Published online Sep 14, 2008. doi: 10.3748/wjg.14.5301
Revised: July 3, 2008
Accepted: July 10, 2008
Published online: September 14, 2008
AIM: To provide a specific review and meta-analysis of the available evidence for continuous wound infusion of local anaesthetic agents following midline laparotomy for major colorectal surgery.
METHODS: Medline, Embase, trial registries, conference proceedings and article reference lists were searched to identify randomised, controlled trials of continuous wound infusion of local anaesthetic agents following colorectal surgery. The primary outcomes were opioid consumption, pain visual analogue scores (VASs), return to bowel function and length of hospital stay. Weighted mean difference were calculated for continuous outcomes.
RESULTS: Five trials containing 542 laparotomy wounds were eligible for inclusion. There was a significant decrease in post-operative pain VAS at rest on day 3 (weighted mean difference: -0.43; 95% CI: -0.81 to -0.04; P = 0.03) but not on post-operative day 1 and 2. Local anaesthetic infusion was associated with a significant reduction in pain VAS on movement on all three post-operative days (day 1 weighted mean difference: -1.14; 95% CI: -2.24 to -0.041; P = 0.04, day 2 weighted mean difference: -0.97, 95% CI: -1.91 to -0.029; P = 0.04, day 3 weighted mean difference: -0.61; 95% CI: 1.01 to -0.20; P = 0.0038). Local anaesthetic wound infusion was associated with a significant decrease in total opioid consumption (weighted mean difference: -40.13; 95% CI: -76.74 to -3.53; P = 0.03). There was no significant decrease in length of stay (weighted mean difference: -20.87; 95% CI: -46.96 to 5.21; P = 0.12) or return of bowel function (weighted mean difference: -9.40; 95% CI: -33.98 to 15.17; P = 0.45).
CONCLUSION: The results of this systematic review and meta-analysis suggest that local anaesthetic wound infusion following laparotomy for major colorectal surgery is a promising technique but do not provide conclusive evidence of benefit. Further research is required including cost-effectiveness analysis.
- Citation: Karthikesalingam A, Walsh SR, Markar SR, Sadat U, Tang TY, Malata CM. Continuous wound infusion of local anaesthetic agents following colorectal surgery: Systematic review and meta-analysis. World J Gastroenterol 2008; 14(34): 5301-5305
- URL: https://www.wjgnet.com/1007-9327/full/v14/i34/5301.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.5301
| Outcome measure | Weighted mean difference | 95% CI | P | Heterogeneity | Bias |
| Opioid consumption | |||||
| Total | -40.13 | -76.74 to -3.53 | 0.03 | P = 0.02 | P = 0.27 |
| Postoperative day 1 | -8.34 | -16.38 to -0.31 | 0.04 | P = 0.019 | P = 0.48 |
| Postoperative day 2 | -9.41 | -20.37 to 1.39 | 0.087 | NA | NA |
| Postoperative day 3 | -4.8 | -11.72 to 2.13 | 0.17 | NA | NA |
| Visual analogue pain score at rest | |||||
| Postoperative day 1 | -0.18 | -1.31 to 0.95 | 0.75 | P < 0.05 | P = 0.80 |
| Postoperative day 2 | -0.20 | -1.06 to 0.66 | 0.65 | P < 0.05 | P = 0.47 |
| Postoperative day 3 | -0.43 | -0.81 to -0.044 | 0.029 | NA | P = 0.63 |
| Visual analogue pain score on coughing or movement | |||||
| Postoperative day 1 | -1.14 | -2.24 to -0.041 | 0.04 | NA | NA |
| Postoperative day 2 | -0.97 | -1.91 to -0.029 | 0.04 | NA | NA |
| Postoperative day 3 | -0.61 | 1.01 to -0.20 | 0.0038 | NA | NA |
| Duration of hospital stay | -20.87 | -46.94 to 5.21 | 0.12 | P = 0.016 | P = 0.30 |
| Time to return of bowel function | -9.4 | -33.98 to 15.17 | 0.45 | NA | NA |
Open surgery comprising colonic resection and primary bowel anastomosis accounts for up to a third of elective general surgical admissions[1]. The control of pain following these operations represents a major challenge as highly complex nociceptive pathways are involved[2-4]. Pain control following abdominal laparotomy and bowel anastomosis is therefore not amenable to pharmacological monotherapy and modern analgesic strategies following major colorectal surgery involve the combination of many agents including parenteral opiates, nonsteroidal anti-inflammatory drugs (NSAIDs), paracetamol and epidural infusion techniques[5].
Unfortunately, there is no ideal analgesic regimen - all current techniques have disadvantages in the form of important side-effects, cost, patient compliance, procedural complications and delays in discharge[6]. Suboptimal post-operative pain control is of great clinical consequence and has been associated with cardiovascular and respiratory complications and increased gastrointestinal paralysis[5].
A recent systematic review[7] has revealed the promise of continuous wound infusion of local anaesthetic agents to provide improved pain control following thoracic[8-10], abdominal[11-13], gynaecological[14-16], and orthopaedic[17-19] operations, but there is a need for a more focused review of the evidence specific to colorectal laparotomy.
An electronic search was performed using the Embase and Medline databases from 1966 until 2007. The search terms “postoperative pain”, “postoperative analgesia”, “local anesthetics”, “continuous”, “infusion”, “perfusion”, “irrigation”, “patient-controlled”, and MeSH headings “Colorectal Surgery” (MeSH), “Laparotomy” (MeSH), were used in combination with the Boolean operators AND or OR. Two authors independently performed electronic searches in March 2008. The electronic search was supplemented by a hand search of published abstracts from meetings of the Surgical Research Society, the Society of Academic and Research Surgery, the American Society of Anesthesiologists, the Anaesthetic Research Society and the Association of Surgeons of Great Britain and Ireland from 1980 to 2007. The reference lists of articles obtained were also searched to identify further relevant citations. Finally, the search included the Current Controlled Trials Register (http://www.controlled-trials.com) and the Cochrane Database of Controlled Trials.
Abstracts of the citations identified by the search were then scrutinised by two observers (SRW and AK) in order to determine eligibility for inclusion in the meta-analysis. Studies were included if they met each of the following criteria: randomised controlled trial, patients undergoing midline laparotomy for colorectal surgery, randomisation to groups with or without continuous wound infusion of local anaesthetic.
The primary outcome measure for the meta-analysis was the opioid consumption in each arm. Data from eligible trials were entered into a computerized spreadsheet for analysis. The quality of each trial was assessed using the Jadad scoring system[20]. The statistical analysis was performed using Statsdirect 2.5.7 (Statsdirect Ltd., UK). Weighted mean difference were calculated for the effect of local anaesthetic infusion on opioid consumption and linear analogue pain scores on post-operative days 1, 2 and 3. Further pooled outcome measures were duration of hospital stay and time to return of bowel function. All pooled outcome measures were determined using random-effects models as described by Der Simonian and Laird[21]. Heterogeneity amongst the trials was assessed by Cochran’s Q statistic, a null hypothesis test in which P < 0.05 is taken to indicate the presence of significant heterogeneity. The Egger test was used to assess the funnel plot for significant asymmetry, indicating possible publication or other biases.
The initial search identified 590 papers. After screening, 5 randomised controlled trials were identified[22-26]. The five trials included 542 laparotomy wounds, of which 259 were randomised to infusion of local anaesthetic agents.
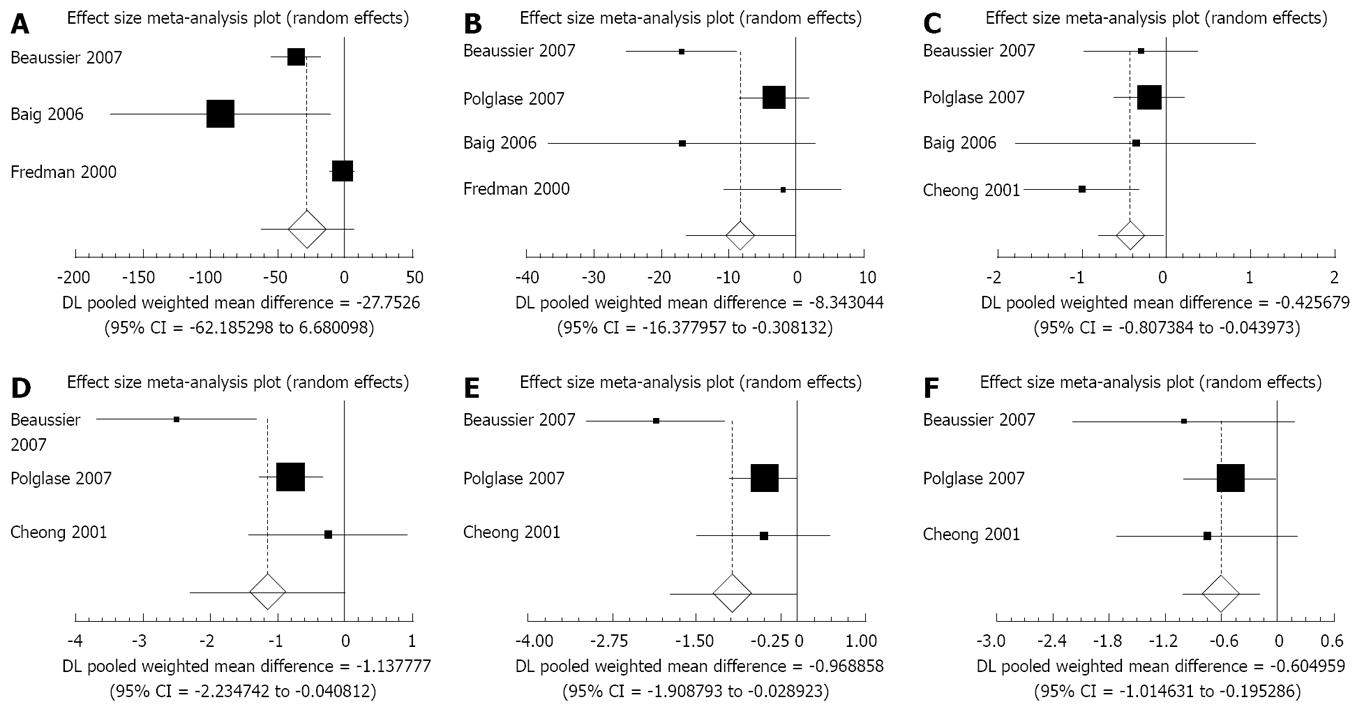
Opioid consumption: Four of the five trials reported total opioid consumption with or without local anaesthetic wound infusions[22-25] (Figure 1A). Local anaesthetic wound infusion was associated with a significant decrease in total opioid consumption (weighted mean difference: -40.13; 95% CI: -76.74 to -3.53; P = 0.03). This outcome measure was associated with significant statistical heterogeneity (Cochran’s Q = 45.31, P = 0.02) but not significant bias (Egger Test = -4.69, P = 0.27).
Four of the five trials reported separate data for opioid consumption with or without local anaesthetic wound infusion on post-operative day 1[22,23,25,26] (Figure 1B). Local anaesthetic wound infusion was associated with a significant decrease in opioid consumption on post-operative day 1 (weighted mean difference: -8.34; 95% CI: -16.38 to -0.31; P = 0.04). There was significant statistical heterogeneity (Cochran’s Q = 9.98, P = 0.019) but not significant bias (Egger test: -2.11, P = 0.48).
Three trials reported opioid consumption on post-operative days 2 and 3[22,23,26] (Table 1). There was no significant effect on opioid consumption (d 2 weighted mean difference: -9.49; 95% CI: -20.37 to 1.39; P = 0.087; day 3 weighted mean difference: -4.80; 95% CI: -11.72 to 2.13; P = 0.17). Two trials did not report this outcome measure rendering calculation of statistical heterogeneity or bias impossible.
Four of the five trials reported visual analogue scores (VASs) of pain on post-operative days 1, 2 and 3[22-24,26]. Post-operative pain was reduced with local anaesthetic infusion on d 1 and 2 but the difference was not significant (Table 1) (d 1 weighted mean difference: -0.18; 95% CI: -1.31 to 0.95; P = 0.75 and d 2 weighted mean difference: -0.20; 95% CI: -1.06 to 0.66; P = 0.65). However, these outcome measures were associated with significant statistical heterogeneity (Cochran’s Q 18.15 and 15.42, P < 0.05). The use of local anaesthetic wound infusions was associated with a significant decrease in post-operative pain at rest on d 3 (Figure 1C) (weighted mean difference: -0.43; 95% CI: -0.81 to -0.044; P = 0.0288). There was no evidence of bias for days 1, 2 or 3 (day 1 Egger test 0.99, P = 0.80; day 2 Egger test 2.75, P = 0.47; day 3 Egger test -1.00, P = 0.63).
Three of the five trials reported pain VAS on coughing or movement, grouped for this analysis as a composite endpoint[23,24,26]. Local Anaesthetic infusion was associated with a significant reduction in pain VAS on all three post-operative days (Figures 1D to F) (day 1 weighted mean difference: -1.14; 95% CI: -2.24 to -0.041; P = 0.04, day 2 weighted mean difference: -0.97, 95% CI: -1.91 to -0.029; P = 0.04, day 3 weighted mean difference: -0.61; 95% CI: 1.01 to -0.20; P = 0.0038). Two trials did not report this pain on movement, rendering calculation of statistical heterogeneity or bias impossible.
All five trials reported length of stay. There was no significant decrease in length of stay (Table 1) (weighted mean difference: -20.87; 95% CI: -46.96 to 5.21; P = 0.12). This outcome measure was associated with significant statistical heterogeneity (Cochran’s Q: 12.20, P = 0.016) without significant bias (Egger test: -1.12, P = 0.30).
Mean time to production of faeces was reported by three trials[22-24]. There was no significant effect of local anaesthetic wound infusion (Table 1) (weighted mean difference: -9.40; 95% CI: -33.98 to 15.17; P = 0.45). Two trials did not report this outcome measure rendering calculation of statistical heterogeneity or bias impossible.
The results of our meta-analysis suggest that wound infusions are a promising adjunct to existing analgesic regimens following laparotomy for major colorectal surgery. The results do not, however, provide conclusive evidence of significant benefit conferred by this technique and it is doubtful whether the data gathered are sufficient to support generalisation of this conclusion to routine practice. The number of eligible trials (5) and total abdominal wounds (542) is small, and meta-analyses on small samples may be vulnerable to confounding if one or two of the eligible trials demonstrate a strong trend for or against the intervention under investigation.
For the purpose of this meta-analysis, the outcome measure “opioid consumption” was chosen to reflect opioid-sparing effect provided by local anaesthetic infusions. However, the significant statistical heterogeneity associated with this outcome measure reflects a variety of background analgesic regimens used in both control and treatment groups. Polglase et al utilized a multimodal analgesic regimen whereas the other trials studied used only patient controlled opioid analgesia to provide background analgesia. This degree of methodological heterogeneity between the trials may have influenced the meta-analysis.
Analysis of pain VAS may also have been affected by methodological heterogeneity between the trials studied. Furthermore, pain VAS is a non-parametric variable whereas the meta-analysis models used assume parametric distribution of the variables under study. The variable “length of stay” reflects a composite endpoint that may have been affected by several factors other than the presence of local anaesthetic infusions, and therefore it is not possible to draw causative inferences from the results of this pooled outcome measure with great validity. It was not possible to obtain sufficient data for all the trials under study to provide a reliable analysis of return to bowel function.
An economic analysis of local anaesthetic wound infusions is also needed - it seems likely that a greater amount of data is needed to clarify any trends in post-operative complications that may support the use of these infusions. Further large randomised controlled trials are required to investigate the promise of local anaesthetic wound infusions in major colorectal surgery, using standardized local anaesthetic agents, background analgesic regimens, experimental protocols, discharge criteria and anatomical site for wound infusion delivery.
In conclusion, Although suboptimal postoperative pain control is associated with cardiovascular, respiratory and gastrointestinal complications, many multimodal regimens for analgesia following major colorectal laparotomy provide inadequate pain relief. Although the number of trials available for meta-analysis is small, the available data demonstrate potential benefit in terms of reduction in opioid consumption following laparotomy for major colorectal surgery. Further large-scale studies will be needed to ascertain if any clear benefit or harm is conferred by the prophylactic use of local anaesthetic wound infusions in major colorectal surgery. Future research on this topic should also address the inaccuracies introduced by the methodological heterogeneity pre-viously addressed in available trials, and provide a cost-effectiveness analysis of the use of continuous wound infusions in colorectal surgery.
Pain control following abdominal laparotomy and bowel anastomosis in colorectal surgery is a complex challenge not amenable to pharmacological monotherapy. Modern multimodal analgesic regimens may provide suboptimal post-operative pain control, which is associated with cardiovascular and respiratory complications and increased gastrointestinal paralysis.
Continuous wound infusions of local anaesthetic agents have been suggested to provide improved pain control following a broad range of surgical incisions, both alone and as part of a multimodal analgesic regimen.
There is a need for a focused and quantitative review of the evidence for the analgesic benefit of continuous wound infusion of local anaesthetics specific to colorectal laparotomy, which is provided by this meta-analysis.
The meta-analysis demonstrates potential benefit in terms of reduction in opioid consumption following laparotomy for major colorectal surgery. The review highlights the need for future research on this topic and identifies that such future research should address the inaccuracies introduced by the methodological heterogeneity identified in available trials, and provides a cost-effectiveness analysis of the use of continuous wound infusions in colorectal surgery.
Visual analogue scale (VAS) is a validated research tool used to quantitatively assess the subjective experience of patients’ pain perception.
The authors present a systematic analysis of local anaesthetics in wounds after open colorectal surgery. This is an area that has not been addressed by systematic analysis previously. It is a well-done and timely review.
| 1. | Gendall KA, Kennedy RR, Watson AJ, Frizelle FA. The effect of epidural analgesia on postoperative outcome after colorectal surgery. Colorectal Dis. 2007;9:584-598; discussion 598-600. |
| 2. | Kehlet H. Surgical stress: the role of pain and analgesia. Br J Anaesth. 1989;63:189-195. |
| 3. | Stein C. Peripheral mechanisms of opioid analgesia. Anesth Analg. 1993;76:182-191. |
| 4. | Dahl JB, Kehlet H. Non-steroidal anti-inflammatory drugs: rationale for use in severe postoperative pain. Br J Anaesth. 1991;66:703-712. |
| 5. | PROSPECT working group. Procedure specific post-operative pain management: open colonic resection. Available from: URL: http: //www.postoppain.org/frameset.htm Accessed 28th January 2008; . |
| 6. | Kehlet H, Liu SS. Continuous local anesthetic wound infusion to improve postoperative outcome: back to the periphery? Anesthesiology. 2007;107:369-371. |
| 7. | Liu SS, Richman JM, Thirlby RC, Wu CL. Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: a quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg. 2006;203:914-932. |
| 8. | Barron DJ, Tolan MJ, Lea RE. A randomized controlled trial of continuous extra-pleural analgesia post-thoracotomy: efficacy and choice of local anaesthetic. Eur J Anaesthesiol. 1999;16:236-245. |
| 9. | Dowling R, Thielmeier K, Ghaly A, Barber D, Boice T, Dine A. Improved pain control after cardiac surgery: results of a randomized, double-blind, clinical trial. J Thorac Cardiovasc Surg. 2003;126:1271-1278. |
| 10. | Francois T, Blanloeil Y, Pillet F, Moren J, Mazoit X, Geay G, Douet MC. Effect of interpleural administration of bupivacaine or lidocaine on pain and morphine requirement after esophagectomy with thoracotomy: a randomized, double-blind and controlled study. Anesth Analg. 1995;80:718-723. |
| 11. | Chester JF, Ravindranath K, White BD, Shanahan D, Taylor RS, Lloyd-Williams K. Wound perfusion with bupivacaine: objective evidence for efficacy in postoperative pain relief. Ann R Coll Surg Engl. 1989;71:394-396. |
| 12. | Lau H, Patil NG, Lee F. Randomized clinical trial of postoperative subfascial infusion with bupivacaine following ambulatory open mesh repair of inguinal hernia. Dig Surg. 2003;20:285-289. |
| 13. | Levack ID, Holmes JD, Robertson GS. Abdominal wound perfusion for the relief of postoperative pain. Br J Anaesth. 1986;58:615-619. |
| 14. | Fredman B, Shapiro A, Zohar E, Feldman E, Shorer S, Rawal N, Jedeikin R. The analgesic efficacy of patient-controlled ropivacaine instillation after Cesarean delivery. Anesth Analg. 2000;91:1436-1440. |
| 15. | Gupta A, Perniola A, Axelsson K, Thorn SE, Crafoord K, Rawal N. Postoperative pain after abdominal hysterectomy: a double-blind comparison between placebo and local anesthetic infused intraperitoneally. Anesth Analg. 2004;99:1173-1179, table of contents. |
| 16. | Kushner DM, LaGalbo R, Connor JP, Chappell R, Stewart SL, Hartenbach EM. Use of a bupivacaine continuous wound infusion system in gynecologic oncology: a randomized trial. Obstet Gynecol. 2005;106:227-233. |
| 17. | Alford JW, Fadale PD. Evaluation of postoperative bupivacaine infusion for pain management after anterior cruciate ligament reconstruction. Arthroscopy. 2003;19:855-861. |
| 18. | Barber FA, Herbert MA. The effectiveness of an anesthetic continuous-infusion device on postoperative pain control. Arthroscopy. 2002;18:76-81. |
| 19. | Bianconi M, Ferraro L, Ricci R, Zanoli G, Antonelli T, Giulia B, Guberti A, Massari L. The pharmacokinetics and efficacy of ropivacaine continuous wound instillation after spine fusion surgery. Anesth Analg. 2004;98:166-172, table of contents. |
| 20. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. |
| 21. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. |
| 22. | Baig MK, Zmora O, Derdemezi J, Weiss EG, Nogueras JJ, Wexner SD. Use of the ON-Q pain management system is associated with decreased postoperative analgesic requirement: double blind randomized placebo pilot study. J Am Coll Surg. 2006;202:297-305. |
| 23. | Beaussier M, El'Ayoubi H, Schiffer E, Rollin M, Parc Y, Mazoit JX, Azizi L, Gervaz P, Rohr S, Biermann C. Continuous preperitoneal infusion of ropivacaine provides effective analgesia and accelerates recovery after colorectal surgery: a randomized, double-blind, placebo-controlled study. Anesthesiology. 2007;107:461-468. |
| 24. | Cheong WK, Seow-Choen F, Eu KW, Tang CL, Heah SM. Randomized clinical trial of local bupivacaine perfusion versus parenteral morphine infusion for pain relief after laparotomy. Br J Surg. 2001;88:357-359. |
| 25. | Fredman B, Zohar E, Tarabykin A, Shapiro A, Mayo A, Klein E, Jedeikin R. Bupivacaine wound instillation via an electronic patient-controlled analgesia device and a double-catheter system does not decrease postoperative pain or opioid requirements after major abdominal surgery. Anesth Analg. 2001;92:189-193. |
| 26. | Polglase AL, McMurrick PJ, Simpson PJ, Wale RJ, Carne PW, Johnson W, Chee J, Ooi CW, Chong JW, Kingsland SR. Continuous wound infusion of local anesthetic for the control of pain after elective abdominal colorectal surgery. Dis Colon Rectum. 2007;50:2158-2167. |
Peer reviewer: Conor P Delaney, MD, MCh, PhD, FRCSI, FACS, Professor of Surgery, Case Western Reserve University, Chief, Division of Colorectal Surgery, Vice-Chairman, Department of Surgery, Director, Institute for Surgery and Innovation, University Hospitals, Case Medical Center, 11100 Euclid Avenue Cleveland, Cleveland 44106-5047, United States
S- Editor Li DL L- Editor Lutze M E- Editor Ma WH