Published online Jul 28, 2008. doi: 10.3748/wjg.14.4473
Revised: July 14, 2008
Accepted: July 21, 2008
Published online: July 28, 2008
AIM: To clarify whether Lysophosphatidic acid (LPA) activates the nuclear translocation of nuclear factor-κB (NF-κB) in pancreatic cancer.
METHODS: Panc-1, a human pancreatic cancer cell line, was used throughout the study. The expression of LPA receptors was confirmed by reverse-transcript polymerase chain reaction (RT-PCR). Cytosolic free calcium was measured by fluorescent calcium indicator fura-2, and the localization of NF-κB was visualized by immunofluorescent method with or without various agents, which effect cell signaling.
RESULTS: Panc-1 expressed LPA receptors, LPA1, LPA2 and LPA3. LPA caused the elevation of cytosolic free calcium dose-dependently. LPA also caused the nuclear translocation of NF-κB. Cytosolic free calcium was attenuated by pertussis toxin (PTX) and U73122, an inhibitor of phospholipase C. The translocation of NF-κB was similarly attenuated by PTX and U73122, but phorbol ester, an activator of protein kinase C, alone did not translocate NF-κB. Furthermore, the translocation of NF-κB was completely blocked by Ca2+ chelator BAPTA-AM. Thapsigargin, an endoplasmic-reticulum Ca2+-ATPase pump inhibitor, also promoted the translocation of NF-κB. Staurosporine, a protein kinase C inhibitor, attenuated translocation of NF-κB induced by LPA.
CONCLUSION: These findings suggest that protein kinase C is activated endogenously in Panc-1, and protein kinase C is essential for activating NF-κB with cytosolic calcium and that LPA induces the nuclear translocation of NF-κB in Panc-1 by mobilizing cytosolic free calcium.
- Citation: Arita Y, Ito T, Oono T, Kawabe K, Hisano T, Takayanagi R. Lysophosphatidic acid induced nuclear translocation of nuclear factor-κB in Panc-1 cells by mobilizing cytosolic free calcium. World J Gastroenterol 2008; 14(28): 4473-4479
- URL: https://www.wjgnet.com/1007-9327/full/v14/i28/4473.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4473
Lysophosphatidic acid (LPA) is the smallest and structurally simplest of all glycerophospholipids, and exists in serum at concentrations between 1-5 &mgr;mol/L[1] LPA is mainly released by activated platelets[2], and immediately complexes with high affinity to serum albumin[3]. It is well known that LPA exhibits hormone- and growth factor-like activities[45]. LPA represents a major bioactive constituent of serum. LPA increases [3H]-thymidine incorporation, inositol phosphates, intracellular calcium, and protein kinase C activities[45]. Indeed, LPA acts on a large number of cells to achieve a broad range of immediate and long lasting effects. Specific responses to LPA include changes in cell shape and tension, chemotaxis, proliferation and differentiation[67]. LPA binds to putative G protein-coupled receptors found on nearly all cell types, including cancer cell lines. Numerous other cellular and biochemical responses to LPA have also been documented[8].
The molecular actions of LPA have been characterized best in rodent fibroblasts, where LPA stimulates phosphoinositide hydrolysis[4] and promotes the Rho-dependent formation of stress fibers and focal adhesions[9]. The stimulation of phosphoinositide hydrolysis is thought to occur through the GTP-binding regulatory protein (G protein) Go or Gq. The formation of stress fibers and focal adhesions is consistent with activation of Rho through G12 or G13[8]. Whether G proteins are sufficient for this action is unclear, but the sensitivity of the phenomenon to pertussis toxin (PTX) implies that Gi/o represents at least one necessary input. Receptors that recognize LPA have been identified that conform to the seven-transmembrane domain motif characteristic of G protein-coupled receptors, and identified as LPA1/Edg-2, LPA2/Edg-4, LPA3/Edg-7[10].
On the other hand, nuclear factor-κB (NF-κB) is the prototype of a family of dimers whose constituents are members of the Rel family of transcription factors[11]. In most types of cells, NF-κB is present as a heterodimer comprising p50 (NF-κB1) and p65 (RelA). NF-κB is normally retained in the cytosol in an inactive form through interaction with IκB inhibitory proteins. Release of NF-κB for translocation to the nucleus and interaction with cognate DNA sequences is accomplished through a signal-induced phosphorylation and subsequent degradation of IκB. Originally described as a necessary element for expression of the immunoglobulin gene in mature B cells, NF-κB is now recognized to be an important transcriptional regulatory protein in a variety of cell types.
It is reported that LPA translocates NF-κB to the nucleus in fibroblasts[12], lymphocytes[13], breast cancer cells[14], colon cancer cells[15], prostate cancer cells[16], and ovarian cancer cells[17]. However, until now it is not clear whether LPA translocates NF-κB in pancreatic cancer cells or not. In this study, we demonstrate for the first time that LPA induces translocation of NF-κB to nucleus in the pancreatic cancer cell line, Panc-1, by mobilizing cytosolic free calcium.
The human pancreatic cancer cell line, Panc-1 (ATCC CRL 1469), was obtained from American Type Culture Collection (MD, USA). Media and supplements were from GIBCO BRL (New York, USA). Fetal bovine serum and fetal calf serum were from Hyclone (Utah, USA). Glass-bottomed chambers were from Costar (Massachusetts, USA). LPA and fatty-acid free albumin were from Sigma (Montana, USA). PTX, staurosporine, genistein and PD98059 were from Calbiochem (California, USA). U73122, U73343 and Fura-2/AM were from Seikagakukogyo (Tokyo, Japan). Rabbit anti-human NF-κB p65 polyclonal antibody was obtained from Upstate Biotechnology (New York, USA); and rhodamine-conjugated donkey anti-rabbit polyclonal antibody and normal donkey serum from Chemicon International (California, USA), respectively.
Cell culture: Panc-1 was maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with penicillin (50 units/mL), streptomycin (50 units/mL), and 10% fetal bovine serum (FBS). Panc-1 was maintained in DMEM without FBS for 48 h prior to use for the following experiments.
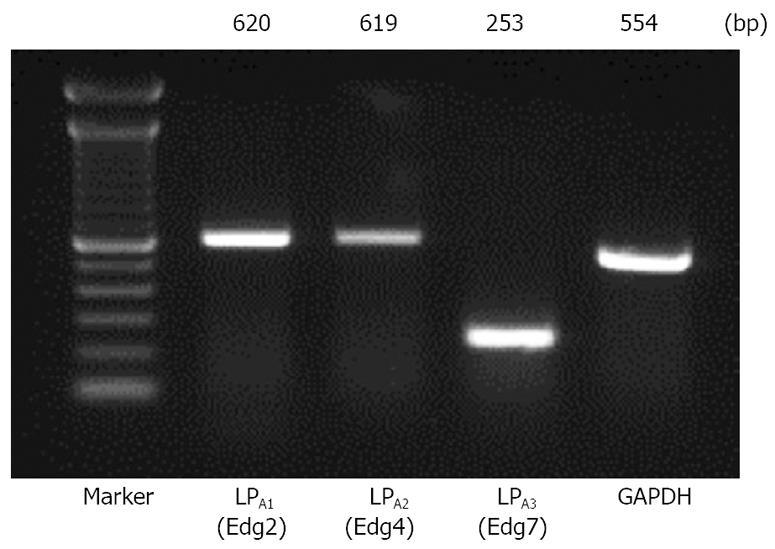
Total RNA extraction and reverse-transcription polymerase chain reaction (RT-PCR) analysis for detection of LPA receptors: Total RNA was extracted from Panc-1 using ISOGEN (Nippon Gene, Tokyo, Japan). The first strand cDNA synthesis was carried out using the SuperScript™ First-Strand Synthesis System for RT-PCR (Invitrogen™ life technologies, California, USA). One hundred nanogram of the synthesized first strand cDNA was subjected to PCR using Platinum® Taq DNA Polymerase (Invitrogen™ Life Technologies, California, USA). The first strand cDNA was then amplified with specific primers; GAPDH (as an internal control), Edg-2, Edg-4, and Edg-7. The PCR primers and conditions are listed in Table 1. In all cases after the RT step the templates were heated at 94°C for 30 s, annealed for another 30 s, and finally subjected to a 72°C extension for 60 s. The PCR products were separated in 2% agarose gel and visualized under UV illumination.
| Sense primer | Antisense primer | Temperature (°C) | Size (bp) | |
| GAPDH | 5’-AATGCATCCTGCACCACCAACTGC-3’ | 5’-GGAGGCCATGTAGGCCATGAGGTC-3’ | 59 | 554 |
| Edg-2 | 5’-TCCACACACGGATGAGCAAC-3’ | 5’-GTGATCATTGCTGTGAACTCC-3’ | 62 | 620 |
| Edg-4 | 5’-CCACCAGCCCATCTACTACCT-3’ | 5’-CTCACAGCCTAAACCATCCAG -3’ | 62 | 619 |
| Edg-7 | 5’-GCTGGAATTGCCTCTGCAAC -3’ | 5’-ACCACAAACGCCCCTAAGAC -3’ | 62 | 253 |
Measurement of cytosolic free calcium: Cytosolic free calcium concentration was measured as previously described[18]. Confluent monolayers of Panc-1 cells grown in 175 cm2 flasks were harvested by incubation in 0.9% (w/v) NaCl, 0.02% (w/v) EGTA and 10 mmol/L Hepes, pH7.2. Cells were washed twice with PBS, resuspended to a density of 1 × 106 cells/mL, and then incubated with 6 &mgr;mol/L fura-2/AM for 1 h at room temperature. Aliquots of this suspension were washed twice with Krebs-Ringer’s HEPES buffer in a stirred fluorimetry cuvette at 37°C. Fluorescence of fura-2 was measured with Shimazu RF-5000 luminescence spectrometer (alternate excitation wavelengths of 340 nm and 380 nm: emission wavelength, 505 nm). Rmax was determined by the addition of 0.1% Triton X-100, then Rmin was estimated using 4 mmol/L EGTA.
Immunofluorescence: Panc-1 cells were cultured on glass-bottomed culture dishes, and experiments were conducted as described above. Panc-1 cells were washed twice with ice-cold PBS and fixed with methanol for 5 min, permeabilized with 0.2% Triton X-100. Once the dishes had air-dried, the cells were incubated in 10% FCS for 2 h to block nonspecific antibody binding. The cells were then incubated with rabbit anti-human NF-κB p65 polyclonal antibody (1:50) in PBS containing 0.2% BSA for 6 h at room temperature. The dishes were then washed three times in PBS with 0.2% BSA for 5 min at room temperature. Cells were then incubated with rhodamine-conjugated donkey anti-rabbit polyclonal antibody (1:100) in PBS containing 0.2% BSA for 1 h at room temperature. The dishes were then washed three times in PBS containing 0.2% BSA for 5 min at room temperature and mounted with glass coverslips using Vectashield mounting medium from Vector Laboratories (California, USA). Immunofluorescence was observed by using an LSM410 Confocal Laser Scanning Microscope from Carl Zeiss, Inc. (Oberkochen, Germany).
Treatment of Panc-1 cells: Panc-1 cells were treated with various agents before measuring cytosolic free calcium or detecting translocation of NF-κB. For PTX-pretreatment, monolayers of Panc-1 cells were cultured in DMEM with 100 ng/mL PTX overnight, and then washed twice with PBS. Panc-1 cells were treated with other agents; 10 &mgr;mol/L U73122 for 10 min, 10 &mgr;mol/L U73343 for 10 min, 10 &mgr;mol/L BAPTA for 15 min, 10 nmol/L staurosporine for 10 min, 50 &mgr;g/mL genistein for 1 h, and 10 nmol/L PD98059 for 1 h before stimulation of LPA.
The Student’s t-test was used to determine significant differences between the groups.
The expression of LPA1/Edg-2, LPA2/Edg-4, and LPA3/Edg-7 receptors was determined by RT-PCR in Panc-1 cells. As shown in Figure 1, a significant expression of mRNA encoding LPA1/Edg-2, LPA2/Edg-4, and LPA3/Edg-7 was observed, as judged from the appearance of unique cDNA bands of the expected size.
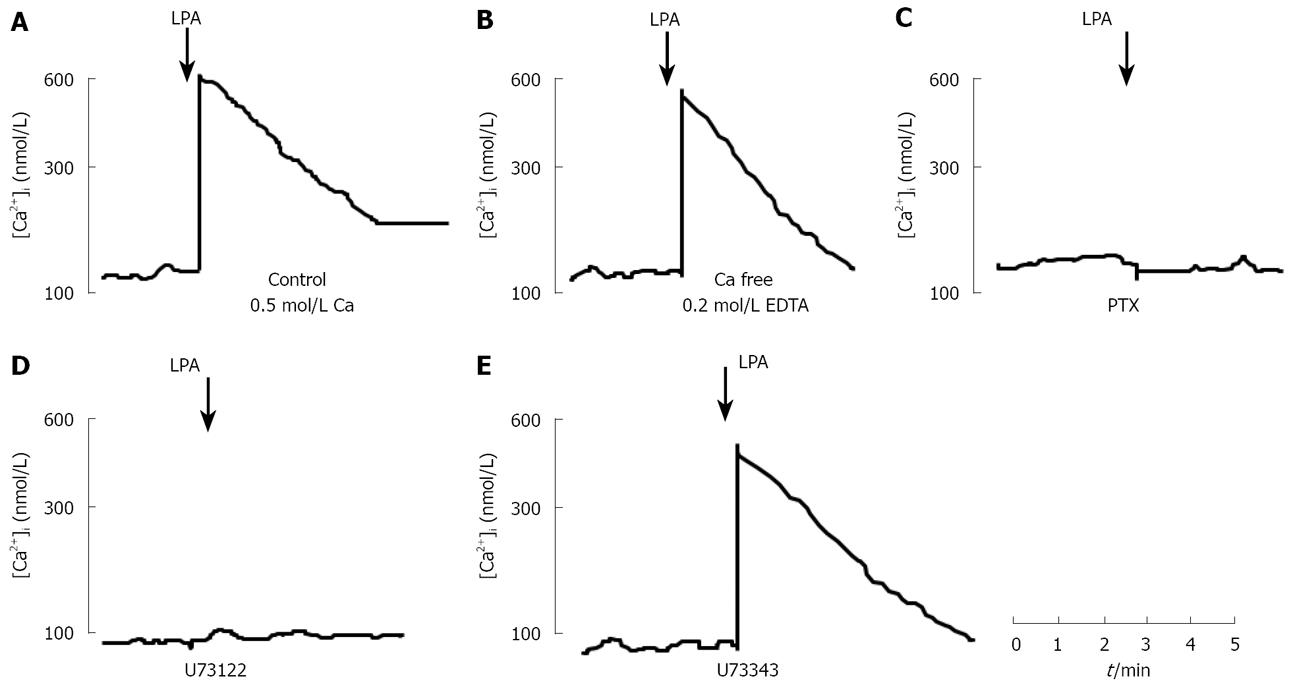
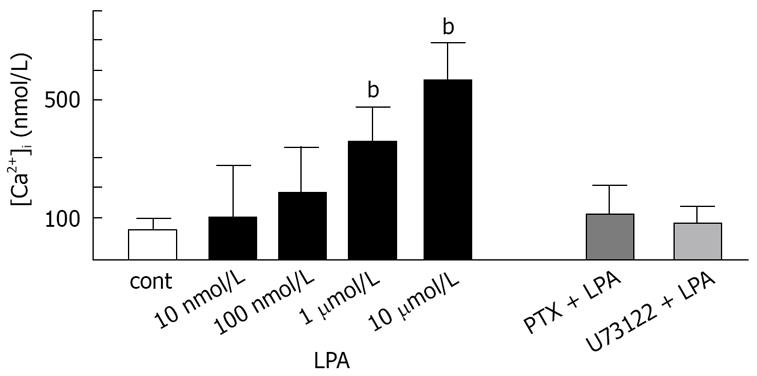
LPA, at concentration of 10 &mgr;mol/L, elevated [Ca2+]i by 600 ± 45 nmol/L (n = 5) above a resting [Ca2+]i of 108 ± 30 nmol/L within 20 s of addition to intact fura2-loaded Panc-1 cell suspension (Figure 2A). This increase in [Ca2+]i was followed by a return to near resting levels within 3 min. The elevation of [Ca2+]i by LPA in Panc-1 cells was concentration-dependent, with an EC50 of 870 nmol/L (Figure 3). Responses to LPA were dependent upon the presence of fatty acid-free BSA (0.1% w/v), without which [Ca2+]i increases were a quarter-maximal. Fatty acid-free BSA itself did not cause a significant increase in [Ca2+]i for 30 min (data not shown), as other researchers previously reported[4]. LPA-induced elevation of [Ca2+]i resulted predominantly from mobilization of intracellular [Ca2+]i store. In the condition of Ca2+-free with 0.2 mmol/L EDTA, LPA increased [Ca2+]i with a similar potency and maximal effect to the condition with extracellular Ca2+ (Figure 2B). After overnight treatment with PTX (100 ng/mL), LPA did not evoke [Ca2+]i in Panc-1 cells (Figures 2C and 3). Treatment of Panc-1 cells with a phospholipase C inhibitor, U73122, at a concentration of 10 &mgr;mol/L for 10 min, abolished LPA-induced increase in [Ca2+]i (Figure 2D and 3). Pretreated with U73343, an inactive analogue of U73122, at a concentration of 10 &mgr;mol/L for 10 min had no effect on LPA-induced elevation of [Ca2+]i in Panc-1 cells (Figure 2E). These findings suggest that LPA induces elevation of [Ca2+]i by mobilizing Ca2+ from Ca2+ store through PTX-sensitive G-protein (Gi or Go) and phospholipase C. Treatment of Panc-1 cells with thapsigargin, the endoplasmic-reticulum Ca2+-ATPase pump inhibitor, at a concentration of 500 nmol/L caused a slow increase in [Ca2+]i followed by a sustained plateau (data not shown).
It has been reported that LPA activates NF-κB in fibroblasts[19] and endothelial cells[20]. We, therefore, investigated the possibility that LPA activates the transcription factor NF-κB in Panc-1 cells.
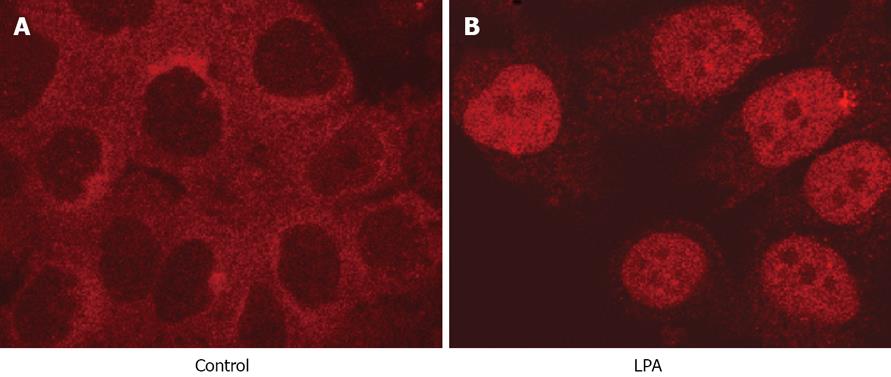
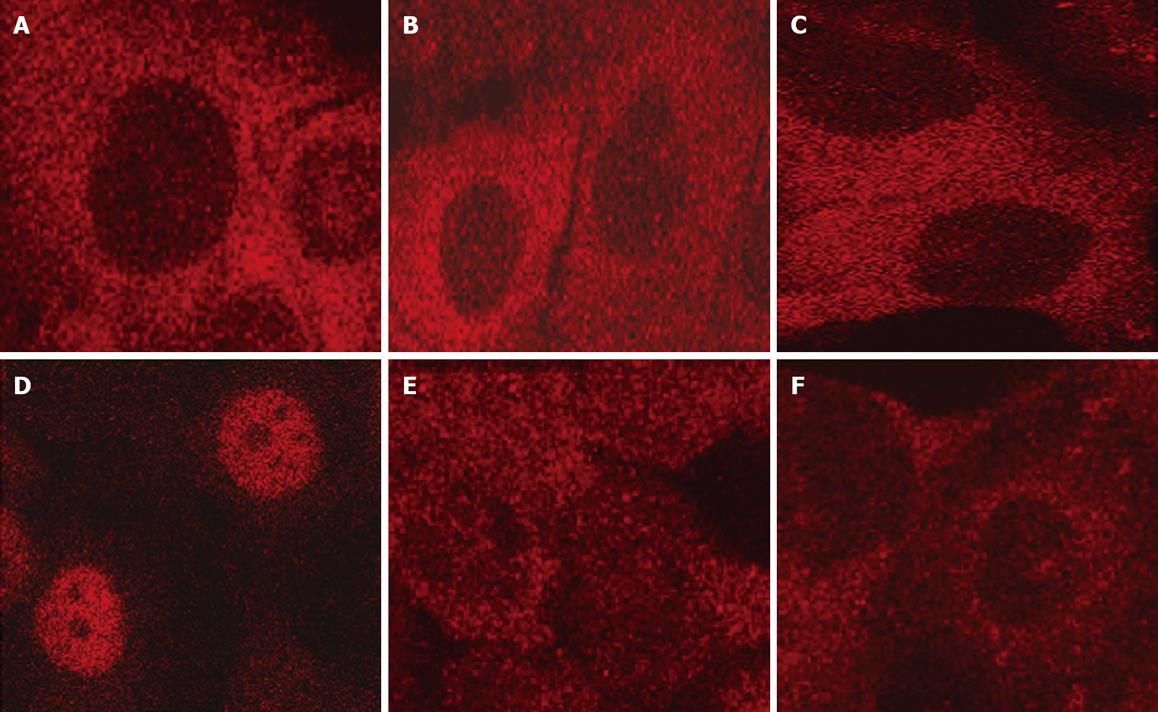
NF-κB is normally retained in the cytosol in an inactive form in Panc-1 cells (Figure 4A). LPA, at a concentration of 10 &mgr;mol/L, translocated NF-κB to the nucleus within 30 min (Figure 4B). NF-κB was re-established in the cytosol after 3 h (data not shown).
After Panc-1 cells were preincubate with 100 ng/mL PTX, LPA did not translocated NF-κB to nuclei (Figure 5A). A phospholipase C inhibitor, U73122, also abolished the translocation induced by LPA (Figure 5B). Pretreatment of Panc-1 with BAPTA-AM, an intracellular calcium chelator, at a concentration of 10 &mgr;mol/L for 15 min, completely abolished NF-κB translocation (Figure 5C). Thapsigargin is known to block a Ca2+-ATP pump of calcium stores, and elevates cytosolic free calcium slowly. In the present study, thapsigargin alone caused NF-κB translocation in Panc-1 cells at a concentration of 500 nmol/L (Figure 5D). These data suggest that elevation of cytosolic free calcium is necessary for activation of NF-κB in Panc-1 cells.
Phorbol myristate, which is well known to be a potent activator of protein kinase C, alone failed to induce the translocation at a concentration of 100 nmol/L (Figure 5E). On the other hand, staurosporine, a protein kinase C inhibitor, attenuated translocation of NF-κB induced by LPA (Figure 5F). These findings suggested that protein kinase C is activated endogenously in cancer cells, such as Panc-1, and that protein kinase C is essential for activating NF-κB with cytosolic calcium. It is reported that PMA could activate NF-κB in some types of cells including rat pancreatic acinar cells. However, the present data revealed that cytosolic calcium might have a more crucial role in activating NF-κB than protein kinase C in Panc-1 cells.
A tyrosine kinase inhibitor genistein or a MEK inhibitor PD98059 did not have significant effects on the translocation of NF-κB induced by LPA (data not shown).
In the present study, we first confirmed that Panc-1 cells express LPA receptors, and then showed that LPA induced Ca2+ mobilization and translocation of NF-κB into the nucleus.
LPA acts as an intercellular messenger molecule bound to serum albumin[3]. LPA exerts multiple biological functions through G protein-coupled receptors. It has been identified a new family of receptor genes for LPA. Members of this family include at least three G-protein-coupled receptors belonging to the endothelial differentiation gene (Edg) family, Edg-2/LPA1, Edg-4/LPA2, and Edg-7/LPA3[10]. Recent investigation has revealed that Panc-1 cells also express functionally active LPA receptors (LPA1/Edg-2, LPA2/Edg-4, LPA3/Edg-7)[21]. LPA has a major role in migration of Panc-1 cells via phosphorylation of ERK[21], but was reported to not act as mitogen in pancreatic cancer cell lines, Panc-1 cells and Bx-PC cells[21]. LPA also mobilized cytosolic calcium in Panc-1 cells as neuropeptides including neurotensin, bombesin, cholecystokinin, and vasopressin[22]. However, it is still unclear of roles of calcium mobilization in Panc-1 cells.
Some researchers have tried to identify which subtype of LPA receptors is crucial for calcium mobilization. An investigation using human-recombinant G protein has suggested that LPA1/EDG-2 transduces Ca2+ mobilization largely through PTX sensitive Gi/o proteins[18]. LPA2/Edg-4 was supposed to be linked with Gq and phospholipase C[18] and cooperated with other G proteins[19]. Another study, using a knock-out technique, revealed that nearly all PLC activation in response to LPA is dependent on endogenous expression of LPA1 and LPA2[20].
The results presented in this paper show that PTX and U73122 attenuated LPA-induced calcium mobilization in Panc-1 cells, which suggests that LPA evokes cytosolic calcium via PTX-sensitive G protein and inositol phosphate production. We have not yet determined which subtypes of LPA receptors have a major role in mobilizing cytosolic calcium in Panc-1 cells. It is likely that LPA1 or LPA2 might have a major role in mobilization of cytosolic calcium in Panc-1 cells.
The present data suggests that LPA-induced cytosolic calcium mobilization is necessary for activation of NF-κB. PTX and U73122 attenuated both cytosolic calcium mobilization and nuclear translocation of NF-κB induced by LPA. Thapsigargin exerted more potent and long lasting actions than those achieved by LPA. PMA alone failed to stimulate the nuclear translocation of NF-κB. This result suggests that evoked cytosolic calcium concentration is crucial for this translocation, and that a resting level of cytosolic calcium might be insufficient for activation of NF-κB by phorbol ester-stimulated protein kinase C.
Activation of NF-κB by LPA may function as a counterpart to proliferative signaling. It is well known that activation of NF-κB is related to resistance to apoptosis. Recent studies have revealed that chemosensitivity of human pancreatic cancer cells, including Panc-1, is enhanced by I-κBα super-repressor[23] and that NF-κB has a major role in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) resistance of pancreatic cancer cell lines[24].
LPA is supposed to utilize G proteins to achieve its activation of NF-κB. The fact that PTX attenuates activation of NF-κB would suggest a role for Gi/o. NF-κB can be activated by oncogenic Ras[25], and both Ras and ERKs are activated by LPA through pathways partly sensitive to PTX[20]. Cummings et al has shown that protein kinase C is also related with activation of NF-κB, which was coupled to Gi/o and G12/13 proteins[25].
Gi/o might have a major role in the activation of NF-κB[25]. It is conceivable that low concentrations of LPA activates Gi/o and that higher concentrations activates Gq. Gq protein may be responsible for the activation of protein kinase C and mobilization of Ca2+ of sufficient magnitude or duration to bring, together with signals from Gi/o, activation of NF-κB[26].
In conclusion, our results show for the first time that LPA induces nuclear translocation of NF-κB in Panc-1 cells by mobilizing cytosolic free calcium. This effect might be caused by activation of phospholipase C via PTX-sensitive G protein.
Lysophosphatidic acid (LPA) is a growth factor that exerts a number of biological actions on some cancer cell lines. However, the effect of LPA on pancreatic cancer cells has not been well estimated. In the present study, the authors investigate the effects of LPA on cytosolic calcium and translocation of NF-κB in Panc-1 cells.
The article focuses on the relationship of cytosolic calcium mobilization and NF-κB in Panc-1 cells induced by LPA.
The present study shows that LPA-induced cytosolic calcium mobilization is necessary for activation of NF-κB in pancreatic cancer cells, and that a resting level of cytosolic calcium might be insufficient for activation of NF-κB by phorbol ester-stimulated protein kinase C. It is suggested that protein kinase C is activated endogenously in Panc-1, and protein kinase C is essential for activating NF-κB with cytosolic calcium and that LPA induces the nuclear translocation of NF-κB in Panc-1 by mobilizing cytosolic free calcium.
It is known that LPA represents a major bioactive constituent of serum, and that activation of NF-κB is related to resistance to apoptosis. By knowing the mechanism of action of LPA, it can be used that target apoptosis in pancreatic cancer cells.
The authors investigated the effect of LPA on calcium mobilization, and localization of the transcription factor, NF-κB in the pancreatic cell line, Panc-1. This is an interesting paper. They demonstrated that LPA stimulates calcium mobilization from intracellular stores via PTX sensitive G proteins and PLC.
| 1. | Eichholtz T, Jalink K, Fahrenfort I, Moolenaar WH. The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem J. 1993;291:677-680. |
| 2. | Gerrard JM, Robinson P. Lysophosphatidic acid can activate platelets without increasing 32P-labelling of phosphatidic acid. Biochim Biophys Acta. 1984;795:487-492. |
| 3. | Tigyi G, Miledi R. Lysophosphatidates bound to serum albumin activate membrane currents in Xenopus oocytes and neurite retraction in PC12 pheochromocytoma cells. J Biol Chem. 1992;267:21360-21367. |
| 4. | van Corven EJ, Groenink A, Jalink K, Eichholtz T, Moolenaar WH. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell. 1989;59:45-54. |
| 5. | Moolenaar WH, Kruijer W, Tilly BC, Verlaan I, Bierman AJ, de Laat SW. Growth factor-like action of phosphatidic acid. Nature. 1986;171-173. |
| 6. | Jalink K, Hordijk PL, Moolenaar WH. Growth factor-like effects of lysophosphatidic acid, a novel lipid mediator. Biochim Biophys Acta. 1994;1198:185-196. |
| 7. | Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389-399. |
| 8. | Moolenaar WH, Kranenburg O, Postma FR, Zondag GC. Lysophosphatidic acid: G-protein signalling and cellular responses. Curr Opin Cell Biol. 1997;9:168-173. |
| 9. | Kumagai N, Morii N, Fujisawa K, Nemoto Y, Narumiya S. ADP-ribosylation of rho p21 inhibits lysophosphatidic acid-induced protein tyrosine phosphorylation and phosphatidylinositol 3-kinase activation in cultured Swiss 3T3 cells. J Biol Chem. 1993;268:24535-24538. |
| 10. | Contos JJ, Ishii I, Chun J. Lysophosphatidic acid receptors. Mol Pharmacol. 2000;58:1188-1196. |
| 11. | Karin M. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene. 1999;18:6867-6874. |
| 12. | Shahrestanifar M, Fan X, Manning DR. Lysophosphatidic acid activates NF-kappaB in fibroblasts. A requirement for multiple inputs. J Biol Chem. 1999;274:3828-3833. |
| 13. | Goetzl EJ, Kong Y, Mei B. Lysophosphatidic acid and sphingosine 1-phosphate protection of T cells from apoptosis in association with suppression of Bax. J Immunol. 1999;162:2049-2056. |
| 14. | Goetzl EJ, Dolezalova H, Kong Y, Zeng L. Dual mechanisms for lysophospholipid induction of proliferation of human breast carcinoma cells. Cancer Res. 1999;59:4732-4737. |
| 15. | Shida D, Kitayama J, Yamaguchi H, Okaji Y, Tsuno NH, Watanabe T, Takuwa Y, Nagawa H. Lysophosphatidic acid (LPA) enhances the metastatic potential of human colon carcinoma DLD1 cells through LPA1. Cancer Res. 2003;63:1706-1711. |
| 16. | Raj GV, Sekula JA, Guo R, Madden JF, Daaka Y. Lysophosphatidic acid promotes survival of androgen-insensitive prostate cancer PC3 cells via activation of NF-kappaB. Prostate. 2004;61:105-113. |
| 17. | Fang X, Yu S, Bast RC, Liu S, Xu HJ, Hu SX, LaPushin R, Claret FX, Aggarwal BB, Lu Y. Mechanisms for lysophosphatidic acid-induced cytokine production in ovarian cancer cells. J Biol Chem. 2004;279:9653-9661. |
| 18. | An S, Bleu T, Zheng Y, Goetzl EJ. Recombinant human G protein-coupled lysophosphatidic acid receptors mediate intracellular calcium mobilization. Mol Pharmacol. 1998;54:881-888. |
| 19. | Yuan J, Slice LW, Gu J, Rozengurt E. Cooperation of Gq, Gi, and G12/13 in protein kinase D activation and phosphorylation induced by lysophosphatidic acid. J Biol Chem. 2003;278:4882-4891. |
| 20. | Contos JJ, Ishii I, Fukushima N, Kingsbury MA, Ye X, Kawamura S, Brown JH, Chun J. Characterization of lpa(2) (Edg4) and lpa(1)/lpa(2) (Edg2/Edg4) lysophosphatidic acid receptor knockout mice: signaling deficits without obvious phenotypic abnormality attributable to lpa(2). Mol Cell Biol. 2002;22:6921-6929. |
| 21. | Stahle M, Veit C, Bachfischer U, Schierling K, Skripczynski B, Hall A, Gierschik P, Giehl K. Mechanisms in LPA-induced tumor cell migration: critical role of phosphorylated ERK. J Cell Sci. 2003;116:3835-3846. |
| 22. | Ryder NM, Guha S, Hines OJ, Reber HA, Rozengurt E. G protein-coupled receptor signaling in human ductal pancreatic cancer cells: neurotensin responsiveness and mitogenic stimulation. J Cell Physiol. 2001;186:53-64. |
| 23. | Sato T, Odagiri H, Ikenaga SK, Maruyama M, Sasaki M. Chemosensitivity of human pancreatic carcinoma cells is enhanced by IkappaBalpha super-repressor. Cancer Sci. 2003;94:467-472. |
| 24. | Khanbolooki S, Nawrocki ST, Arumugam T, Andtbacka R, Pino MS, Kurzrock R, Logsdon CD, Abbruzzese JL, McConkey DJ. Nuclear factor-kappaB maintains TRAIL resistance in human pancreatic cancer cells. Mol Cancer Ther. 2006;5:2251-2260. |
| 25. | Cummings R, Zhao Y, Jacoby D, Spannhake EW, Ohba M, Garcia JG, Watkins T, He D, Saatian B, Natarajan V. Protein kinase Cdelta mediates lysophosphatidic acid-induced NF-kappaB activation and interleukin-8 secretion in human bronchial epithelial cells. J Biol Chem. 2004;279:41085-41094. |
| 26. | Chen BC, Lin WW. PKC- and ERK-dependent activation of I kappa B kinase by lipopolysaccharide in macrophages: enhancement by P2Y receptor-mediated CaMK activation. Br J Pharmacol. 2001;134:1055-1065. |