Published online Aug 14, 2008. doi: 10.3748/wjg.14.4816
Revised: July 14, 2008
Accepted: July 21, 2008
Published online: August 14, 2008
AIM: To obtain evidence for selection of antigens used in genetically engineered vaccine against Helicobacter pylori (H pylori).
METHODS: Enzyme linked immunoabsorbent assay (ELISA) was established on the basis of recombinant protein antigens rUreB, rHpaA, rVacA, rCagA1, rNapA, rFlaA and rFlaB of H pylori to detect expression rates of the antigens in bacterial isolates as well as positive rates of the antibodies in sera from H pylori-infected patients. PCR was applied to the detection of carrying rates of the genes encoding antigens in the isolates.
RESULTS: The outputs of rUreB, rHpaA, rVacA, rCagA1, rNapA, rFlaA and rFlaB were approximately 35%, 32%, 15%, 23%, 56%, 25% and 20% of the total bacterial proteins, respectively. One hundred and fifty-one strains of H pylori were isolated from 347 biopsy specimens of chronic gastritis, peptic ulcer or gastric adenocarcinoma, with a positive rate of 43.5%. All of the isolates expressed UreB, HpaA, FlaA and FlaB while 52.3%, 92.1% and 93.4% of the isolates expressed VacA, CagA and NapA, respectively. In the sera of 151 H pylori-infected patients, the positive rates of IgG antibodies against UreB, HpaA, VacA, CagA, NapA, FlaA and FlaB were 100%, 87.4%, 43%, 71.5%, 89.4%, 84.8% and 79.5%, respectively. Furthermore, the expression frequencies of VacA and NapA were found to be relative to the severity of gastric diseases (P = 0.016 and P < 0.0001, respectively).
CONCLUSION: UreB antigen is the top option of developing genetically engineered vaccine against H pylori followed by NapA or HpaA.
-
Citation: Tang RX, Luo DJ, Sun AH, Yan J. Diversity of
Helicobacter pylori isolates in expression of antigens and induction of antibodies. World J Gastroenterol 2008; 14(30): 4816-4822 - URL: https://www.wjgnet.com/1007-9327/full/v14/i30/4816.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4816
| Target genes | Primer sequences (5’-3’) | Reference | Tm (°C) | Products (bp) |
| ureB | F: CGTCCGGCAATAGCTGCCATAGT | [18] | 55 | 463 |
| R: GTAGGTCCTGCTACTGAAGCCTTA | ||||
| hpaA | F: CTTTTAGGTGCGAGCGTGGTG | [19] | 54 | 716 |
| R: AATTCCTTGGCGTCTTTTTGATAA | ||||
| vacA | F: ATGGAAATACAACAAACACACCG | [20] | 54 | 337 |
| R: CAACCTCCATCAATCTTACTGGA | ||||
| CagA | F: ATAAGTCTAAATTAGACAACTTGAGCGA | [21] | 56 | 297 |
| R: TTAGAATAATCAACAAAC ATCACGCCAT | ||||
| napA | F: TGCGATCGTGTTGTTTATG | [22] | 50 | 344 |
| R: GATCGTCCGCATAAGTTAC | ||||
| flaA | F: GCTTTTTCAGGTCAATAC | [13] | 52 | 524 |
| R: GTAGCGATACGAACCTGA | ||||
| flaB | F: GATAAATACCAATATCGC | [13] | 52 | 241 |
| R: CTCATCCATCGCTTTATC |
| Target gene (positive/negative) | Positive rate (%) | Target antigen (positive/negative) | Positive rate (%) | Target antibody (positive/negative) | Positive rate (%) |
| ureB (151/0) | 100 | UreB (151/0) | 100 | Anti-UreB (151/0) | 100 |
| hpaA (151/0) | 100 | HpaA (151/0) | 100 | Anti-HpaA (132/19) | 87.4 |
| vacA (151/0) | 100 | VacA (79/72) | 52.3 | Anti-VacA (65/86) | 43.0 |
| CagA (146/5) | 96.7 | CagA (139/12) | 92.1 | Anti-CagA (108/43) | 71.5 |
| napA (148/3) | 98.0 | NapA (141/10) | 93.4 | Anti-NapA (135/16) | 89.4 |
| flaA (151/0) | 100 | FlaA (151/0) | 100 | Anti-FlaA (128/23) | 84.8 |
| flaB (151/0) | 100 | FlaB (151/0) | 100 | Anti-FlaB (120/31) | 79.5 |
| Disease | Patient | Positive number/rate of bacteria, n/% | Positive number/rate of virulence factor, n/% | |||
| UreB | VacA | CagA | NapA | |||
| Chronic gastritis | 240 | 102/42.5 | 102/100 | 47/46.1a | 93/91.2 | 94/92.2 |
| Surface gastritis | 93 | 38/40.9 | 38/100 | 13/34.2 | 34/89.5 | 36/94.7d |
| Active gastritis | 120 | 52/43.3 | 52/100 | 30/59.6c | 48/92.3 | 50/96.2d |
| Atrophic gastritis | 27 | 12/44.4 | 12/100 | 4/33.3 | 11/91.7 | 8/66.7 |
| Peptic ulcer | 92 | 43/46.7 | 43/100 | 29/67.4 | 40/93.0 | 41/95.4 |
| Stomach | 35 | 16/45.7 | 16/100 | 11/68.9 | 15/93.8 | 15/93.8 |
| Duodenum | 39 | 18/46.2 | 18/100 | 11/61.1 | 16/88.9 | 17/94.4 |
| Complexity | 18 | 9/50.0 | 9/100 | 7/77.8 | 9/100 | 9/100 |
| Gastric adenocarcinoma | 15 | 6/40.0 | 6/100 | 3/50.0 | 6/100 | 6/100 |
In 1983, for the first time, Barry J. Marshall and J. Robin Warren successfully isolated Helicobacter pylori (H pylori) from the gastric mucosa of patients with chronic active gastritis or peptic ulcer[1,2], for which they were awarded the Nobel Prize in Physiology or Medicine in 2005. Thereafter, lots of findings have confirmed that H pylori is strongly associated with chronic gastritis, peptic ulcer[3-5], gastric adenocarcinoma, mucosa-associated lymphoid tissue (MALT) lymphoma and primary gastric non-Hodgkin’s lymphoma[6,7]. Vaccination is by far the most effective and economical method in infection prevention, though the vaccine against H pylori is scarcely touched up to date, which is probably due to the culture of H pylori characterized by enriched media, slow growth and incubation in a microaerophilic environment, plus difficulty in storage, etc. Thus, it may be a cost-effective strategy in developing a genetically engineered vaccine against H pylori.
The selection for antigenic targets is critical in the design of a genetically engineered vaccine against H pylori. The best ascertained antigens out of the outer membrane protein antigens are UreB and HpaA followed by VacA, CagA, NapA, FlaA and FlaB[8-13]. Tomb reported all the genomic sequences of H pylori in 1997[14], and in our previous study, these genomic prokaryotic expression systems were constructed.
The expression rates of outer membrane protein antigens are known to be diverse in the same bacterial isolates from various area, and so are the levels of immune response to different protein antigens, all of which will ultimately affect the selection of vaccine antigens. In order to develop effective genetically engineered vaccines, the encoding gene of the antigen should be widely located at the genome of clinical strains. The gene sequence should be conservative, the natural expression rate should also be high, and the immunogenicity of expression products should be potent. Our research focused on the carrying rates and expression level of 7 genes of H pylori from the clinical H pylori in China, including rUreB, rHpaA, rVacA, rCagA1, rNapA, rFlaA and rFlaB. Meanwhile, the levels of specific antibodies against the 7 antigens in serum were measured. This research will further our understanding of the diversity of carrying rates of the encoding genes, the expression levels and immunogenicity of the antigens, and provide evidence for the selection of antigens employed in genetically engineered vaccines against H pylori.
Three hundred and forty-seven biopsy specimens and serum samples were collected gastroscopically from patients with chronic gastritis, peptic ulcer or gastric adenocarcinoma in hospitals between January, 2005 and December, 2006, and 35 serum samples were collected from healthy children. All the patients were not treated with antibiotics, anti-inflammatory agents or antacids within a month prior to the sample collections. H pylori NCTC11637 strain, 10 clinical strains of Bacillus coli and 10 Staphylococcus aureus strains were offered by Department of Pathogenic Biology, Zhejiang University School of Medicine. Urease test kit was from Sanqiang BioChem Co., Ltd., Fujian Province, China. Oxidase test kit was from Hangzhou Tianhe Microorganism Reagent Co., Ltd., Zhejiang Province, China. Gel image analysis system was from Bio-Rad, and Ni-NTA affinity column was from BioColor. Rabbit anti-H pylori antibody was from DAKO. HRP-labeling goat anti-rabbit IgG and HRP-labeling goat anti-human IgG were from Jackson ImmunoResearch. The primer and PCR SuperMix High Fidelity were from TaKaRa Biotechnology, Dalian, China.
Each biopsied specimen from the patients was divided into two parts. One was detected by urease test to check whether the urease test is positive. If it was positive, the other was then used to isolate H pylori as previously described[15]. Biopsy specimens were homogenized with a tissue grinder and inoculated onto Columbia agar plates supplemented with 8% defibrinated sheep blood, 5 mg/L TMP, 10 mg/L vancomycin, 2500 U/L bacillosporin B and 2.5 mg/L amphotericin B. The plates were incubated at 37°C under microaerophilic conditions (5% O2, 10% CO2, 85% N2) for 3-5 d. Isolates were identified as H pylori with Gram-staining. The positive strains were stored in Brucella broth at -70°C.
The gene clones of ureB, hpaA, vacA, cagA, napA, flaA and flaB were from H pylori NCTC11637 strain. The plasmids pET32a or pET42a and an Escherichia coli (E.coli) BL21DE3 (presented by the Department of Pathogenic Biology, Zhejiang University School of Medicine) were used as the expression system and host cells, respectively. The expression system of recombinant antigens was inoculated in LB medium with 0.5 mmol/L IPTG and shaken at 250 r/min and cultured at 30°C for 4-5 h. The expression of rUreB (569 aa, about 63 kDa), rHpaA (260 aa, about 29 kDa), rVacA (747 aa, about 82 kDa), rCagA1 (716 aa, about 79 kDa), rNapA (144 aa, about 16 kDa), rFlaA (510 aa, about 56 kDa) and rFlaB (514 aa, about 57 kDa) were detected with SDS-PAGE and gel image analysis system. These recombinant protein antigens were purified by Ni-NTA affinity column. The purity quotient of recombinant protein antigens was detected with SDS-PAGE. The recombinant antigens were identified by Western blot. Rabbit anti-H pylori were used as the first antibody and HRP-labeling goat anti-rabbit IgG as the second antibody. The density of extracted proteins was determined by ultraviolet spectrophotometry[16].
Each of these recombinant proteins was respectively mixed with Freund’s complete adjuvant, and thereafter administered into the New Zealand rabbits for immunization with multiple dorsal ID, four times, once every two weeks. Rabbit serum was collected by cardiac puncture two weeks after the final immunization. The titer of antiserum was determined by double immunodiffusion.
The purified rUreB, rHpaA, rVacA, rCagA1, rNapA, rFlaA and rFlaB were diluted to 20 μg/mL with carbonic acid buffer (pH 9.6), coated in a 96-well plate with 0.1 mL of the dilution per well overnight. Based on the result of the preliminary experiment, serum samples from patients with chronic gastritis, peptic ulcer or gastric adenocarcinoma, and those from healthy people (1:200 dilution in both) were used as the first antibody, and HRP-labeling goat anti-human IgG (1:4000 dilution) as the second antibody. The ELISA result of the serum sample from a patient was considered positive if the optical density at 490 nm (A490) was over the mean ± 3SD of negative serum sample[17].
H pylori NCTC11637 strain, clinical strains of H pylori, 10 Bacillus coli strains and 10 Staphylococcus aureus strains were ultrasonically broken up and centrifuged at 1000 r/min to obtain supernatant, which was later determined by ultraviolet spectrophotometry[16]. The proteins of H pylori, Bacillus coli and Staphylococcus aureus were diluted to 50 μg/mL with carbonic acid buffer (pH 9.6), coated in a 96-well plate with 0.1 mL of the dilution per well overnight. Based on the result of the preliminary experiment, the self-prepared rabbit antiserum against the recombinant protein (1:200 or 1:400 dilution) was used as the first antibody, and HRP-labeling goat antiserum against rabbit IgG (1:3000 dilution) as the second antibody, respectively. The ELISA result of an ultrasonic supernatant sample of H pylori was considered positive if the A490 value was over the mean ± SD of 20 ultrasonic supernatant samples at the same protein concentration of Bacillus coli and Staphylococcus aureus[17].
The genes of clinical H pylori ureB, hpaA, vacA, cagA, napA, flaA and flaB were detected by PCR. Each reaction volume was 50 μL with an amplification of 35 cycles. Both the upstream and downstream primer concentrations were 20 pmol. Primer sequences, main reaction values and the product are shown in Table 1[13,18-22]. The PCR products were identified with 2% agarose gel staining using the fluorescent dye ethidium bromide.
The positive rates of target genes and their products of clinical H pylori, as well as specific antibodies against target antigens in patients’ sera were compared, which would benefit the comprehension of the diversity of positive rates of target genes, the expression levels and immunogenicity of their antigens.
HpaA, FlaA and FlaB are structural proteins essential to the outer membrane of H pylori, while UreB, VacA, CagA and NapA are virulent factors of functional proteins. Accordingly, the positive H pylori infection rates were compared with the expression rates of virulent factors and the severity of gastric diseases.
Data were analyzed with SPSS10.0 statistical software. The positive ratio was performed by chi square test. P < 0.05 was considered statistically significant.
One hundred and fifty-one strains of H pylori were successfully isolated from 347 biopsy specimens of chronic gastritis, peptic ulcer or gastric adenocarcinoma with a positive rate of 43.5% (Figure 1). Of the 151 H pylori-infected patients, 102 were diagnosed to have chronic gastritis (38 with superficial gastritis, 52 with active gastritis and 12 with atrophic gastritis), and 43 patients were diagnosed to have peptic ulceration (16 with gastrelcosis, 18 with duodenobulbar ulcer and 9 with complex ulcer), and 6 patients with gastric adenocarcinoma.
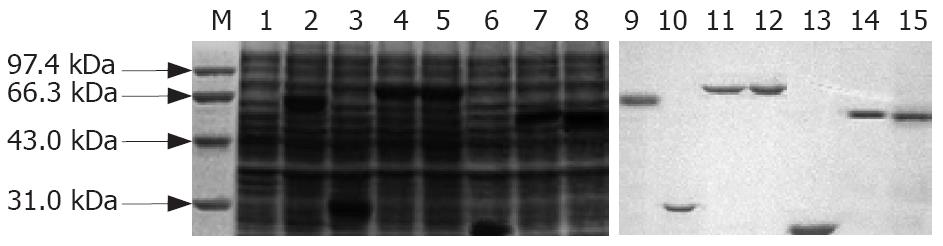
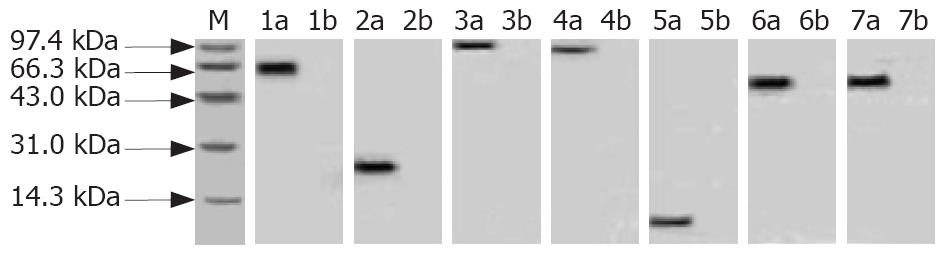
The expression rate of rUreB, rHpaA, rVacA, rCagA1, rNapA, rFlaA and rFlaB was approximately 35%, 32%, 15%, 23%, 56%, 25% and 20%, respectively. The purified recombinant proteins were shown as a single band detected by SDS-PAGE (Figure 2). The recombinant protein antigens could be recognized specifically by the rabbit antiserum against the whole cells of H pylori, and the results were shown to be positive by Western Blot analysis (Figure 3).
All the rUreB, rHpaA, rVacA, rCagA1, rNapA, rFlaA and rFlaB could efficiently induce the rabbits to produce specific antibodies, and the titer of antisera was 1:8, 1:8, 1:4, 1:4, 1:8, 1:4 and 1:4, respectively.
The A490 (mean ± SD) of the negative control sera from the 35 healthy children was 0.23 ± 0.05, and the A490 of the positive standard was 0.38. In the sera from 151 H pylori-infected patients, the positive rate of IgG antibodies against UreB, HpaA, VacA, CagA, NapA, FlaA and FlaB was 100% (151/151), 87.4% (132/151), 43% (65/151), 71.5% (108/151), 89.4% (135/151), 84.8% (128/151) and 79.5% (120/151), respectively. The total range of the A490 was 0.27-2.05.
The A490 (mean ± SD) of the negative control proteins from 10 Bacillus coli strains and 10 Staphylococcus aureus strains was 0.21 ± 0.03, and the A490 of the positive standard was 0.30. UreB, HpaA, FlaA and FlaB were expressed in 100% of the isolates (151/151), while VacA, CagA and NapA were expressed in 52.3% (79/151), 92.1% (139/151) and 93.4% (141/151) of the isolates. The total range of the A490 was 0.33-1.97.
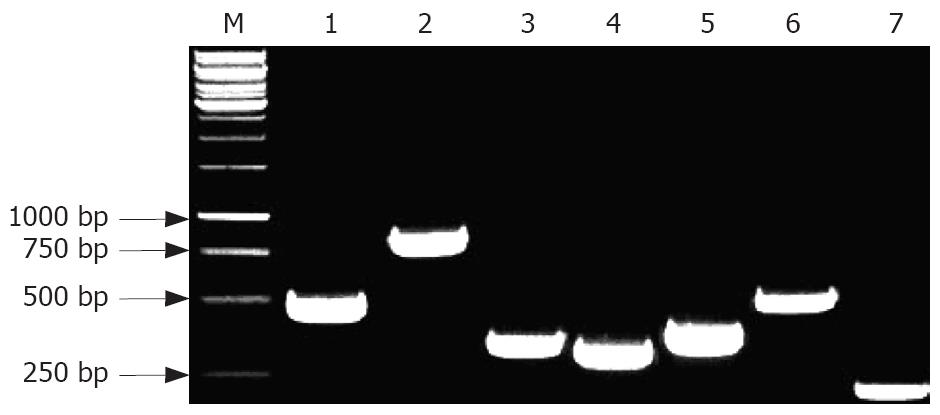
In the 151 clinical H pylori strains, the PCR products showed that the positive rate of ureB, hpaA, vacA, flaA and flaB genes was 100%, respectively, while the positive rate of CagA and napA genes was 96.7% (146/151) and 98% (148/151), respectively. The representative amplified product of each gene is shown in Figure 4.
In the 151 clinical strains of H pylori, the positive rates of ureB, hpaA, vacA, cagA, napA, flaA and flaB genes and their expression products as well as the specific corresponding antibodies are listed in Table 2. The positive rate of ureB gene and its expression product was completely coincident with that of the antibody. The positive rate of antibody against CagA was obviously lower than the positive rate and expression rate of cagA. The expression rate of vacA gene and the positive rate of corresponding antibody were obviously lower than the positive rate of vacA gene. There was a sequential decrease in the positive rate of the remaining genes, expression products and specific antibodies.
There was no statistically significant difference in the positive rates of H pylori isolated from the specimens of different gastric diseases. The expression rate of VacA gene in H pylori isolated from specimens of chronic gastritis was lower than that isolated from specimens of peptic ulcer (χ2 = 5.55, P = 0.019). The expression rate of VacA gene in superficial and atrophic gastritis was lower than that in active gastritis (χ2 = 5.76, P = 0.016). The expression rate of NapA gene in H pylori isolated from atrophic gastritis (66.7%) was lower than that in H pylori isolated from superficial and active gastritis (94.7%, 96.2%; χ2 = 6.81, P = 0.009; χ2 = 9.98, P = 0.002). There was no statistically significant difference in the expression rate of UreB and CagA genes and in their virulence factors in the specimens from different gastric diseases (Table 3).
It was reported that there are many protective antigens against H pylori, such as UreB, HpaA, VacA, CagA, NapA, FlaA, FlaB and heat shock protein (HSP)[8-13,23]. Due to cross-antigens against HSP in different sibling bacteria[23], 7 kinds of protective antigens of H pylori except for HSP were selected in our research. In order to develop effective genetically engineered vaccines, the encoding gene of the antigen should be widely located at the genome of clinical strains. The gene sequence should be conservative, the natural expression rate should also be high, and the immunogenicity of expression products should be potent. In addition, the high expression rate of recombinant proteins is also one of the most important problems to be solved in preparing a genetically engineered vaccine. Thus, it is essential to understand the above-mentioned characteristics of protein antigens of H pylori.
The biological characteristics of bacteria and other microbes exhibit an obvious diversity in different regions, mainly manifested as different dominant serological types or genotypes according to their different antigenicity, toxicity and drug resistance. So far, the gene orders of ureB, hpaA, napA, flaA and flaB have been confirmed as highly conservative, while the gene order of vacA can mutate moderately between signal peptide zone (S) and intermedial zone (M), and the mutations of CagA gene order are mainly located at the 3' terminal repeat[8,9,12,13,15]. Our research focused on the carrying rates and expression level of the 7 kinds of genes of H pylori from the clinical H pylori in China. The PCR products showed the the carrying rate of ureB, hpaA, vacA, flaA and flaB genes was 100%, respectively, while the carrying rate of cagA and napA genes was 96.7% and 98%, respectively. However, the expression rates of these genes were significantly different. As shown above, all the strains (100%) could express UreB, HpaA, FlaA and FlaB, and more than 90% strains (92.1% and 93.4%) could express CagA and NapA, but the expression rate of VacA was only 52.3%. While the unique epitoxin of H pylori, VacA, has always been the selective antigen against gene vaccine[11,24], our study demonstrated that VacA was not the ideal antigen on the grounds that the carrying rate (100%) was not consistent with the expression rate (approximately 50%) of the gene. It was reported that the activity of vacuolus toxin is high if the vacA genotype is s1/m1, and moderate or low if the vacA genotype is s1/m2[25]. The expression rate of vacA gene was low in our study, which is relevant to the vacA genotype s1a/m2, because the vacA genotype of 55.1% H pylori strains is a low virulent s1a/m2 in China[15]. Almost 100% of the H pylori strains could carry napA gene, while the expression rate of NapA is only 60%[12]. In our research, the expression rate of napA gene was high (98%), which might be related to the protein expression of diverse epidemic strains in different regions.
In vaccination, antibodies mainly play their preventive function, while cell-mediated immunity generally eliminates the pathogenic microorganisms which invade the human body. Because of their high level and long duration in all antibodies, IgG antibodies are regarded as the most important antibodies, thus IgG antibodies against UreB, HpaA, VacA, CagA, NapA, FlaA and FlaB in serum samples were detected in our study. The results show that the positive rate of IgG antibodies against UreB, NapA, HpaA and FlaA was 100%, 89.4%, 87.4% and 84.8%, respectively, while the positive rate of IgG antibodies against FlaB, CagA and VacA was 79.5%, 71.5% and 43%, respectively. The positive rate of IgG antibodies against HpaA, CagA, FlaA and FlaB was 71.5%-87.4%, while the carrying rate (96.7%-100%) and expression rate (92.1%-100%) of these genes were higher. This discrepancy might be related to the weak antigenicity and low natural expression of these proteins, presumably due to the insufficient exposure of these proteins to the surface of bacteria. For example, HpaA is usually called an adhesin of H pylori, and in fact, it is a tunica vaginalis protein expressed in the flagellae of H pylori[9]. FlaA and FlaB expressed in the flagella are subunits of flagellin[13]. As an intein of H pylori, CagA is a non-exocrine protein and only when H pylori has contact with host cells, does it act on the target cells through type III secretory system[10,11]. However, the high expression rate of rNapA, rUreB and rHpaA and the low expression rate of the other 4 kinds of proteins would consequently affect the selection of genetically engineered vaccines against H pylori.
Moreover, the relationship between the infection rate of H pylori, virulence factors and various gastropathies was also taken into account in our research. As mentioned above, VacA and NapA are the most important virulence factors. The former can induce vacuolar degeneration of target cells, while the latter can activate leukocytes to evoke inflammatory reactions. Urease (Ure) is the key colonization factor for H pylori, which is considered closely related to the virulence for its resistance to the acid environment. CagA is not directly cytotoxic, though it is the most important component of cag pathogenicity island (CPI), and thus also regarded as a reference index to bacterial virulence[26]. No statistically significant difference in the positive rate of H pylori isolated the specimens of different gastric diseases was found in our study. All of the strains could express UreB, and the expression rate of cagA gene was not statistically significant in the specimens of different gastric diseases. However, the expression rate of vacA gene in H pylori isolated from specimens of chronic gastritis was lower than that isolated from specimens of peptic ulcer (P = 0.019), and its expression rate in superficial and atrophic gastritis was lower than that in active gastritis (P = 0.016). The expression rate of NapA gene in H pylori isolated from specimens of atrophic gastritis was lower than that in H pylori isolated from specimens of superficial gastritis and active gastritis (P < 0.0001). These results suggest that different gastrosis might not be related to the infection rate of H pylori but related to the virulence of VacA and NapA.
In conclusion, according to the expression rate of the 7 kinds of genes, the positive rates of serum antibodies, quantities of the recombinant proteins, UreB is the top option for antigens in developing genetically engineered vaccines against H pylori followed by NapA and HpaA.
Lots of findings have confirmed that Helicobacter pylori (H pylori) is strongly associated with chronic gastritis, peptic ulcer, gastric adenocarcinoma, mucosa-associated lymphoid tissue (MALT) lymphoma and primary gastric non-Hodgkin’s lymphoma. Vaccination is by far the most effective and economical method in infection prevention, though the vaccine against H pylori is scarcely touched up to date. So, it is a very important task to search the vaccine against H pylori nowadays.
The vaccine against H pylori is scarcely touched nowadays, probably due to the culture of H pylori characterized by enriched media, slow growth and incubation in a microaerophilic environment, plus difficulty in storage, etc. Thus, it may be a cost-effective strategy in developing a genetically engineered vaccine against H pylori. The selection of antigenic targets is critical in the design of a genetically engineered vaccine against H pylori.
In this study, the expression rate of antigens in bacterial isolates and the positive rate of antigenic antibodies in sera from H pylori-infected patients were detected by ELISA according to the recombinant protein antigens rUreB, rHpaA, rVacA, rCagA1, rNapA, rFlaA and rFlaB of H pylori. Meanwhile, PCR was performed to detect the carrying rate of genes encoding antigens in the isolates. Our research focused on the carrying rate and expression level of 7 kinds of genes of H pylori from the clinical H pylori in China.
The experimental results of this study indicate that UreB is the first choice of the 7 kinds of recombinant protein antigens in developing a genetically engineered vaccine against H pylori followed by NapA or HpaA. Our results also indicate that different diseases are no correlated to the H pylori infection rate but to the virulence of H pylori strains. These results provide additional evidence for selection of antigens used in genetically engineered vaccines against H pylori.
It is an interesting paper with almost novel findings which may help develop H pylori vaccines.
Peer reviewers: Sun-Lung Tsai, Professor, Department of Medical Research, Chi Mei Medical Center, 901 Chu-Hwa Road, Young-Kang City 710, Taiwan, China; Didier Merlin, Associate Professor, Emory University, 615 Michael Street, Atlanta, GA 300322, United States
S- Editor Zhong XY L- Editor Wang XL E- Editor Yin DH
| 1. | Warren JR, Marshall BJ. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273-1275. |
| 2. | Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-1315. |
| 3. | Hunt RH. Eradication of Helicobacter pylori infection. Am J Med. 1996;100:42S-50S; discussion 50S-51S. |
| 4. | Kuipers EJ, Klinkenberg-Knol EC, Vandenbroucke-Grauls CM, Appelmelk BJ, Schenk BE, Meuwissen SG. Role of Helicobacter pylori in the pathogenesis of atrophic gastritis. Scand J Gastroenterol Suppl. 1997;223:28-34. |
| 5. | Redeen S, Petersson F, Jonsson KA, Borch K. Relationship of gastroscopic features to histological findings in gastritis and Helicobacter pylori infection in a general population sample. Endoscopy. 2003;35:946-950. |
| 6. | Sipponen P, Hyvarinen H. Role of Helicobacter pylori in the pathogenesis of gastritis, peptic ulcer and gastric cancer. Scand J Gastroenterol Suppl. 1993;196 Suppl:3-6. |
| 7. | Dragosics B. Significance of Helicobacter pylori infection for stomach lymphoma and stomach carcinoma. Wien Med Wochenschr. 2002;152:135-140. |
| 8. | Tanahashi T, Kita M, Kodama T, Yamaoka Y, Sawai N, Ohno T, Mitsufuji S, Wei YP, Kashima K, Imanishi J. Cytokine expression and production by purified Helicobacter pylori urease in human gastric epithelial cells. Infect Immun. 2000;68:664-671. |
| 9. | Mao YF, Yan J, Li LW, Li SP. Construction of hpaA gene from a clinical isolate of Helicobacter pylori and identification of fusion protein. World J Gastroenterol. 2003;9:1529-1536. |
| 10. | Stein M, Rappuoli R, Covacci A. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc Natl Acad Sci USA. 2000;97:1263-1268. |
| 11. | Ito Y, Azuma T, Ito S, Miyaji H, Hirai M, Yamazaki Y, Sato F, Kato T, Kohli Y, Kuriyama M. Analysis and typing of the vacA gene from cagA-positive strains of Helicobacter pylori isolated in Japan. J Clin Microbiol. 1997;35:1710-1714. |
| 12. | Satin B, Del Giudice G, Della Bianca V, Dusi S, Laudanna C, Tonello F, Kelleher D, Rappuoli R, Montecucco C, Rossi F. The neutrophil-activating protein (HP-NAP) of Helicobacter pylori is a protective antigen and a major virulence factor. J Exp Med. 2000;191:1467-1476. |
| 13. | Yan J, Liang SH, Mao YF, Li LW, Li SP. Construction of expression systems for flaA and flaB genes of Helicobacter pylori and determination of immunoreactivity and antigenicity of recombinant proteins. World J Gastroenterol. 2003;9:2240-2250. |
| 14. | Tomb JF, White O, Kerlavage AR, Clayton RA, Sutton GG, Fleischmann RD, Ketchum KA, Klenk HP, Gill S, Dougherty BA. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539-547. |
| 15. | Chen XJ, Yan J, Shen YF. Dominant cagA/vacA genotypes and coinfection frequency of H. pylori in peptic ulcer or chronic gastritis patients in Zhejiang Province and correlations among different genotypes, coinfection and severity of the diseases. Chin Med J (Engl). 2005;118:460-467. |
| 16. | Sambrook J, Fritsch EF, Maniatis T, Molecular Cloning [M]; A Laboratory Manual. 2nd edition. New York: Cold Spring Harbor Laboratory Press, 1989, 1.21-1.52, 2.60-2.80, 7.3-7.35, 9.14-9.22. . |
| 17. | Lewis SM, Osei-Bimpong A. Haemoglobinometry in general practice. Clin Lab Haematol. 2003;25:343-346. |
| 18. | Velapatino B, Balqui J, Gilman RH, Bussalleu A, Quino W, Finger SA, Santivanez L, Herrera P, Piscoya A, Valdivia J. Validation of string test for diagnosis of Helicobacter pylori infections. J Clin Microbiol. 2006;44:976-980. |
| 19. | Voland P, Hafsi N, Zeitner M, Laforsch S, Wagner H, Prinz C. Antigenic properties of HpaA and Omp18, two outer membrane proteins of Helicobacter pylori. Infect Immun. 2003;71:3837-3843. |
| 20. | Ito Y, Azuma T, Ito S, Miyaji H, Hirai M, Yamazaki Y, Sato F, Kato T, Kohli Y, Kuriyama M. Analysis and typing of the vacA gene from cagA-positive strains of Helicobacter pylori isolated in Japan. J Clin Microbiol. 1997;35:1710-1714. |
| 21. | Jenks PJ, Megraud F, Labigne A. Clinical outcome after infection with Helicobacter pylori does not appear to be reliably predicted by the presence of any of the genes of the cag pathogenicity island. Gut. 1998;43:752-758. |
| 22. | Evans DJ Jr, Evans DG, Takemura T, Nakano H, Lampert HC, Graham DY, Granger DN, Kvietys PR. Characterization of a Helicobacter pylori neutrophil-activating protein. Infect Immun. 1995;63:2213-2220. |
| 23. | Du RJ, Ho B. Surface localized Heat Shock Protein 20 (HslV) of Helicobacter pylori. Helicobacter. 2003;8:257-267. |
| 24. | Del Giudice G, Covacci A, Telford JL, Montecucco C, Rappuoli R. The design of vaccines against Helicobacter pylori and their development. Annu Rev Immunol. 2001;19:523-563. |
| 25. | Atherton JC, Cao P, Peek RM Jr, Tummuru MK, Blaser MJ, Cover TL. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771-17777. |
| 26. | Censini S, Lange C, Xiang Z, Crabtree JE, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648-14653. |