Published online Aug 21, 2008. doi: 10.3748/wjg.14.4903
Revised: August 4, 2008
Accepted: August 11, 2008
Published online: August 21, 2008
AIM: To investigate the changing pattern of α-catenin expression and its relationship to clinical and pathological features of colorectal cancer (CRC) patients.
METHODS: Archival tumor samples were analyzed using immunohistochemistry (IHC) for α-catenin in 91 patients with advanced CRC.
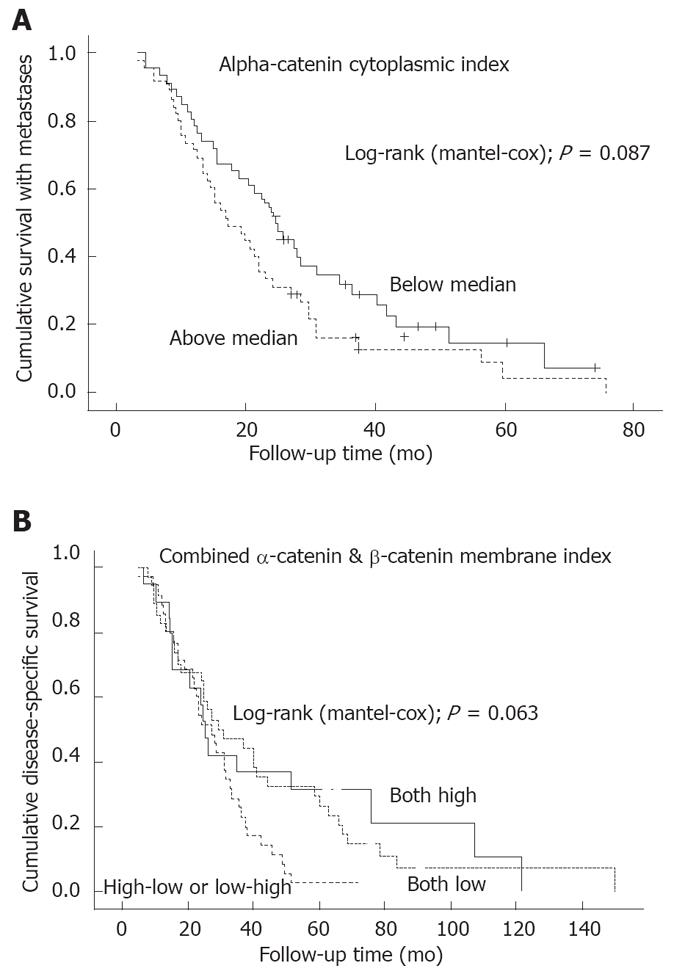
RESULTS: The values of α-catenin membrane index (MI) and cytoplasmic index (CI) were significantly related to the depth of tumor invasion (P = 0.027, P = 0.020, respectively), high indices being associated with increased depth of the primary tumor invasion (T3 and T4). Similarly, patients with high α-catenin expression had a significantly increased risk of lymph node metastasis (32/39 vs 37/52 for MI and 37/45 vs 32/46 for CI) (P = 0.001, P = 0.0001, respectively, for LNN status). An altered expression (i.e., cytoplasmic pattern) was also related (P = 0.047) to the response to chemotherapy; patients with low CI were more responsive (CR: 7/46) than patients with high CI values (CR: 0/45). There was a marginal effect on survival in patients time with metastases (SWM) (P = 0.087); patients with low CI showing slightly longer SWM, but no such effect on disease free survival (DFS) or disease specific survival (DSS). As to co-expression with another member of the adhesion complex (β-catenin), high α-catenin/β-catenin MI index was of marginal significance in predicting longer DSS (P = 0.063, log-rank).
CONCLUSION: The results implicate that high α-catenin expression is intimately involved in the key regulatory mechanisms leading to invasive phenotype, lymph node metastases, and progressive disease in CRC.
- Citation: Elzagheid A, Buhmeida A, Korkeila E, Collan Y, Syrjänen K, Pyrhönen S. Up-regulation of α-catenin is associated with increased lymph node involvement in colorectal cancer. World J Gastroenterol 2008; 14(31): 4903-4908
- URL: https://www.wjgnet.com/1007-9327/full/v14/i31/4903.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4903
| Variable | n or value | %1 |
| Patients | 91 | |
| Male | 57 | 63.0 |
| Female | 34 | 37.0 |
| Age (yr) | ||
| Median (range) | 60.7 (24-80) | |
| Primary tumour status2 | 91 | |
| T1 | 1 | 1.0 |
| T2 | 6 | 6.5 |
| T3 | 59 | 64.8 |
| T4 | 16 | 17.6 |
| Tx | 9 | 9.8 |
| Primary nodal status2 | 91 | |
| N0 | 22 | 24.0 |
| N+ | 49 | 54.0 |
| Nx | 20 | 22.0 |
| Metastases at diagnosis | 91 | |
| M0 | 34 | 37.0 |
| M1 | 57 | 63.0 |
| Histological grade | 91 | |
| Gr I | 11 | 12.1 |
| Gr II | 62 | 68.1 |
| Gr III | 18 | 19.8 |
| Stage | 91 | |
| Stage II | 14 | 15.0 |
| Stage III | 19 | 21.0 |
| Stage IV | 58 | 64.0 |
| Survival (mo) | ||
| From primary diagnosis | ||
| Median (range) | 27.3 (3-150) | |
| From metastasis | ||
| Median (range) | 21.5 (3-80) |
Under normal conditions, cell-cell adhesion molecules maintain epithelial cell integrity and cellular architecture. The process of tumor invasion and metastasis is associated with alterations in the functions of several adhesion molecules. In general, tumor cells lose their capacity for normal adherence, which facilitates their detachment from their site of origin[1,2].
Homotypic cell-cell adhesion is regulated mostly by the cadherin-catenin complex. Alterations in E-cadherin and catenins have been linked with more aggressive behavior of several human tumors[3-6]. Catenins are divided according to their molecular weight as α-catenin (102 kDa), β-catenin (88 kDa) and γ-catenin (80 kDa)[7-9].
α-catenin links E-cadherin to the actin cytoskeleton via its association with either β- or γ-catenin[10,11]. Abnormal α-catenin expression has been reported in many human cancers[12-15]. Reduced α-catenin expression was associated with tumor invasion and metastases in colorectal cancer (CRC)[6].
In this study, we examined α-catenin expression in a series of CRC, and analyzed the relationship with clinical and pathological features of CRC patients.
The material of the present study consists of a series of 91 patients with advanced CRC, enrolled among the consecutive CRC patients attending our clinic for therapeutic procedures. Of these 91 patients, 58 had metastases at diagnosis (Stage IV disease), while the remaining 33 patients (with stage II and III disease at baseline) subsequently developed a metastatic disease during the mean follow-up (FU) time of 25.1 ± 27.8 mo. All patients were treated for advanced and metastatic disease at the Department of Oncology and Radiotherapy, Turku University Hospital, according to the protocols used for CRC patients with stage II, III or IV disease at that time. These 91 patients included in the present study were enrolled into this prospective cohort between October 1998 and August 2003. All patients have been prospectively followed-up until death or when last seen alive at their clinical visit (March 2007), with the median FU-time of 27.6 mo (range 3-150 mo). The study was approved by the TUH Ethics Committee and was conducted in accordance with the Declaration of Helsinki. Samples were collected with the endorsement of the National Authority for Medico-legal Affairs.
The key clinical data of the patients are shown in Table 1. Of these 91 cases, 34 were women and 57 were men. The mean age was 61.5 years (range 24-78 years). The majority (n = 38) of the tumors were localized in the left colon, followed in order of frequency by the right colon (n = 23), rectum (n = 22), and colon transversum (n = 7). At the time of diagnosis, 14 patients had Stage II disease, 19 Stage III and 58 tumors were at Stage IV. The majority ( = 59, 64.8%) were T3 tumors, and almost half (n = 46) had known lymph node involvement at the time of diagnosis, including the cases with Nx status. The patients were selected into the cohort on the basis of both the diagnosis and treatment received, and were assigned to one of the two treatment arms: (1) 20 were treated with irinotecan alone; and (2) 71 received a combination of irinotecan and 5-fluorouracil (5-FU) as the first line treatment.
Formalin-fixed, paraffin-embedded primary colorectal tumor tissue was obtained from 91 patients. Sections were cut serially at 5μm for routine haematoxylin and eosin staining and for immunohistochemical (IHC) analysis. An experienced pathologist confirmed all histological diagnoses. IHC analysis was done using the automatic system (BenchMark XT, Ventana Medical Systems, Inc. Tucson, Arizona, USA). This fully automated processing of bar code labeled slides included baking of the slides, solvent free deparaffinization, antigen retrieval in a cell conditioning buffer CC2 (Mild: 36 min conditioning, and standard: 60 min conditioning), incubation with the monoclonal mouse α-catenin antibody (clone α CAT-7A4, isotype IgG1-κ, Zymed Laboratories, San Franscisco, CA), at a dilution 1:100 (32 min, 37°C). The dilution of the primary antibody was based on dilution experiments. Application of ultraViewTM Universal DAB (a biotin-free, Multimer-based detection system for the specific and sensitive detection of mouse IgG, mouse IgM, and rabbit IgG primary antibodies). UltraView DAB includes: ultraView Universal HRP, ultraView Universal DAB Inhibitor, ultraView Universal DAB Chromogen, ultraView Universal DAB H2O2, and ultraView Universal DAB Copper. Counterstaining with haematoxylin (2021) took 4 min, and post-counterstaining with bluing reagent (2037) took 4 min as well. After staining, the sections were dehydrated in ethanol, cleared in xylene, and covered with Mountex and cover slips.
The α-catenin staining was evaluated by observers blinded to the clinical data using regular light microscopy. Membranous and cytoplasmic expression was evaluated separately. For the cell membrane staining, four categories were used, (+++, ++, +, -), starting from equivalent to normal to entirely negative[16]. The cytoplasmic staining was also graded into four categories: (0) Negative, no detectable staining; (1) Weak, but still detectable staining; (2) Moderate, clearly positive but still weak; (3) Heavy staining, intense[17]. In calculating the staining indexes: membrane index (MI), and cytoplasmic index (CI), both the intensity of staining and the fraction of positively-stained cells were taken into account, using the following formula: Ι = 0 * f0 + 1 * f1 + 2 * f2 + 3 * f3, where I is the staining index, f0-f3 are the fractions of the cells showing a defined level of staining intensity (from 0 to 3). Theoretically, the index could vary between 0 and 3n[18]. The α-catenin expression was evaluated independently by two observers (AE, AB). The agreement of the evaluation of α-catenin staining indices was tested between two observers, and the agreement was good as suggested by the correlation coefficient (Pearson’s r: MI, CI, and NI were 0.77, 0.91, and 0.90, respectively, P < 0.001).
Statistical analyses were performed using the SPSS® (SPSS, Inc., Chicago, USA) and STATA (Stata Corp., Texas, USA) software packages (SPSS for Windows, version 14.0.1 and STATA/SE 9.2). Frequency tables were analyzed using the χ2 test, which evaluation included likelihood ratio (LR), or Fischer's exact test to assess the significance of the correlation between the categorical variables. Odds ratios and their 95% confidence intervals (95%CI) were calculated where appropriate, using the exact method. Differences in the means of continuous variables were analyzed using non-parametric tests (Mann-Whitney or Kruskal-Wallis) for 2- and K-independent samples, respectively. ANOVA (analysis of variance) was only used for deriving the mean values (and their SD) of each individual category. Univariate survival (life-table) analysis for the outcome measure (disease specific survival, DSS; disease free survival, DFS) was based on Cox's method (indices treated as continuous variables), and/or using Kaplan-Meier analysis (indices with Median as cut-off). In all tests, P < 0.05 was regarded statistically significant.
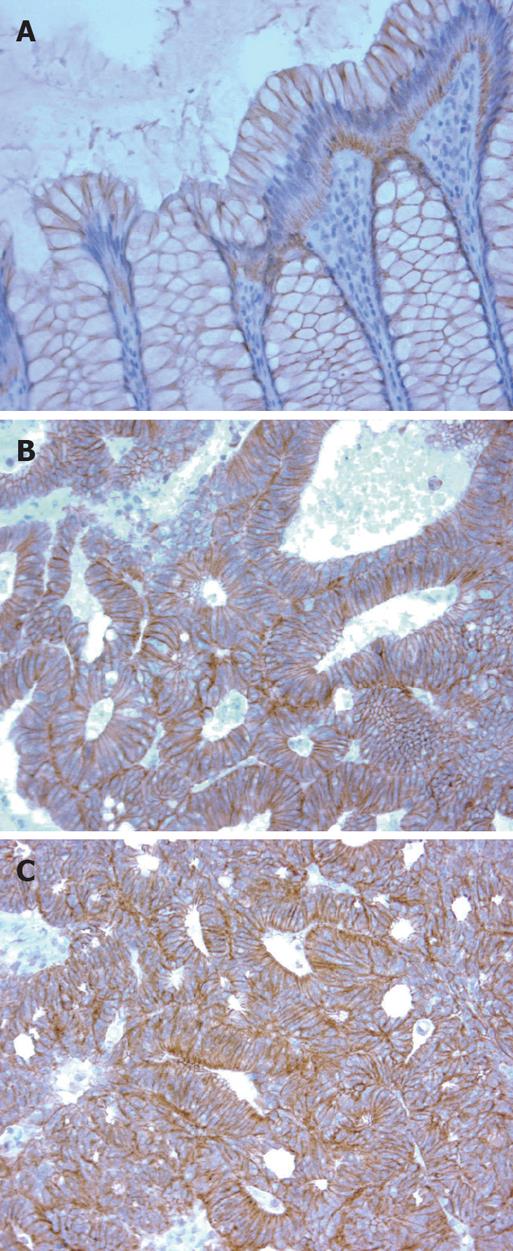
In normal colonic mucosa, α-catenin expression was predominantly membranous but this pattern was disturbed (diffuse cytoplasmic and membranous) in the tumor tissues (Figure 1). The mean values of MI and CI were 1.3 and 1.1, respectively.
The two expression patterns of α-catenin were related to all clinical and tumor variables recorded in this series. MI was significantly (P = 0.03) related to the localization of the primary tumor, being more intense in carcinomas of the descending colon (n = 38) and rectum (n = 22) than in those of the ascending (n = 23) and transverse colon (n = 7). Both MI and CI were also correlated with the depth of the primary tumor (ANOVA; P = 0.027, P = 0.020, respectively), higher mean index values being associated with increased depth of the primary tumor invasion (i.e., T3 and T4). Interestingly, patients with high α-catenin expression (MI, CI) had a significantly increased lymph node metastasis (32/39 vs 37/52 for MI and 37/45 32/46 for CI) (P = 0.001, P = 0.0001, respectively). On the other hand, there was no correlation between α-catenin expression and most of the clinical variables (e.g. age, sex, and stage). However, there was a marginal relation between MI and CI (P = 0.08, P = 0.07, respectively) and the grade of the primary tumor.
Cytoplasmic α-catenin expression was also significantly (P = 0.04) related to the response to treatment in that the patients with low CI were more responsive (CR: 7/46) than patients with high CI values (CR: 0/45). No such association was established for the MI.
In univariate survival analysis, neither MI nor CI was a significant predictor of disease outcome. As to the survival with metastases, there was a marginal difference in the survival curves, patients with low CI showing slightly longer survival with metastases (SWM) (Figure 2A). Finally, when α-catenin expression was analyzed jointly with β-catenin expression (with the combined MI and CI indices built up using the same criteria as described for α-catenin), only the combined MI index was of marginal prognostic value in predicting DSS, those with high MI surviving longer (Figure 2B).
As compared with the sub-cellular distribution of α-catenin in normal colonic mucosa, neoplastic cells demonstrated a distinct shift from the membranous localization to more widespread distribution (membranous, cytoplasmic) in cancer cells. This is in line with the previous reports describing this type of altered pattern of α-catenin expression in cancer cells[19,20].
Interestingly, α-catenin expression (MI) was more intense in carcinomas of the descending colon and rectum as compared with the lesions localized in the ascending and transverse colon. Similar observation was reported for β-catenin[21,22], but not for α-catenin. There is increasing evidence to suggest that molecular mechanisms and molecular phenotypes differ in carcinomas arising in the proximal and distal segments of the large bowel[23]. The involvement of different molecular pathways in colorectal carcinogenesis is exemplified by the fact that cancers of “mutator” phenotypes preferentially occur in the proximal colon, whereas the adenoma-to-carcinoma sequence phenotype is characteristic of carcinomas in the distal colon and rectum[24,25]. Corresponding differences have also been demonstrated with other potential prognosticators[26].
The correlation between α-catenin expression pattern and the TNM categories is a controversial issue. Some studies report that there is no correlation between α-catenin and these clinicopathological variables[3]. On the other hand, Gofuku et al 1999 demonstrated that reduced expression of α-catenin was significantly correlated with the depth of invasion. Moreover, the frequency of lymph node metastases was significantly higher in those tumors with reduced α-catenin expression[6]. Our data show that higher indices in both (MI, CI) have a correlation with increased depth of tumor invasion and to increased lymph node involvement. There are multiple reasons that could explain these inconsistent and in part discrepant results reported in these different studies[3,6]. Such potential confounding factors might include the size of tissue sample, intrinsic tumor heterogeneity, lack of standardization of the positive and negative results, and different immunohistochemical staining and grading methods, with varying degree of sensitivity. In addition, our patients represent advanced CRC, the majority of patients with stage IV disease, as compared e.g. with Gofuku’s material, in which only 24/100 patients had stage IV CRC[6].
To our knowledge, this is the first study to investigate the relation between α-catenin expression and response to treatment. Interestingly, a significant relation was observed between CI and the response to treatment. Accordingly, the patients with low CI were more responsive to treatment than patients with high CI values. The significance of these observations remains to be elucidated in a larger study, however.
As to the relation between α-catenin expression pattern and clinical outcome, a marginally significant association was observed to survival with metastases. When we combined both MI and CI of α-catenin and MI and CI of β-catenin (using 3 categories; high/high; high/low or low/high; low/low), only the combined MI index was of marginal prognostic value, high MI predicting longer DSS. Such information has not been reported previously. As shown by the data (Figure 2B), it seems feasible to speculate that patients with high MI of both α- and β-catenin survive longer, because this is the normal expression pattern of these two adhesion molecules, and retaining this pattern could indicate a less pronounced dedifferentiation of the cancer cells.
Taken together, the present study examined the predictive and prognostic value of α-catenin expression in advanced CRC. Our results substantiate the emerging evidence on different molecular events operating in CRC at different localizations, while demonstrating a significant difference in α-catenin expression between tumors of the proximal and distal sites. High α-catenin expression was typical in advanced invasion of the primary tumour (i.e., T3 and T4) as well as in appearance of LNN metastases. An altered expression (i.e., cytoplasmic) pattern was also related to the response to treatment, and there was some marginal association with the SWM, but not with DFS or DSS. The latter was marginally predicted by the combined (α-catenin/β-catenin) MI, however, patients with high MI showing longer DSS. The results implicate that α-catenin expression is associated with the regulatory mechanisms leading to invasive phenotype and progressive disease in CRC. The full prognostic value of this adhesion molecule as a single marker remains to be established, however, and there is some circumstantial evidence (Figure 2B) that probably more valuable prognostic information could be obtained when α-catenin is combined with the other constituents of the cell adhesion complex, e.g. β-catenin and the cadherins.
Process of tumor invasion and metastasis is associated with alterations in the functions of several adhesion molecules. In general, tumor cells lose their capacity for normal adherence, which facilitates their detachment from their site of origin. α-catenin links E-cadherin to the actin cytoskeleton via its association with either β- or γ-catenin. Reduced α-catenin expression was associated with tumor invasion and metastases in colorectal cancer (CRC). We examined α-catenin expression in a series of 91 patients with advanced colorectal carcinoma, and analyzed the relationship with clinical and pathological features of CRC patients.
CRC is one of the commonest malignant tumours and has a relatively poor prognosis, where the outcome depends on the extent of local and particularly metastatic tumour spread. For example, in locally advanced disease (Dukes C), the 5-year relative survival rate is 65% but goes down to 8% in metastatic disease (Dukes D). Metastatic disease causes the majority of cancer-related deaths, either as a result of tumour involvement of critical organs or due to complications of therapy to control tumour growth and spread. This dramatic difference emphasizes the importance of delineating predictive factors capable of reliably distinguishing CRC patients at risk for developing a metastatic phenotype.
Patients with high α-catenin expression (MI, CI) had a significantly increased lymph node metastasis. There is also significant difference in α-catenin expression between tumors of the proximal and distal sites. Interestingly, α-catenin expression (MI) was more intense in carcinomas of the descending colon and rectum as compared with the lesions localized in the ascending and transverse colon. Similar observation was reported for β-catenin, but not for α-catenin. There is increasing evidence to suggest that molecular mechanisms and molecular phenotypes differ in carcinomas arising in the proximal and distal segments of the large bowel. The involvement of different molecular pathways in colorectal carcinogenesis is exemplified by the fact that cancers of “mutator” phenotypes preferentially occur in the proximal colon, whereas the adenoma-to-carcinoma sequence phenotype is characteristic of carcinomas in the distal colon and rectum. Corresponding differences have also been demonstrated with other potential prognosticators. To our knowledge, this is the first study to investigate the relation between α-catenin expression and response to treatment. Interestingly, a significant relation was observed between CI and the response to treatment. Accordingly, the patients with low CI were more responsive to treatment than patients with high CI values. The significance of these observations remains to be elucidated in a larger study, however.
Valuable prognostic information could be obtained when α-catenin is combined with the other constituents of the cell adhesion complex, e.g. β-catenin and the cadherins.
The manuscript is well written and the results implicate that high α-catenin expression is associated with invasive phenotype, lymph node metastases, and progressive disease in CRC. It includes interesting new data of α-catenin expression in colon carcinomas of different locations. This is also the first study to investigate the relation between α-catenin expression and the response to treatment.
| 1. | Breen E, Steele G Jr, Mercurio AM. Role of the E-cadherin/alpha-catenin complex in modulating cell-cell and cell-matrix adhesive properties of invasive colon carcinoma cells. Ann Surg Oncol. 1995;2:378-385. |
| 2. | Ahmad A, Hart IR. Mechanisms of metastasis. Crit Rev Oncol Hematol. 1997;26:163-173. |
| 3. | Shiozaki H, Iihara K, Oka H, Kadowaki T, Matsui S, Gofuku J, Inoue M, Nagafuchi A, Tsukita S, Mori T. Immunohistochemical detection of alpha-catenin expression in human cancers. Am J Pathol. 1994;144:667-674. |
| 4. | van der Wurff AA, Vermeulen SJ, van der Linden EP, Mareel MM, Bosman FT, Arends JW. Patterns of alpha- and beta-catenin and E-cadherin expression in colorectal adenomas and carcinomas. J Pathol. 1997;182:325-330. |
| 5. | Takayama T, Shiozaki H, Doki Y, Oka H, Inoue M, Yamamoto M, Tamura S, Shibamoto S, Ito F, Monden M. Aberrant expression and phosphorylation of beta-catenin in human colorectal cancer. Br J Cancer. 1998;77:605-613. |
| 6. | Gofuku J, Shiozaki H, Tsujinaka T, Inoue M, Tamura S, Doki Y, Matsui S, Tsukita S, Kikkawa N, Monden M. Expression of E-cadherin and alpha-catenin in patients with colorectal carcinoma. Correlation with cancer invasion and metastasis. Am J Clin Pathol. 1999;111:29-37. |
| 7. | Hugh TJ, Dillon SA, O'Dowd G, Getty B, Pignatelli M, Poston GJ, Kinsella AR. beta-catenin expression in primary and metastatic colorectal carcinoma. Int J Cancer. 1999;82:504-511. |
| 8. | Conacci-Sorrell M, Zhurinsky J, Ben-Ze'ev A. The cadherin-catenin adhesion system in signaling and cancer. J Clin Invest. 2002;109:987-991. |
| 9. | Ozawa M, Kemler R. Molecular organization of the uvomorulin-catenin complex. J Cell Biol. 1992;116:989-996. |
| 10. | Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61:514-523. |
| 11. | Adams CL, Nelson WJ, Smith SJ. Quantitative analysis of cadherin-catenin-actin reorganization during development of cell-cell adhesion. J Cell Biol. 1996;135:1899-1911. |
| 12. | Aaltomaa S, Lipponen P, Ala-Opas M, Eskelinen M, Kosma VM. Alpha-catenin expression has prognostic value in local and locally advanced prostate cancer. Br J Cancer. 1999;80:477-482. |
| 13. | Bohm J, Niskanen L, Kiraly K, Kellokoski J, Eskelinen M, Hollmen S, Alhava E, Kosma VM. Expression and prognostic value of alpha-, beta-, and gamma-catenins indifferentiated thyroid carcinoma. J Clin Endocrinol Metab. 2000;85:4806-4811. |
| 14. | Li CC, Xu B, Hirokawa M, Qian Z, Yoshimoto K, Horiguchi H, Tashiro T, Sano T. Alterations of E-cadherin, alpha-catenin and beta-catenin expression in neuroendocrine tumors of the gastrointestinal tract. Virchows Arch. 2002;440:145-154. |
| 15. | Koksal IT, Ates M, Danisman A, Sezer C, Ciftcioglu A, Karpuzoglu G, Sevuk M. Reduced E-cadherin and alpha-catenin expressions have no prognostic role in bladder carcinoma. Pathol Oncol Res. 2006;12:13-19. |
| 16. | Elzagheid A, Kuopio T, Ilmen M, Collan Y. Prognostication of invasive ductal breast cancer by quantification of E-cadherin immunostaining: the methodology and clinical relevance. Histopathology. 2002;41:127-133. |
| 17. | Elzagheid A, Algars A, Bendardaf R, Lamlum H, Ristamaki R, Collan Y, Syrjanen K, Pyrhonen S. E-cadherin expression pattern in primary colorectal carcinomas and their metastases reflects disease outcome. World J Gastroenterol. 2006;12:4304-4309. |
| 18. | Lipponen P, Collan Y. Simple quantitation of immuno-histochemical staining positivity in microscopy. Acta Stereol. 1992;11:125-132. |
| 19. | El-Bahrawy MA, Poulsom R, Jeffery R, Talbot I, Alison MR. The expression of E-cadherin and catenins in sporadic colorectal carcinoma. Hum Pathol. 2001;32:1216-1224. |
| 20. | El-Bahrawy MA, Talbot IC, Poulsom R, Jeffery R, Alison MR. The expression of E-cadherin and catenins in colorectal tumours from familial adenomatous polyposis patients. J Pathol. 2002;198:69-76. |
| 21. | Baldus SE, Monig SP, Huxel S, Landsberg S, Hanisch FG, Engelmann K, Schneider PM, Thiele J, Holscher AH, Dienes HP. MUC1 and nuclear beta-catenin are coexpressed at the invasion front of colorectal carcinomas and are both correlated with tumor prognosis. Clin Cancer Res. 2004;10:2790-2796. |
| 22. | Feng Han Q, Zhao W, Bentel J, Shearwood AM, Zeps N, Joseph D, Iacopetta B, Dharmarajan A. Expression of sFRP-4 and beta-catenin in human colorectal carcinoma. Cancer Lett. 2006;231:129-137. |
| 23. | Chung DC. The genetic basis of colorectal cancer: insights into critical pathways of tumorigenesis. Gastroenterology. 2000;119:854-865. |
| 24. | Loeb LA. A mutator phenotype in cancer. Cancer Res. 2001;61:3230-3239. |
| 25. | Yashiro M, Carethers JM, Laghi L, Saito K, Slezak P, Jaramillo E, Rubio C, Koizumi K, Hirakawa K, Boland CR. Genetic pathways in the evolution of morphologically distinct colorectal neoplasms. Cancer Res. 2001;61:2676-2683. |
| 26. | Hilska M, Roberts PJ, Collan YU, Laine VJ, Kossi J, Hirsimaki P, Rahkonen O, Laato M. Prognostic significance of matrix metalloproteinases-1, -2, -7 and -13 and tissue inhibitors of metalloproteinases-1, -2, -3 and -4 in colorectal cancer. Int J Cancer. 2007;121:714-723. |
Peer reviewers: Minna Nyström, PhD, Department of Biological and Environmental Sciences, PO Box 56 (Viikinkaari 5 D), University of Helsinki, Helsinki FI-00014, Finland; John K Marshall, MD, Associate Professor of Medicine, Division of Gastroenterology (4W8), McMaster University Medical Centre, 1200 Main Street West, Hamilton, Ontario L8N 3Z5, Canada
S- Editor Li DL L- Editor Li M E- Editor Lin YP