Published online Jul 14, 2008. doi: 10.3748/wjg.14.4227
Revised: June 17, 2008
Accepted: June 24, 2008
Published online: July 14, 2008
AIM: To investigate the effects of aspirin (acetylsalicylic acid) on proliferation and apoptosis of colorectal cancer cell line SW480 and its mechanism.
METHODS: Cyclooxygenase (COX)-2 negative colorectal cancer cell line SW480 was treated with aspirin at concentrations of 2.5 mmol/L, 5.0 mmol/L, 10.0 mmol/L for different periods in vitro. Anti-proliferation effect of aspirin on SW480 was detected by 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cell cycle and apoptosis were observed by flow cytometry (FCM). Transmission electron microscope (TEM) was used for morphological study. Apoptosis-associated genes were detected by immunohistochemical staining and Western blotting.
RESULTS: Aspirin inhibited SW480 proliferation and induced apoptosis in a dose- and time-dependent manner. Treatment with different concentrations of aspirin significantly increased the proportions of cells at the G0/G1 phase and decreased the proportions of cells at the S- and G2/M phases in a concentration-dependent manner. Aspirin not only induced apoptosis but also caused cell necrosis at a high concentration as well. After treatment with aspirin, SW480 cells displayed typically morphological features of apoptosis and necrosis under TEM, and increased the Bcl-2 expression in cells, but the expression of Bax was down regulated.
CONCLUSION: Aspirin inhibits proliferation and induces apoptosis of SW480 cells. Its anti-tumor mechanism may arrest cell cycle and shift Bax/Bcl-2 balance in cells.
- Citation: Lai MY, Huang JA, Liang ZH, Jiang HX, Tang GD. Mechanisms underlying aspirin-mediated growth inhibition and apoptosis induction of cyclooxygenase-2 negative colon cancer cell line SW480. World J Gastroenterol 2008; 14(26): 4227-4233
- URL: https://www.wjgnet.com/1007-9327/full/v14/i26/4227.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4227
Colorectal cancer is a common disease that remains the major cause of cancer-related mortality in developed countries. With improvement in economic status, its incidence increases in developing countries including China and severely threatens the health of human beings. Increasing evidence from human epidemiological studies, animal models, and experiments in vitro revealed that administration of nonsteroidal anti-inflammatory drugs (NSAIDs) including aspirin (acetylsalicylic acid) represents a treatment of choice for colon cancer[1–3]. Data indicate that use of NSAIDs, including aspirin, is inversely associated with the risk of colorectal cancer, and clinical trials in patients with familial adenomatous polyposis showed that use of NSAIDs can lead to the regression of colorectal adenomas by exerting their protective effects on the stomach and esophagus[4]. However, the molecular mechanism by which aspirin exhibits its anticancer effects is not completely clear.
The best-defined molecular target for aspirin and other NSAIDs is cyclooxygenase (COX). COX-2, the regulated isoform of COX, plays an important role in the carcinogenesis[5] and angiogenesis[6]. A possible mechanism underlying the antitumor properties of aspirin has been ascribed to its direct inhibition of COX-2 in colorectal cancer tissue[7]. However, several lines of evidence suggest that the wide range of antiproliferative potencies of aspirin does not correlate exclusively with COX-2 inhibitory activity, because NSAIDs can induce apoptosis in colon cancer cell lines that lack detectable expression of COX-2 protein[89]. Until now, most researchers recruit COX-2 positive colon cancer cell lines to study the effect of aspirin on their apoptosis and proliferation. However, few studies of the effect of aspirin on COX-2 negative colon cancer cells and few systematic analyses of aspirin-induced morphologic changes are available[10–12]. Furthermore, the results of certain studies are controversial[1213]. The purpose of this study was to investigate the effect of aspirin on proliferation, and apoptosis of SW480 cells, a COX-2 negative human colon cancer cell line.
Aspirin, 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenylte-trazolium bromide (MTT), penicillin/streptomycin and RNase A were from Sigma (St. Louis, MO, USA). Mouse monoclonal antibodies against Bcl-2 and Bax as well as biotin-labeled anti-mouse IgG, were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). RPMI1640 medium was purchased from Life Technologies, Inc (Grand Island, NY) and fetal bovine serum was from Gibco (GIBCO BRL). Annexin V-Cy5 apoptosis detection kit was purchased from PharMingen (San Diego, CA).
The human colon cancer cell line SW480 was obtained from the American Type Culture Collection and cultured in RPMI 1640 medium containing 10% fetal bovine serum and 1% penicillin/streptomycin solution at 37°C in a humidified atmosphere containing 50 mL/L CO2.
We dissolved aspirin in 1 mol/L Tris-HCl (pH 7.5) to a stock concentration of 1 mol/L and adjusted the pH to 7.2 with 4 mol/L HCl. Twenty-four hours before aspirin treatment, exponentially growing human colon cancer cells were seeded at a density of 105 cells/100 mm culture dish (Becton Dickinson, Franklin Lakes, NJ). The cells were washed once with phosphate buffered saline (PBS) and treated for an indicated time by adding various volumes of stock to obtain the final aspirin concentrations of 1 mmol/L, 2.5 mmol/L, 5 mmol/L, or 10 mmol/L, respectively. Control cells were treated with an equivalent volume of Tris-HCl (pH 7.2).
SW480 colon cancer cells were seeded at a density of 3 × 104 cells/well onto a 96-well plate. Twenty-four hours after seeding, the cells were exposed to different concentrations of aspirin (0 mmol/L, 2.5 mmol/L, 5 mmol/L, 10 mmol/L) and maintained in culture for 1-7 d at 37°C in a humidified atmosphere containing 50 mL/L CO2. Individual cells were taken from suspension after exposure to trypsin/EDTA in PBS. Aliquots were counted with a haemocytometer and tested for their viability by trypan blue exclusion. Each assay was performed in triplicate.
The effect of aspirin on cellular viability was also evaluated by MTT assay. SW480 cells were plated in 96-well microtiter plates at a density of 104 cells/well in a final volume of 100 &mgr;L of RPMI 1640 medium. Twenty-four hours after the initial seeding, the cells were treated with various concentrations of aspirin for 1-7 d. The untreated cells (appropriate volume of buffer solution was added) served as controls. After treatment, the cells were incubated for 3 h at 37°C with a solution of MTT at a concentration of 50 &mgr;g/100 &mgr;L, lysed for 12 h at room temperature in a buffer containing 10% SDS and 0.01 mol/L HCl as previously described[14]. For each sample, the absorbance of the reduced intracellular formazan product was read at 570 nm on a microtiter plate reader (Bio-Rad Laboratories, Hercules, CA). Each assay was performed in triplicate.
SW480 cells were plated at a density of 106 cells/10 cm dishes and treated with aspirin at the concentration of 0, 2.5, 5 and 10 mmol/L, respectively. After treatment, the cells were harvested by trypsinization and washed once with 10 mL of PBS. We used the Annexin V-Cy5 apoptosis detection kit according to the manufacturer’s instructions to stain the apoptosis marker and cell surface phosphatidylserine. Briefly, the cells were resuspended in 200 &mgr;L of 1 × annexin binding buffer containing 10 mmol/L HEPES, 140 mmol/L NaCl, 2.5 mmol/L CaCl2 (pH 7.4) and stained with 5 &mgr;L of annexin V-Cy5 (1 &mgr;mol/mL). After incubation for 15 min at room temperature, the cells were incubated with 10 &mgr;L of 7-AAD for another 15 min at 25°C in the dark. The cell suspension was gently centrifuged, into which 400 mL of 1 × annexin binding buffer was added. The cells were analyzed by flow cytometry (FCM) within 1 h of 7-AAD staining. Appropriate controls were used to subtract background counts. We used the FACScalibur flow cytometer (Becton Dickinson, San Jose, CA) for two-color analysis of apoptosis. Fluorescence compensation was adjusted to minimize overlap of the Cy5 and 7-AAD signals.
The effect of aspirin treatment on cell proliferation was evaluated by measuring the distribution of cells in different phases of the cell cycle by FCM based on the measurement of the DNA content of nuclei labeled with propidium iodide (PI). Cell suspension from either aspirin-treated or untreated cell cultures was prepared by trypsinizing the cells and washing them twice with cold PBS. The cells were resuspended at a density of 106 cells/mL in cold PBS and fixed with 75% ethanol overnight at -20°C. Ethanol-fixed cells were washed with cold PBS, resuspended in 300 &mgr;L of PBS containing 0.15% boiled and renatured RNase A at 37°C for 30 min, and stained with 80 &mgr;g/mL of PI for 30 min. The cells were analyzed for DNA content with the FACScalibur flow cytometer at an excitation of 488 nm with detection at 620 nm for red fluorescence. The cell cycle data were analyzed using the Multicycle Software Autofit Version 2.50 (Phoenix Flow Systems, San Diego, CA).
For electron microscopic analysis of apoptosis, pretreated SW480 cells were fixed in 1% glutaraldehyde and 4% paraformaldehyde in PBS, postfixed in 1% osmium tetroxide in PBS, dehydrated and subsequently embedded in epoxy resin. Ultrathin sections (80 nm) were stained with uranyl and lead acetates and examined under a Hitachi H-600 electron microscope at 80 kV (Hitachi, Tokyo, Japan).
SW480 cells were seeded at a density of 4 × 105 cells/well onto 24-well plates and cultured for 24 h. After treatment for 48 h with ASPIRIN at final concentration of 2.5, 5.0 and 10.0 mmol/L, respectively, the untreated cells (appropriate volumes of buffer solution added) served as controls. The cells were fixed for 10 min in 100% methanol at room temperature, washed with PBS, treated with 3% hydrogen peroxide to block the endogenous peroxidase activity and incubated with 2% nonfat dry milk. After incubated for 2 h at room temperature with primary antibody, the cells were washed three times with PBS (10 min each time) and incubated with biotin-labeled anti-mouse IgG for 20 min at room temperature. After washed three times with PBS (10 min each time), the cells were stained using a streptavidin-peroxidase detection system (Novo Castra, Newcastle, UK) and viewed under a Hoffman modulation contrast microscope.
Bax, Bcl-2 and β-actin protein levels in cells were measured by Western blotting. Twenty micrograms of protein from a 20% (wt/vol) cell homogenate was separated on a 15% (wt/vol) polyacrylamide denaturing gel, and then electroblotted onto Hybone-enhanced chemiluminescence (ECL) nitrocellulose (Amersham Pharmacia Biotech Ltd). The membranes were blocked by addition of 3% (wt/vol) bovine serum albumin (BSA) into 0.1% (vol/vol) Tween-20 tris-buffered saline (TBS) at 4°C overnight. Western blots were probed with a polyclonal rabbit anti-rat Bax diluted at 1:2000 (P-19) or a monoclonal mouse anti-rat Bcl-2 diluted at 1:2000 at room temperature for 2 h. Primary Bax or Bcl-2 antibody binding was revealed using an anti-rabbit-HRP or an anti-mouse-HRP antibody diluted at 1:2000 or at 1:1000 for 1 h and the ECL detection system. Developed films were quantitatively analyzed using a volume densitometer and Molecular Analyst version 4 software (Bio-Rad Laboratories Ltd). The Bax to Bcl-2 ratio was determined for each sample individually by reprobing the same membrane and dividing the volume density for Bax by that for Bcl-2.
Data were expressed as mean ± SE. Statistical difference was assessed by a single factor variance (ANOVA) or the Student t test. For each test, P < 0.05 was considered statistically significant.
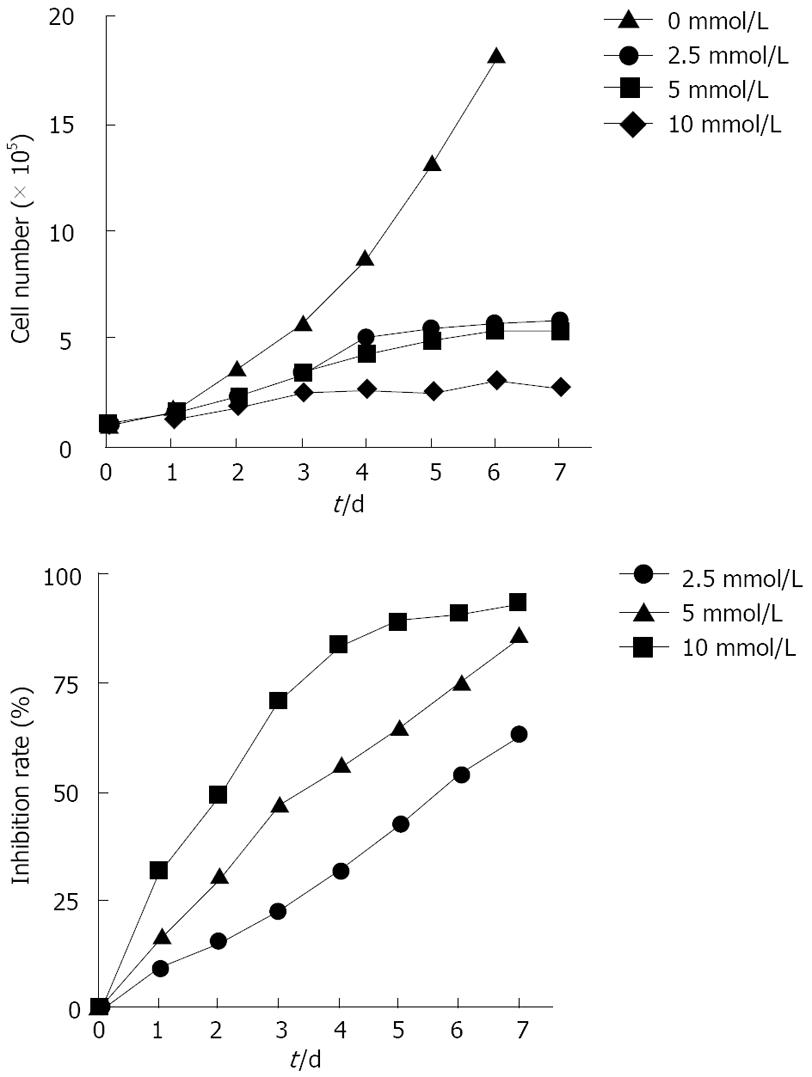
In the present study, SW480 cell proliferation was assessed by counting the cells after stimulation of aspirin at different concentrations for 7 d. As shown in Figure 1, the untreated cells grew in a linear manner. After aspirin treatment, the growth of SW480 cells was inhibited in a concentration-dependent manner. Moreover, aspirin modulated the growth of SW480 cells in a time-dependent manner. Being consistent with the results of cell counting, aspirin showed an anti-proliferation effect on the growth of SW480 cells in a concentration-and time-dependent manner.
To study the effect of aspirin treatment on cell proliferation at different phases of the cell cycle, we treated exponentially growing cells with aspirin at different concentrations for 72 h. Conventional DNA FCM showed that 39%-46% of the untreated SW480 cells were found at the G0/G1 phase, 31%-35% at the S phase, and the remaining cells at the G2/M phase, depending on the dose of aspirin used and the percentage of cells decreased (Table 1). However, the presence of aspirin at different concentrations resulted in an accumulation of cells at the G0/G1 phase. The fraction of sub-G1 DNA content accounted for 3.17%, indicating that apoptosis was decayed in the untreated control cells. After treatment with aspirin at the concentrations of 2.5, 5.0 and 10.0 mmol/L, the fraction of sub-G1 DNA content increased to 8.9%, 13.1% and 39.9%, respectively, indicating that aspirin could not only arrest cell cycle at the G0/G1 phase, but also induce apoptosis in a concentration-dependent manner (Table 1).
| Aspirin Conc (mmol/L) | Cell cycle | Proliferation index (%) | Apoptosis rate (%) | ||
| G0/G1 (%) | S (%) | G2/M (%) | |||
| 0 | 42.3 ± 2.49 | 33.0 ± 2.16 | 24.7 ± 0.47 | 57.7 ± 2.49 | 3.17 ± 0.37 |
| 2.5 | 61.0 ± 1.63b | 24.3 ± 1.25b | 14.7 ± 1.70b | 39.0 ± 1.63b | 8.92 ± 0.49b |
| 5 | 73.3 ± 3.36b | 13.7 ± 1.24b | 13.0 ± 2.42b | 26.6 ± 3.63b | 13.14 ± 1.29b |
| 10 | 80.3 ± 2.49b | 8.7 ± 0.47b | 11.0 ± 2.94b | 19.7 ± 2.49b | 37.92 ± 1.37b |
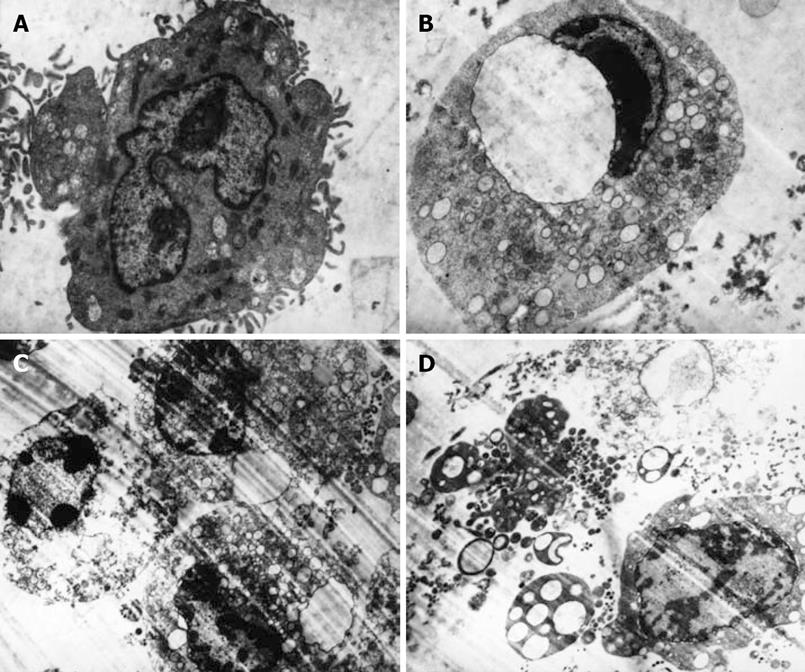
Electron microscopy of the aspirin-treated SW480 cells showed typical apoptosis characterized by volume reduction, chromatin condensation, nuclear fragmentation, and presence of apoptotic bodies (Figure 2) when compared to the untreated cells (Figure 2A). The severity of morphological changes increased in a dose-dependent manner. After 72 h of treatment with 2.5 mmol/L aspirin, the SW480 cells showed early changes in apoptosis with loss of microvilli, nuclear chromatin condensation that formed a crescent-like clump (Figure 2B). Intermediate and late stages of apoptosis, characterized by more extensive nuclear chromatin condensation, cytoplasmic blebbing, nuclear membrane splitting, fragmentation of nuclei, and formation of apoptotic bodies, were induced by treatment with 5 mmol/L aspirin for 72 h (Figure 2C). Aspirin induced secondary postapoptotic necrosis, defined as degeneration of subcellular organelles and rupture of the plasma membrane despite the presence of apoptotic condensation of chromatin when its concentration was increased to 10 mmol/L. Certain SW480 cells showed the features of oncotic necrosis, such as oncotic nuclear change, disrupted plasma membrane, degeneration of cytoplasmic organelles, swollen mitochondria with amorphous dense bodies (Figure 2D).
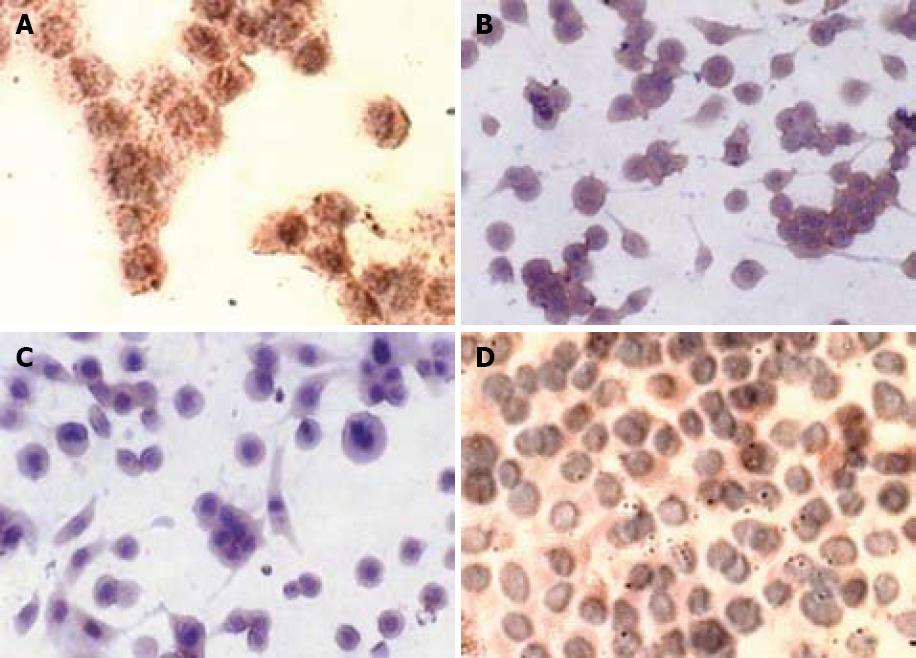
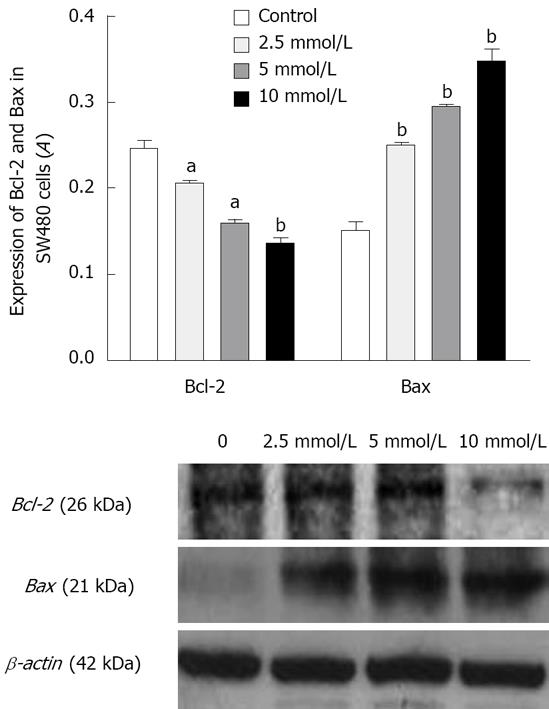
In the untreated SW480 cells, there was a strong cytoplasmic staining for Bcl-2 as detected by immunocytochemistry (Figure 3). After incubation with 5 mmol/L aspirin for 72 h, cytoplasmic Bcl-2 staining was substantially diminished (Figure 3) while immunocytochemistry staining for Bax was significantly increased after treatment with aspirin. Being consistent with immunocytochemistry staining, the expression of Bcl-2 protein measured by Western blotting, was reduced in aspirin-treated SW480 cells while that of Bax protein was increased, suggesting that aspirin could down-regulate Bcl-2 protein expression and up-regulate Bax protein expression (Figure 4).
The molecular mechanism underlying the chemo-preventive effects of NSAIDs is not well understood. One most widely accepted mechanism underlying the anticancer effect of NSAIDs is the reduced PG synthesis by inhibiting the COX activity. In the present study, we used a COX-2 negative colon cancer cell line SW480 as a model to exclude the pathway of COX-2. Being consistent with previous findings[101115], treatment with aspirin at different concentrations inhibited the proliferation of SW480 cells in a dose- and time-dependent, arrested the cell cycle at the G0/G1 phase, decreased the cell proportions at the S- and G2/M- phases in a concentration-dependent manner. SW480 cell apoptosis induced by aspirin was confirmed by flow cytometric analyses of cellular DNA content and transmission electron microscopy (TEM). After treatment with aspirin at the concentration of 2.5-10 mmol/L for 72 h, typical morphological alterations of apoptosis were observed[16], including cytoplasmic blebbing, nuclear membrane splitting, fragmentation of nuclei, and formation of apoptotic bodies. The mechanism underlying aspirin-induced cell cycle arrest is not known. It was reported that NASAIDs can arrest HT-29 cell cycle at the G0/G1 phase by inhibiting the expression of cdc2, cdc4 and cyclin, decreasing the phosphorylation of Rb, and increasing the p21WAF-1/CIPL expression in HT-29 cells[17]. Moreover, Perugini[18] found that aspirin induces cell cycle arrest at the G1 phase in human pancreatic cancer cells by inhibiting cyclinD1. Whether aspirin shares the same mechanism against SW480 cells still needs to be explored.
Several protein families modulating apoptosis have been described, among which the Bcl-2 family of proteins is best characterized[1920]. Bcl-2 appears to function as an inhibitor of apoptosis whereas Bax promotes this process. It has been shown that Bax can form heterodimers with Bcl-2 to inhibit the anti-apoptotic function of Bcl-2[21]. Our results show that aspirin could inhibit the expression of Bcl-2 in colon cancer cells but increase Bax expression. The Bcl-2/Bax ratio is important for apoptosis and the decline of this ratio contributes to apoptosis induction. In addition, Bax dimers or oligomers directly form channels in mitochondrial outer membrane in apoptosis[2021]. It is reasonable to conclude that, at least in part, aspirin promotes apoptosis of SW480 cells by down-regulating Bcl-2 and up-regulating Bax expression.
In summary, aspirin inhibits the proliferation and induces apoptosis of COX-2 negative colon cancer SW480 cells by down-regulating Bcl-2 and up-regulating Bax expression, which might be a potential mechanism by which aspirin plays a positive role in apoptosis of SW480 cells.
Increasing evidence from human epidemiological studies, animal models, and experiments in vitro reveals that administration of nonsteroidal anti-inflammatory drugs (NSAIDs) including aspirin (acetylsalicylic acid) represents a treatment of choice for colon cancer. Data indicate that use of NSAIDs, including aspirin, is inversely associated with the risk of colorectal cancer, and clinical trials in patients with familial adenomatous polyposis showed that use of NSAIDs can lead to the regression of colorectal adenomas. In addition, data derived from various animal models of chemical carcinogenesis also suggest that NSAIDs can protect the stomach and esophagus against development of cancer. However, the molecular mechanism by which aspirin exhibits its anticancer effects is not completely clear.
A possible mechanism under lying the antitumour properties of aspirin against cancer has been ascribed to its direct inhibition of cyclooxygenase (COX)-2 in colorectal cancer tissue. However, several lines of evidence suggest that the wide range of antiproliferative potencies of aspirin do not correlate exclusively with the COX-2 inhibitory activity, because some reports showed that NSAIDs can induce apoptosis in colon cancer cells that lack detectable expression of COX-2 protein. Until now, most of researchers recruited COX-2 positive colon cancer cells to study the effect of aspirin on apoptosis and proliferation of colon cancer cells. However, few studies on the effect of aspirin on COX-2 negative colon cancer cells and lack systematic analyses of aspirin-induced morphologic changes are available. Meanwhile, whether aspirin induces apoptosis of COX-2 negative colon cancer cells is controversial. The purpose of this study was to investigate the effect of aspirin on proliferation and apoptosis of SW480 cells, a COX-2 negative human colon cancer cell line.
Aspirin could not only induce apoptosis but also necrosis of cells at high concentrations. After treatment with aspirin, SW480 cells displayed a typically morphological feature of apoptosis and necrosis under transmission electron microscope (TEM), and increased the Bcl-2 expression, but decreased the expression of Bax.
The results of this study confirmed that aspirin could inhibit the proliferation and induce apoptosis of COX-2 negative colon cancer cell line SW480 by down-regulating the Bcl-2 expression, and up-regulating the Bax expression, which might be a potential mechanism by which aspirin plays a positive role in apoptosis of this cell line. This may benefit to the exploration of new anticancer drugs in the future.
This paper describes the antiproliferative effect of aspirin on Cox-2 negative colon cancer cell via the activation of apoptosis, cell cycle arrest and change in the Bax/Bcl-2 balance. The manuscript contains interesting data, and benefits to the elucidation of the pathogenesis of colon cancer.
| 1. | Reddy BS, Rao CV, Rivenson A, Kelloff G. Inhibitory effect of aspirin on azoxymethane-induced colon carcinogenesis in F344 rats. Carcinogenesis. 1993;14:1493-1497. |
| 2. | Garcia Rodriguez LA, Huerta-Alvarez C. Huerta-Alvarez C. Reduced incidence of colorectal adenoma among long-term users of nonsteroidal antiinflammatory drugs: a pooled analysis of published studies and a new population-based study. Epidemiology. 2000;11:376-381. |
| 3. | Garcia Rodriguez LA, Huerta-Alvarez C. Reduced risk of colorectal cancer among long-term users of aspirin and nonaspirin nonsteroidal antiinflammatory drugs. Epidemiology. 2001;12:88-93. |
| 4. | Barnes CJ, Lee M. Chemoprevention of spontaneous intestinal adenomas in the adenomatous polyposis coli Min mouse model with aspirin. Gastroenterology. 1998;114:873-877. |
| 5. | Plummer SM, Holloway KA, Manson MM, Munks RJ, Kaptein A, Farrow S, Howells L. Inhibition of cyclo-oxygenase 2 expression in colon cells by the chemopreventive agent curcumin involves inhibition of NF-kappaB activation via the NIK/IKK signalling complex. Oncogene. 1999;18:6013-6020. |
| 6. | Marrogi AJ, Travis WD, Welsh JA, Khan MA, Rahim H, Tazelaar H, Pairolero P, Trastek V, Jett J, Caporaso NE. Nitric oxide synthase, cyclooxygenase 2, and vascular endothelial growth factor in the angiogenesis of non-small cell lung carcinoma. Clin Cancer Res. 2000;6:4739-4744. |
| 7. | Janne PA, Mayer RJ. Chemoprevention of colorectal cancer. N Engl J Med. 2000;342:1960-1968. |
| 8. | Richter M, Weiss M, Weinberger I, Furstenberger G, Marian B. Growth inhibition and induction of apoptosis in colorectal tumor cells by cyclooxygenase inhibitors. Carcinogenesis. 2001;22:17-25. |
| 9. | Thompson WJ, Piazza GA, Li H, Liu L, Fetter J, Zhu B, Sperl G, Ahnen D, Pamukcu R. Exisulind induction of apoptosis involves guanosine 3',5'-cyclic monophosphate phosphodiesterase inhibition, protein kinase G activation, and attenuated beta-catenin. Cancer Res. 2000;60:3338-3342. |
| 10. | Hanif R, Pittas A, Feng Y, Koutsos MI, Qiao L, Staiano-Coico L, Shiff SI, Rigas B. Effects of nonsteroidal anti-inflammatory drugs on proliferation and on induction of apoptosis in colon cancer cells by a prostaglandin-independent pathway. Biochem Pharmacol. 1996;52:237-245. |
| 11. | Zhang X, Morham SG, Langenbach R, Young DA. Malignant transformation and antineoplastic actions of nonsteroidal antiinflammatory drugs (NSAIDs) on cyclooxygenase-null embryo fibroblasts. J Exp Med. 1999;190:451-459. |
| 12. | Smith ML, Hawcroft G, Hull MA. The effect of non-steroidal anti-inflammatory drugs on human colorectal cancer cells: evidence of different mechanisms of action. Eur J Cancer. 2000;36:664-674. |
| 13. | Sharma RA, Gescher A, Plastaras JP, Leuratti C, Singh R, Gallacher-Horley B, Offord E, Marnett LJ, Steward WP, Plummer SM. Cyclooxygenase-2, malondialdehyde and pyrimidopurinone adducts of deoxyguanosine in human colon cells. Carcinogenesis. 2001;22:1557-1560. |
| 14. | Ealey PA, Yateman ME, Sandhu R, Dattani MT, Hassan MK, Holt SJ, Marshall NJ. The development of an eluted stain bioassay (ESTA) for human growth hormone. Growth Regul. 1995;5:36-44. |
| 15. | Goel A, Chang DK, Ricciardiello L, Gasche C, Boland CR. A novel mechanism for aspirin-mediated growth inhibition of human colon cancer cells. Clin Cancer Res. 2003;9:383-390. |
| 16. | Majno G, Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995;146:3-15. |
| 17. | Goldberg Y, Nassif II, Pittas A, Tsai LL, Dynlacht BD, Rigas B, Shiff SJ. The anti-proliferative effect of sulindac and sulindac sulfide on HT-29 colon cancer cells: alterations in tumor suppressor and cell cycle-regulatory proteins. Oncogene. 1996;12:893-901. |
| 18. | Perugini RA, McDade TP, Vittimberga FJ Jr, Duffy AJ, Callery MP. Sodium salicylate inhibits proliferation and induces G1 cell cycle arrest in human pancreatic cancer cell lines. J Gastrointest Surg. 2000;4:24-32, discussion 32-33. |
| 19. | Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nunez G, Thompson CB. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597-608. |