INTRODUCTION
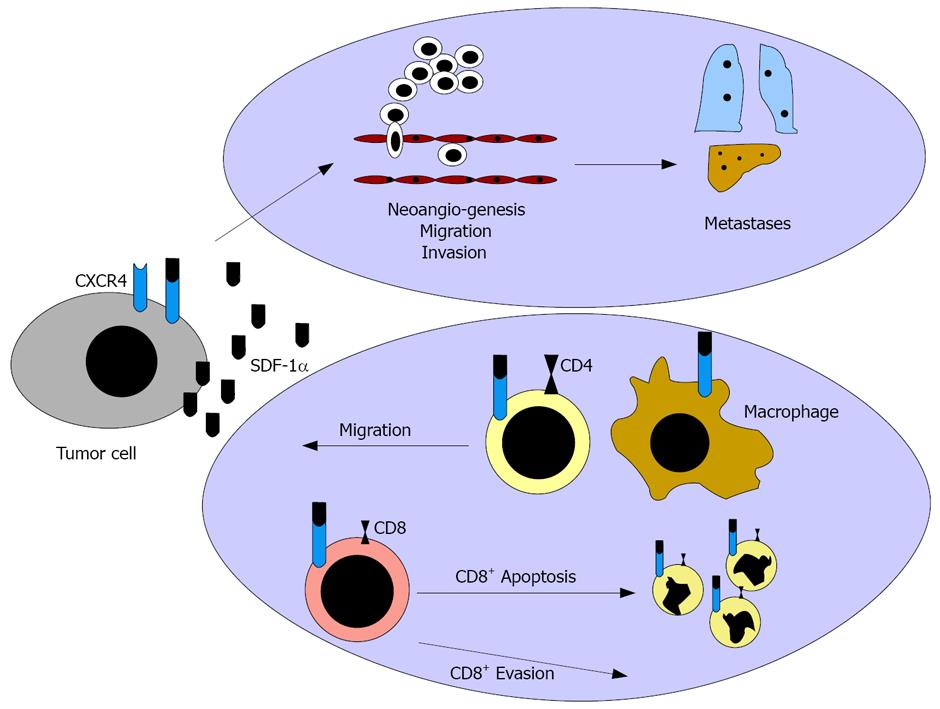
Figure 1 CXCR4 mediates tumor dissemination via induction of tumor cell migration and invasion and via stimulation of neoangiogenesis.
The CXCR4 ligand SDF-1α might act as “chemorepellent” preventing tumor rejection.
Chemokines and their receptors, such as CXCR4, induce chemotaxis of diverse immune cells [CD4+cells, CD8+ cells, macrophages, and dendritic cells (DC)] into areas of inflammation[1]. CXCR4 and CCR5 were initially reported to represent co-receptors for human immunodeficiency virus (HIV) infection in man. Gp120 binds CXCR4 and CCR5, mediating fusion of HIV with the host cell membrane. Therefore, CXCR4 was previously termed Fusin. However, after viral binding to the chemokine receptor CXCR4, HIV induces an immunologic T helper (Th) cell response, called a Th1 to Th2 switch, which clearly supports the immune evasion of HIV-infected lymphocytes[2,3]. In addition, in vitro studies on HIV revealed that the chemokine receptor CXCR4 ligand SDF-1α induced apoptosis of CD8+ cells, again inhibiting strong immune defence mechanisms[4]. In HIV therapy, much attention has been paid to CXCR4, to which the fusion-mediating and migration-inducing chemokine SDF-1α binds selectively. However, the sole blockade of this receptor, which is expressed on CD4+ cells, CD8+ cells, and APC, does not lead to a sufficient inhibition of the fusion and, therefore, to a persisting HIV infection of lymphocytes.
During the last 5 years, diverse human cancers were reported to express high levels of CXCR4 multiplying the metastasis process via their aggressive-invasive and migratory phenotypes[5,6]. We and others recently reported that gastrointestinal cancers co-express CXCR4 and its ligand SDF-1α in an autocrine and paracrine fashion, thereby increasing tumor cell dissemination[7-9].
IMMUNOLOGICAL RELEVANCE OF THE CXCR4/SDF-1 AXIS
The CXCR4/SDF-1 pathway has been reported to impact the progression of various malignomas. However, the influence on the immune system was only analyze at a basic level, so far. Moreover, only brief investigations have focused on the influence of SDF-1α in comparison to the splicing variant SDF-1β on “immune escape” mechanisms (Figure 1). SDF-1 is the only known ligand of CXCR4 and exists in several splicing variants[10]. Its expression is restricted to some cell types, as endothelial cells, epithelial thymus cells, bone marrow cells, mucosal epithelial cells, and tumor cells[11-13]. Increased expression occurs, especially in areas of inflammation and neoangionesis, and thus leads to a fulminate chemotaxis of CXCR4 expressing immune cells, such as CD4+ cells and DC along the SDF-1 gradient[14].
Interestingly, SDF-1α exposition of anti-CD3-activated CD4+ cells induced Th1-(IFN-γ) as well as Th2 (IL-4 and IL-10)-specific cytokine expression[15]. A SDF-1α exposition of activated T cells inhibited CD4+ cell apoptosis by reduction of BCC-2 and activation of PI3-K and MAPK[16]. However, this effect was linked to the SDF-1α concentration and the activation state of the CD4+ cells, as a higher concentration of SDF-1α induced apoptosis of CD4+ Jurkat cells by up-regulation of CD95 and CD95 ligands[17].
Moreover, SDF-1α induced CD8+ apoptosis after up-regulation of membrane-bound TNF-α on APC and TNF-α receptor II on CD8+ cells and a consecutive interaction of the ligand with the receptor[4].
Further results implied that SDF-1α expressing tumor cells may protect themselves from a cytotoxic immune reaction, as SDF-1α promotes apoptosis in cytotoxic T cells and might induce a local Th1 → Th2 shift in the tumor bed. This theory is supported by data reporting that SDF-1α induces a chemorepulsion of CTL supporting an immune evasion in melnoma[18]. In contrast, SDF-1β acts as chemoattractant for CTLs, inducing tumor rejection[19]. Interestingly, CXCR4 blockade not only inhibited dissemination but also sensitized tumor cells for immune therapy[20]. However, the influence on the immune system and differences between the splicing variants SDF-1α and SDF-1β have been analysed only marginally.
ONCOLOGICAL ASPECTS OF THE CXCR4/SDF-1α/β AXIS
The presence of different chemokine/cytokine receptors and their ligands, such as IL-8, IP10, Rantes, or SDF-1α could be confirmed in the tumor bed of diverse malignancies[7]. Current investigations reveal an expression by tumor cells, but also by endothelial cells[7,14]. Thus, these findings suggest that both tumor-expressed CXCR4 and SDF-1α play important roles in local progression, dissemination, and immune evasion of tumor cells.
SDF-1α binding to the second extracellular loop of CXCR4 induces activation of classical G-protein-coupled PI3-K/AKT/mTOR and RAF/MEK/ERK signalling pathways in the respective cells[7,21]. Proliferation, adhesion, migration, and invasion are the most relevant consequences. In addition, current publications report that CXCR4 activation induces EGFR1, HER2neu, and VEGFR transactivation. Most interestingly, CXCR4 upregulation was observed after EGFR/Her2neu activation, implying a compensatory pathway possibly inducing resistance to tyrosine-kinase inhibition[11].
Autocrine SDF-1α expression has initially been described in breast cancer and glioblastoma cell lines, where it has been correlated with an increased migratory and invasive potential of the tumor cells[7-9]. These effects are closely associated with the receptor affinity of SDF-1α, since a SDF-1α gene polymorphism, which is associated with an increased SDF-1α receptor affinity occuring in 5% of the general population[22], can be frequently observed in patients with breast cancer and HIV-associated non-Hodgkin lymphoma[23,24].
The paracrine expression of SDF-1α is also closely associated with neoangiogenesis. On the one side, SDF-1α and VEGFB attract endothelial precursor cells towards the tumor. On the other side, hemangiocytes (VEGFR1+, CXCR4+) are released in the bone marrow by VEGFA and directed towards the tumor by a SDF-1α gradient, where they might differentiate to pericytes stabilizing the neo-vasculature[25,26].
In contrast to benign tissues, tumors often reveal a significant up-regulation of CXCR4. A key investigation concerning the relevance of chemokine receptors links CXCR4 expression with the localization of metastases in breast cancer[27]. In a variety of human tumor entities, an increased CXCR4 expression correlated with hematogenic and lymphogenic metastasis and reduced survival times[5,6,28]. In vitro, the activation of this receptor was associated with increased proliferation, migration, and invasion. These properties could be efficiently inhibited by CXCR4 blockade in tumor models[29,30]. The important role of CXCR4 for dissemination of tumor cells was confirmed in animal models. AMD3100, a recently developed CXCR4-inhibiting “small molecule”, blocked CXCR4 in a glioblastoma model with high receptor affinity and induced a massive tumor regression in vivo[31]. T22, another CXCR4 antagonist, strongly inhibited pulmonal metastasis in a murine B16 melanoma model[32].
INFLUENCE OF SDF-1α/CXCR4 ON THE CLINICAL OUTCOME OF GASTROINTESTINAL TUMORS
The relevance of CXCR4 expression for tumor progression has been described in various gastrointestinal malignancies.
Over-expression of CXCR4 in colorectal cancer was significantly associated with advanced UICC tumor stages III/IV and with lymphatic or haemotogenic metastasis, respectively[28]. Moreover, the CXCR4 ligand SDF-1α stimulated migration and invasion in the CXCR4-expressing colon carcinoma cells SW480 and SW620 in vitro[28].
In esophageal cancer, the median overall survival of patients with CXCR4 expressing tumors was 20 months compared to 76 months for CXCR4 negative cases. CXCR4 expression was furthermore significantly associated with increased lymph node and bone marrow involvement. In multivariable analysis, CXCR4 expression was an independent variable and strongly associated with reduced disease-specific survival and overall survival. In another publication, which discriminated squamous cell from adenocarcinoma, a strong CXCR4 expression revealed a poorer long-term prognosis following curative esophagectomy for both histological subtypes, though with lack of statistical significance[33].
In gastric cancer, CXCR4 expressing primary gastric carcinomas significantly correlated with the development of peritoneal carcinomatosis and malignant ascites which contained high concentrations of CXCL12[34].
In pancreatic cancer, an over-expression of CXCR4 correlated with advanced UICC stages III/IV and revealed a trend for hematogenous metastasis and progressed local tumor stages without affecting survival[35].
In hepatocellular cancer, a high expression of CXCR4 or CCR7 was associated with locally advanced primary tumors and lymphogenic metastasis[36]. Moreover, high expression of CXCR4 led to a significant increase in haematogenic metastasis and to a decreased 3-year survival[36]. In Huh7 hepatoma cells, SDF-1α exposition induced receptor internalization and perinuclear localization as well as an increase of proliferation and invasion. As a result of a CXCR4 receptor defect, these phenomena did not occur in HepG2 cells. While Hep3B and Huh7 showed high levels of autocrine and paracrine SDF-1α expression, this effect could barely be observed in HepG2 cells.
In summary, CXCR4 increases the metastatic phenotype for diverse gastrointestinal malignancies. Current analyses are investigating whether CXCR4 expression might be valuable predictor for tumor recurrence.
Peer reviewers: Kazuma Fujimoto, Professor, Department of Internal Medicine, Saga Medical School, Nabeshima, Saga, Saga 849-8501, Japan; Reinhard Buettner, Professor, Institute of Pathology, University Hospital Bonn, Sigmund-Freud-Str. 25, D-53127 Bonn, Germany; Marc Basson, MD, PhD, MBA, Chief of Surgery, John D. Dingell VA Medical Center, 4646 John R. Street, Detroit, MI 48301, United States
S- Editor Zhong XY L- Editor Mihm S E- Editor Lin YP