dis. 2002;39:937-947. |
| 17. | Kawamata T, Imaizumi H, Yoshida M, Kaneko M. Polymyxin B-immobilized fiber improves hyperdynamic state in MRSA septic patients. Intensive Care Med. 1997;23:130-131. |
| 18. | Kushi H, Nakahara J, Miki T, Okamoto K, Saito T, Tanjo K. Hemoperfusion with an immobilized polymyxin B fiber column inhibits activation of vascular endothelial cells. Ther Apher Dial. 2005;9:303-307. |
| 19. | Ohki S, Oshima K, Takeyoshi I, Matsumoto K, Morishita Y. Endotoxin removal with a polymyxin B-immobilized hemoperfusion cartridge improves cardiopulmonary function after cardiopulmonary bypass. J Surg Res. 2008;145:74-79. |
| 20. | Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815-3822. |
| 21. | Dimopoulou I, Armaganidis A, Douka E, Mavrou I, Augustatou C, Kopterides P, Lyberopoulos P, Tzanela M, Orfanos SE, Pelekanou E. Tumour necrosis factor-alpha (TNFalpha) and interleukin-10 are crucial mediators in post-operative systemic inflammatory response and determine the occurrence of complications after major abdominal surgery. Cytokine. 2007;37:55-61. |
| 22. | Wu B, Iwakiri R, Ootani A, Fujise T, Tsunada S, Fujimoto K. Platelet-activating factor promotes mucosal apoptosis via FasL-mediating caspase-9 active pathway in rat small intestine after ischemia-reperfusion. FASEB J. 2003;17:1156-1158. |
| 23. | Zingarelli B, Yang Z, Hake PW, Denenberg A, Wong HR. Absence of endogenous interleukin 10 enhances early stress response during post-ischaemic injury in mice intestine. Gut. 2001;48:610-622. |
Peer reviewer: Yuji Naito, Professor, Kyoto Prefectural University of Medicine, Kamigyo-ku, Kyoto 602-8566, Japan
S- Editor Li DL L- Editor Negro F E- Editor Yin DH