Published online Jul 14, 2008. doi: 10.3748/wjg.14.4209
Revised: June 2, 2008
Accepted: June 9, 2008
Published online: July 14, 2008
AIM: To study the expression of human insulin gene in gastrointestinal tracts of diabetic rats.
METHODS: pCMV.Ins, an expression plasmid of the human insulin gene, wrapped with chitosan nanoparticles, was transfected to the diabetic rats through lavage and coloclysis, respectively. Fasting blood glucose and plasma insulin levels were measured for 7 d. Reverse transcription polymerase chain reaction (RT-PCR) analysis and Western blot analysis were performed to confirm the expression of human insulin gene.
RESULTS: Compared with the control group, the fasting blood glucose levels in the lavage and coloclysis groups were decreased significantly in 4 d (5.63 ± 0.48 mmol/L and 5.07 ± 0.37 mmol/L vs 22.12 ± 1.31 mmol/L, respectively, P < 0.01), while the plasma insulin levels were much higher (32.26 ± 1.81 &mgr;IU/mL and 32.79 ± 1.84 &mgr;IU/mL vs 14.23 ± 1.38 &mgr;IU/mL, respectively, P < 0.01). The human insulin gene mRNA and human insulin were only detected in the lavage and coloclysis groups.
CONCLUSION: Human insulin gene wrapped with chitosan nanoparticles can be successfully transfected to rats through gastrointestinal tract, indicating that chitosan is a promising non-viral vector.
- Citation: Niu L, Xu YC, Dai Z, Tang HQ. Gene therapy for type 1 diabetes mellitus in rats by gastrointestinal administration of chitosan nanoparticles containing human insulin gene. World J Gastroenterol 2008; 14(26): 4209-4215
- URL: https://www.wjgnet.com/1007-9327/full/v14/i26/4209.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.4209
Type 1 diabetes mellitus is the result of insulin deficiency caused by the autoimmune destruction of insulin-producing pancreatic β cells. Hyperglycemia would cause a lot of long-term clinical problems, including renal failure, retinopathy, neuropathy and heart disease[1]. Although intensive exogenous insulin therapy can delay or prevent the onset of chronic complications, it is rather cumbersome and sometimes would cause hypoglycemia, which could be life-threatening. However, the development of gene therapy has also generated a greater hope and excitement for a possible “cure” of diabetes since insulin gene was first cloned and expressed in cultured cells in the late 1970s[2]. Many attempts have been made, including islet transplantation[3–5], whole pancreas transplantation[67], regeneration of β cells[8–10] and insulin gene therapy[11–13].
In general, whole organ transplants have more sustained and durable function. Advances in islet transplantation procedures now mean that patients with the disease can be cured by transplantation of primary human islets of Langerhans. The major drawbacks of these strategies are the insufficient availability of donor islets, invasive procedure and high cost. Due to the limited available number of donor islet cells, researchers are looking for different sources of pancreatic islet progenitor or stem cells. Stem cells with an extensive proliferative ability may provide a valuable source of islet progenitor cells. Several studies have demonstrated that progenitor/stem cells can be expanded in vitro to generate a large number of islet progenitor cells[14–16]. However, efficient and direct differentiation of these cells to an endocrine pancreatic lineage is difficult to achieve. Insulin gene therapy including any approach involving the introduction of a foreign gene into any cell type in the body can produce insulin[17]. The gene(s) introduced could be the insulin gene itself, perhaps under control of a tissue specific promoter, allowing for expression in a selected non-β-cell type, or in a gene encoding for a factor that activates the insulin gene, thereby allowing for ectopic insulin production. In insulin gene therapy, one of the key issues is the selection of carriers. Conventional viral vectors can introduce exogenous genes into cells precisely and effectively, but they can easily cause immune reactions because of the existence of antiviral immune system. More and more researchers are interested in non-viral vectors.
In this study, we constructed an expression plasmid pCMV.Ins expressing human insulin gene. Then, we wrapped the pCMV.Ins with chitosan nanoparticles, a non-viral vector, and transfected to diabetic rats through gastrointestinal tract to explore the gene therapy for type 1 diabetes mellitus. At present, there is no report on transfection of human insulin gene to gastrointestinal tract and application of chitosan as a vector in gene therapy for type 1 diabetes mellitus.
The cDNA sequences of human insulin gene were cut from the PBAT16, hInsG1.M2 (presented by Doctor Michael German, Department of Hormone Research Institute, University of San Francisco, USA.) using BglII/ NotI enzyme inserted into the expression vector of pCMV.eGFP in the cohesive end. Then the pCMV.Ins was transformed into E.coli. The top10F strains (Invitrogen) were screened. Plasmids isolated with a large-scale alkaline lysis procedure were purified, 0.1 g chitosan was dissolved in 100 mL acetic acid, and the pH was adjusted to 5.5 with sodium hydrate. The solution was stored at 4°C after determined with a micropore film, the aperture of which was 0.22 &mgr;m. Before it was used, the chitosan solution was diluted with ddH2O until the concentration reached 0.02%. One hundred &mgr;L DNA solution at a concentration of 200 &mgr;g/mL was added into 100 &mgr;L sodium sulfate solution at a concentration of 25 mmol/L. After the chitosan and DNA solutions were heated to 55°C in an aqueous bath for 15 min, respectively, they were mixed immediately and put into convolution for 1 min. The volume of reaction system should not excess 500 &mgr;L[18]. Experiments were carried out in 45 male Wistar rats (weighing180-190 g) at the age of 8 wk. After an overnight fasting, the rats were given a single intraperitoneal injection of 60 mg/kg streptozotocin (STZ, Upjohn, Kalamazoo, Ml, USA) in a 0.01 mol/L citrate buffer. Forty rats with their fasting blood glucose level > 16.7 mmol/L were used in the study. The rats were randomly divided into 4 groups. Chitosan-DNA nanoparticles were transfected to the diabetic rats through lavage and coloclysis, respectively. The two control groups were treated with naked chitosan and normal saline respectively. The rats had free access to diets and water.
pCMV.Ins was identified by PCR. The sequences of primers used for human insulin specifically are 5'- ATCACTGTCCTTCTGCCA-3' (forward) and 5'- GGGTGTGTAGAAGAAGCC-3' (reverse). The PCR products were analyzed by a 1.5% agarose gel electrophoresis, and the expected amplification length was 173 bp. The DNA sequencing of pCMV.Ins plasmid was performed in the Shanghai Sangon Sequencing Center.
The fasting blood glucose and plasma insulin levels were measured in all groups. The glucose levels in blood samples obtained from tail veins were measured with the one touch meter and test strips (Lifescan Inc, USA) every morning. The plasma human insulin was detected with a commercial human insulin radioimmunoassay (RIA) kit (Institute of Atomic Energy, China).
RT-PCR was performed to verify the expression of human insulin gene in gastrointestinal tract. Half of the rats in each group were killed on the 4th d after they transfected with chitosan-DNA nanoparticles. Immediately upon death, stomachs, intestines and rectums of the treated and control animals were flash-frozen in liquid nitrogen, and stored at -70°C. Total RNA (5 &mgr;g), obtained using Trizol (Gibco BRL, Gaithersburg, MD, USA) according to the manufacturer’s instructions, was subjected to reverse transcription by using an oligo-dT21 primer, recombinant RNAsin, and AMV reverse transcriptase (all Promega, Madison, WI, USA). AMV RT was inactivated at 95°C, and PCR was performed by using a gene amp PCR system 9600 thermal cycler (Perkin Elmer, Norwalk, CT, USA), and Taq DNA polymerase (Perkin Elmer). The cDNA-mixture was allowed to react for 19 (GAPDH), or 22 (insulin) cycles. The sequences of primers used for human insulin are 5'-ACCATGGCCCTGTGGATGCGC-3' (forward), and 5'-CTAGTTGCA GTAGTTCTCCAG-3' (reverse). The sequences of primers for GAPDH are 5'-ACCACAGTCCATGCCATCAC-3' (forward) and 5'-TCCACCACCCTGTTG CTGTA-3' (reverse). Insulin primers were designed to amplify human insulin specifically. Primers for GAPDH were used in control reactions. The RT-PCR products were analyzed by a 1.5% agarose gel electrophoresis, and the expected amplification lengths were 336 bp and 451 bp, respectively.
Four days after the transfection, stomachs and intestines were harvested and resuspended in a lysis buffer containing 1% nonidet P-40, 50 mmol/L Tris-HCl (pH 7.4), 150 mmol/L NaCl, 200 U/mL aprotinin, 1 mmol/L phenylmethanesulfonyl fluoride. The tissue lysates (50 mg of protein) were separated by 12% polyacrylamide gel electrophoresis and blotted onto poly-vinylidene difluoride membranes. Immunoblotting was performed with the antibody against human insulin (Sigma-Aldrich Corp, St Louis, MO, USA), and the molecular weight of human insulin was known as 56 kDa.
Data were expressed as mean ± SD. The concentrations of blood glucose and plasma insulin were evaluated by one-way ANOVA (SPSS for Windows 11.5). P < 0.05 was considered statistically significant.
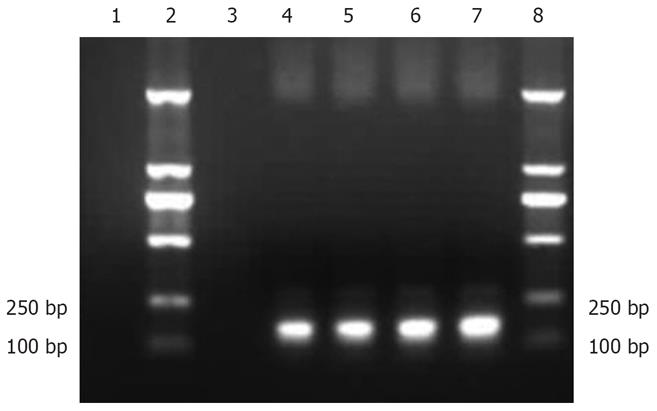
The PCR products of recombinant plasmid pCMV.Ins and bacterial suspension were amplified. One specific band was obtained in lanes 4-7, respectively, corresponding to the expected size of 173 bp. No fragments in lanes 1 and 3 were amplified from pCMV.eGFP and negative control (Figure 1), suggesting that the human insulin gene was successfully inserted into recombinant plasmid pCMV.Ins. The sequencing results also revealed that the recombinant pCMV.Ins plasmid was successfully constructed. The number of sequencing reports was LE142.
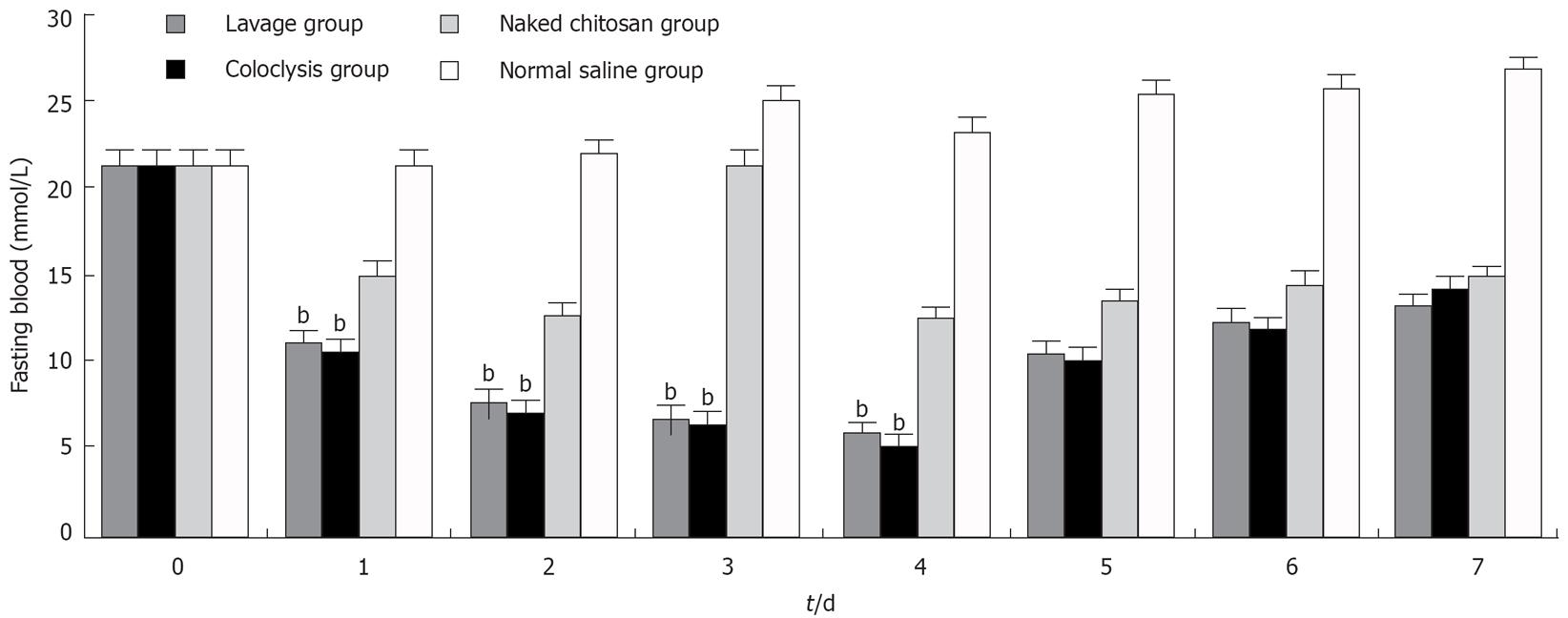
The fasting blood glucose level was decreased in lavage and coloclysis groups from 22.12 ± 1.31 mmol/L to 5.63 ± 0.48 mmol/L and 5.07 ± 0.37 mmol/L, respectively, after transfected (n = 10 each group). The levels of fasting blood glucose were significantly lower in the lavage group and coloclysis group transfected with chitosan-DNA nanoparticles (P < 0.01) after the normal saline treatment from the 1st to 4th d (Figure 2). The blood glucose levels in the naked chitosan group also decreased significantly, because chitosan had the effect of decreasing blood glucose level. The blood glucose levels in the lavage and coloclysis groups were much lower than those in the naked chitosan group, and the differences were significant (P < 0.01). There was no difference in blood glucose levels between the lavage and coloclysis groups.
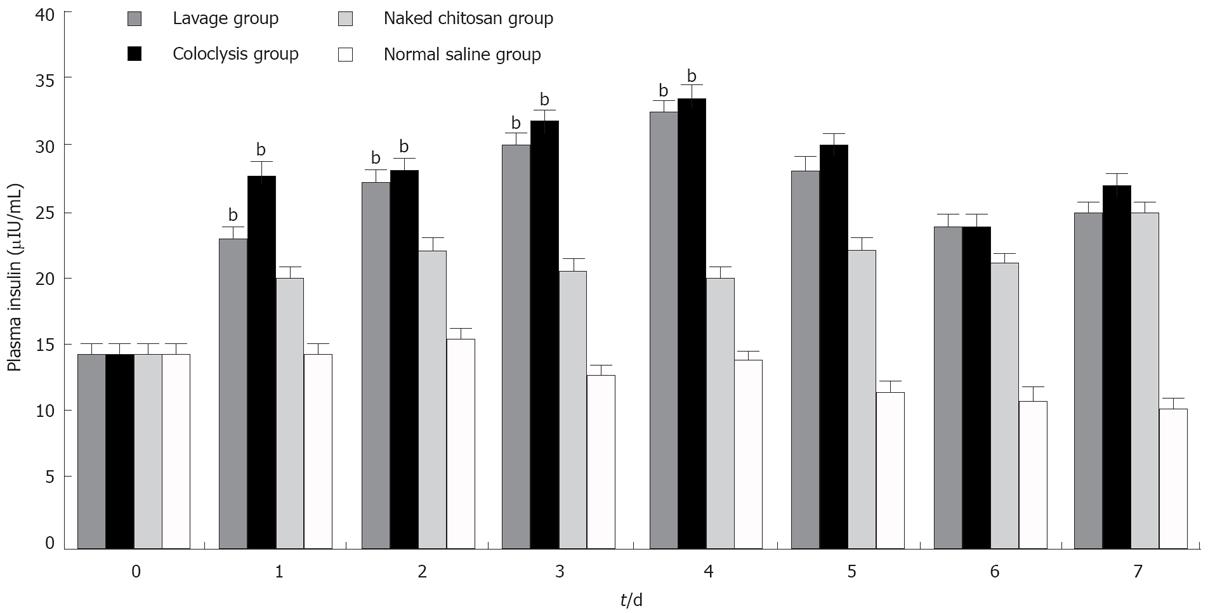
The plasma insulin in the lavage and coloclysis groups increased from 14.23 ± 1.38 &mgr;IU/mL to 32.26 ± 1.81 &mgr;IU/mL and 32.7 ± 1.84 &mgr;IU/mL after transfection (n = 10 each group). The plasma insulin levels in the lavage, coloclysis and naked chitosan groups (Figure 3) were higher than those in the normal saline group from the Day 1 to the Day 4 (P < 0.01). There were remarkable differences among the lavage, coloclysis and naked chitosan groups (P < 0.01). The differences between the lavage and coloclysis groups did not show any statistical significance. These results were consistent with reduction in the fasting blood glucose levels.
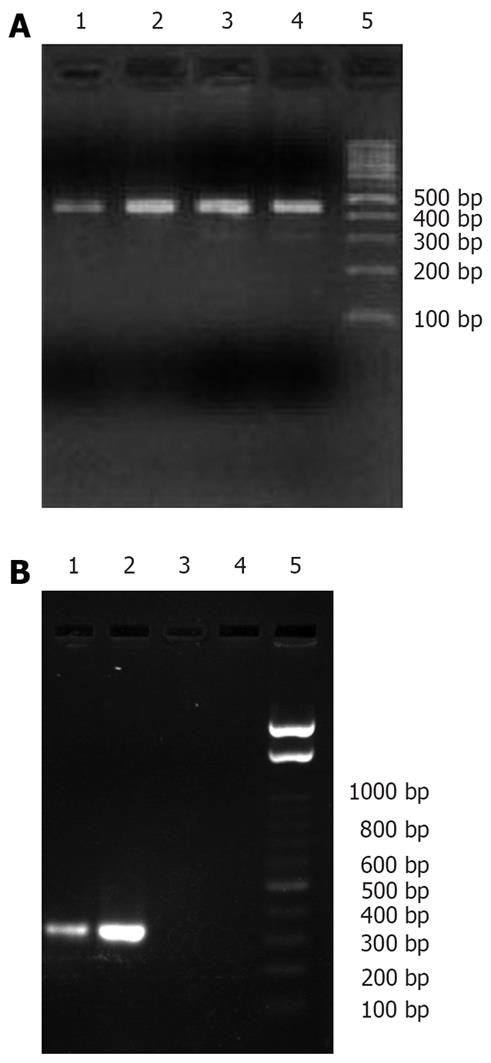
To analyze the expression of the human insulin gene, RT-PCR and Western blot analysis were carried out. Total RNA from the gastrointestinal tract was amplified using primers specific for GAPDH, revealing a 451 bp fragment in all groups (Figure 4A). However, human insulin specific primers produced a fragment, corresponding to the expected size of 336-bp in lanes 1 and 2, only in reactions containing RNA from the lavage and coloclysis groups, respectively (Figure 4B). No products were amplified from cDNA in the naked chitosan group (lane 3) and normal saline group (lane 4).
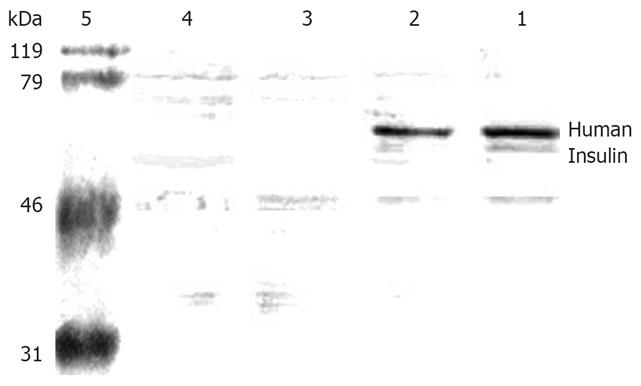
The molecular weight of human insulin was 56 kDa (Figure 5). One specific band was only obtained in the lavage and coloclysis groups in lanes 1 and 2, respectively. No products were obtained in the naked chitosan group (lane 3) and normal saline group (lane 4). These results suggest that the exogenous transferred pCMV.Ins plasmid but not the endogenous insulin gene expressed the mRNA and human insulin.
One factor critical to successful gene therapy is the development of efficient delivery systems. Despite the advances in gene transfer technology including viral and non-viral vectors, no ideal vector system is available at present[19]. Although viral vectors can introduce exogenous genes into cells precisely and effectively, they can easily cause immune reactions because of the existence of antiviral immune system. Due to the growing concerns over the toxicity and immunogenicity of viral DNA delivery systems, DNA delivery via improving viral routes has become more desirable and advantageous[20].
A perfect vector should also be biocompatible, efficient, and modular so that it can be applied both in research and in clinical settings[21]. Taking into account this point, we selected chitosan nanoparticle, a kind of non-viral vector, in the study. We found that human insulin gene wrapped with chitosan nanoparticles could decrease the fasting blood glucose level and increase the insulin level in STZ diabetic rats. The mRNA in human insulin gene and human insulin was detectable in the gastrointestinal tract. These results demonstrate that chitosan nanoparticles can mediate the transfection of human insulin gene and that chitosan nanoparticles can be used as a good vector in gene therapy of type 1 diabetes mellitus. Köping-Höggård et al[22] reported that aerosol delivery formulated with chitosan oligomers can improve the distribution of pDNA polyplexes in the lungs and increase 6-fold of the efficiency of gene delivery in vivo over the commonly used intratracheal instillation method.
Chitosan nanoparticles are a kind of non-viral vector. Non-viral vector includes liposome[23–25], composite[26], microsphere, and nanoparticles, etc[2728], but the cytotoxicity of the bangosome limits its application in vivo. Owing to its loose constitution, the constancy of composites is poor. The diameter of microsphere is bigger than that of nanoparticles. Chitosan nanoparticles are a comparatively promising non-viral vector. Chitosan nanoparticles[29] have a good biocompatibility and no toxicity, and are economically available. The transfection efficiency of chitosan can be regulated by changing its molecular weight, plasmid concentration, and the chitosan/plasmid ratio. After the plasmid is embedded in chitosan, it can resist the degradation of nucleases. It also exhibits an antibacterial activity by inhibiting the bacterial metabolism.
In our study, the fasting blood glucose level was decreased during the first 4 d, due to the regeneration of gastrointestinal tract epithelial cells, which is consistent with the reported data[30].
Further study should be performed to detect the cells intaking the plasmid. We speculate that gut K-cells may intake the expression plasmid. The gut K-cells in the epithelium mucosa of gastrointestinal tract secrete glucose-dependent insulinotropic polypeptide (GIP)[31], an incretin hormone secreted by endocrine K-cells in response to nutrient absorption. GIP can stimulate β cells to release insulin, and promote the regeneration of β cells[32]. GLP accounts for about 60% of the stimulation of insulin by oral glucose, but the determinants of their secretion from the small intestine are poorly understood[33]. There are many similarities between the release of GIP and the secretion of insulin. The concentration of GIP will obviously increase several minutes after glucose ingestion, and return to its basal level after 2 h. It was reported that the antagonist of GIP can remarkably degrade the secretion of insulin[34]. Furthermore, the promoter of GIP can only be activated in K-cells.
One of the key points in gene therapy for diabetes is the modification (processing) of proinsulin to insulin. Most non-β cells functioning as target cells in gene therapy for diabetes are lack of typical prohormone convertases which are essential to the processing of proinsulin, whereas K-cells can produce PC2 and PC3, which can help process the proinsulin correctly[35]. Palizban et al have transfected rat small intestine K-cells with pGIP/Ins plasmid by DOTAP liposome[36]. As mentioned above, K-cells might be the best target cells in gene therapy for type 1 diabetes mellitus due to their response to glucose and resistance to destruction mediated by cytokines and free radicals.
In conclusion, the human insulin gene can be transfected successfully by chitosan-DNA nanoparticles and expressed efficiently in the gastrointestinal tract of diabetic rats, and chitosan is a promising non-viral vector. If it is applied in clinic practice, it would be accepted by patients. Although much work remains to be done, the rapid progress in insulin gene therapy provides an optimistic outlook for its clinical applications in the treatment of type 1 diabetes mellitus.
Gastrointestinal K-cells might be the best target cells in gene therapy for type 1 diabetes mellitus due to their response to glucose and the resistance to the destruction mediated by cytokines and free radicals. A perfect vector should also be biocompatible, efficient, and modular so that it can be applied both in research and in clinical settings. Chitosan is an ideal non-viral vector and has drawn wide attention.
There are considerable endocrine cells in the gastrointestinal tract. Gastrointestinal K-cells are the potential and ideal target cells in gene therapy for diabetes. Progress in gene therapy has produced promising results that translate experimental research into clinical treatment. The main barrier in gene transfer is a safe and effective gene delivery system.
The exogenous insulin genes can be transfected by chitosan nanoparticles and expressed efficiently in the gastrointestinal tract of diabetic rats, indicating that chitosan is a promising non-viral vector. At present, there is no report on the transfection of human insulin gene to the gastrointestinal tract and on the application of chitosan as a vector in gene therapy for type 1 diabetes mellitus.
The superiority of human insulin gene expression in gastrointestinal tract by chitosan nanoparticles is its safety without any wound. If it can be applied in clinic practice, it would be accepted by patients. Chitosan is an ideal non-viral vector and can be widely used in gene transfer.
Gastrointestinal K-cells exist in the epithelium mucosa of gastrointestinal tract and can secrete glucose-dependent insulinotropic polypeptide (GIP), which can stimulate β cells to release insulin and promote the regeneration of β cells. Chitosan nanoparticles are a kind of non-viral vector and have a good biocompatibility without any toxicity, and are economically available. The transfection efficiency of chitosan can be regulated easily. After the plasmid is embedded in chitosan, it can resist the degradation of nucleases.
This paper reports the expression of human insulin gene in the gastrointestinal tract by chitosan nanoparticles, thus providing new technologies of gene transfer to endocrine cells in the gastrointestinal tract. This study is well designed and interesting.
| 1. | Dieterle C, Brendel MD, Seissler J, Eckhard M, Bretzel RG, Landgraf R. [Therapy of diabetes mellitus. Pancreas transplantation, islet transplantation, stem cell and gene therapy]. Internist (Berl). 2006;47:489-496, 498-501. |
| 2. | Samson SL, Chan L. Gene therapy for diabetes: reinventing the islet. Trends Endocrinol Metab. 2006;17:92-100. |
| 3. | Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230-238. |
| 4. | Lau J, Mattsson G, Carlsson C, Nyqvist D, Kohler M, Berggren PO, Jansson L, Carlsson PO. Implantation site-dependent dysfunction of transplanted pancreatic islets. Diabetes. 2007;56:1544-1550. |
| 5. | Li M, Inaba M, Guo KQ, Hisha H, Abraham NG, Ikehara S. Treatment of streptozotocin-induced diabetes mellitus in mice by intra-bone marrow bone marrow transplantation plus portal vein injection of beta cells induced from bone marrow cells. Int J Hematol. 2007;86:438-445. |
| 6. | Sutherland DE, Gruessner RW, Dunn DL, Matas AJ, Humar A, Kandaswamy R, Mauer SM, Kennedy WR, Goetz FC, Robertson RP, Gruessner AC, Najarian JS. Lessons learned from more than 1,000 pancreas transplants at a single institution. Ann Surg. 2001;233:463-501. |
| 7. | Socci C, Orsenigo E, Zuber V, Caldara R, Castoldi R, Parolini D, Secchi A, Staudacher C. Triple arterial reconstruction improves vascularization of whole pancreas for transplantation. Transplant Proc. 2006;38:1158-1159. |
| 8. | Vaca P, Berna G, Araujo R, Carneiro EM, Bedoya FJ, Soria B, Martin F. Nicotinamide induces differentiation of embryonic stem cells into insulin-secreting cells. Exp Cell Res. 2008;314:969-974. |
| 9. | Brenner S, Ryser MF, Whiting-Theobald NL, Gentsch M, Linton GF, Malech HL. The late dividing population of gamma-retroviral vector transduced human mobilized peripheral blood progenitor cells contributes most to gene-marked cell engraftment in nonobese diabetic/severe combined immunodeficient mice. Stem Cells. 2007;25:1807-1813. |
| 10. | Timper K, Seboek D, Eberhardt M, Linscheid P, Christ-Crain M, Keller U, Muller B, Zulewski H. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem Biophys Res Commun. 2006;341:1135-1140. |
| 11. | Fukushima M, Hattori Y, Tsukada H, Koga K, Kajiwara E, Kawano K, Kobayashi T, Kamata K, Maitani Y. Adiponectin gene therapy of streptozotocin-induced diabetic mice using hydrodynamic injection. J Gene Med. 2007;9:976-985. |
| 12. | Yoo HS, Mazda O, Lee HY, Kim JC, Kwon SM, Lee JE, Kwon IC, Jeong H, Jeong YS, Jeong SY. In vivo gene therapy of type I diabetic mellitus using a cationic emulsion containing an Epstein Barr Virus (EBV) based plasmid vector. J Control Release. 2006;112:139-144. |
| 13. | Lu YC, Sternini C, Rozengurt E, Zhukova E. Release of transgenic human insulin from gastric g cells: a novel approach for the amelioration of diabetes. Endocrinology. 2005;146:2610-2619. |
| 14. | Cho YM, Lim JM, Yoo DH, Kim JH, Chung SS, Park SG, Kim TH, Oh SK, Choi YM, Moon SY. Betacellulin and nicotinamide sustain PDX1 expression and induce pancreatic beta-cell differentiation in human embryonic stem cells. Biochem Biophys Res Commun. 2008;366:129-134. |
| 15. | Jafary H, Larijani B, Farrokhi A, Pirouz M, Mollamo-hammadi S, Baharvand H. Differential effect of activin on mouse embryonic stem cell differentiation in insulin-secreting cells under nestin-positive selection and spontaneous differentiation protocols. Cell Biol Int. 2008;32:278-286. |
| 16. | Serafimidis I, Rakatzi I, Episkopou V, Gouti M, Gavalas A. Novel effectors of directed and Ngn3-mediated differentiation of mouse embryonic stem cells into endocrine pancreas progenitors. Stem Cells. 2008;26:3-16. |
| 17. | D'Anneo A, Rood P, Bottino R, Balamurugan AN, He J, Giannoukakis N. Gene therapy for type 1 diabetes: is it ready for the clinic? Immunol Res. 2006;36:83-89. |
| 18. | Mao HQ, Roy K, Troung-Le VL, Janes KA, Lin KY, Wang Y, August JT, Leong KW. Chitosan-DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiency. J Control Release. 2001;70:399-421. |
| 20. | Zaia JA. The status of gene vectors for the treatment of diabetes. Cell Biochem Biophys. 2007;48:183-190. |
| 21. | Prud'homme GJ, Glinka Y, Khan AS, Draghia-Akli R. Electroporation-enhanced nonviral gene transfer for the prevention or treatment of immunological, endocrine and neoplastic diseases. Curr Gene Ther. 2006;6:243-273. |
| 22. | Koping-Hoggard M, Issa MM, Kohler T, Tronde A, Varum KM, Artursson P. A miniaturized nebulization catheter for improved gene delivery to the mouse lung. J Gene Med. 2005;7:1215-1222. |
| 23. | Chaszczewska-Markowska M, Stebelska K, Sikorski A, Madej J, Opolski A, Ugorski M. Liposomal formulation of 5-fluorocytosine in suicide gene therapy with cytosine deaminase--for colorectal cancer. Cancer Lett. 2008;262:164-172. |
| 24. | Bajaj A, Kondaiah P, Bhattacharya S. Gene transfection efficacies of novel cationic gemini lipids possessing aromatic backbone and oxyethylene spacers. Biomacromolecules. 2008;9:991-999. |
| 25. | Kim HS, Kim JS, Lee YK, Koo KH, Park YS. An efficient liposomal gene delivery vehicle using Sendai F/HN proteins and protamine. Cancer Gene Ther. 2008;15:214-224. |
| 26. | Turner P, Petch A, Al-Rubeai M. Encapsulation of viral vectors for gene therapy applications. Biotechnol Prog. 2007;23:423-429. |
| 27. | Ragusa A, Garcia I, Penades S. Nanoparticles as nonviral gene delivery vectors. IEEE Trans Nanobioscience. 2007;6:319-330. |
| 28. | Schmitz T, Bravo-Osuna I, Vauthier C, Ponchel G, Loretz B, Bernkop-Schnurch A. Development and in vitro evaluation of a thiomer-based nanoparticulate gene delivery system. Biomaterials. 2007;28:524-531. |
| 29. | Douglas KL, Piccirillo CA, Tabrizian M. Effects of alginate inclusion on the vector properties of chitosan-based nanoparticles. J Control Release. 2006;115:354-361. |
| 30. | Okamoto R, Watanabe M. Molecular and clinical basis for the regeneration of human gastrointestinal epithelia. J Gastroenterol. 2004;39:1-6. |
| 31. | Cani PD, Holst JJ, Drucker DJ, Delzenne NM, Thorens B, Burcelin R, Knauf C. GLUT2 and the incretin receptors are involved in glucose-induced incretin secretion. Mol Cell Endocrinol. 2007;276:18-23. |
| 32. | Cassidy RS, Irwin N, Flatt PR. Effects of gastric inhibitory polypeptide (GIP) and related analogues on glucagon release at normo- and hyperglycaemia in Wistar rats and isolated islets. Biol Chem. 2008;389:189-193. |
| 33. | Kuo P, Chaikomin R, Pilichiewicz A, O'Donovan D, Wishart JM, Meyer JH, Jones KL, Feinle-Bisset C, Horowitz M, Rayner CK. Transient, early release of glucagon-like peptide-1 during low rates of intraduodenal glucose delivery. Regul Pept. 2008;146:1-3. |
| 34. | McClean PL, Gault VA, Irwin N, McCluskey JT, Flatt PR. Daily administration of the GIP-R antagonist (Pro3)GIP in streptozotocin-induced diabetes suggests that insulin-dependent mechanisms are critical to anti-obesity-diabetes actions of (Pro3)GIP. Diabetes Obes Metab. 2008;10:336-342. |
| 35. | Cheung AT, Dayanandan B, Lewis JT, Korbutt GS, Rajotte RV, Bryer-Ash M, Boylan MO, Wolfe MM, Kieffer TJ. Glucose-dependent insulin release from genetically engineered K cells. Science. 2000;290:1959-1962. |
| 36. | Palizban AA, Salehi R, Nori N, Galehdari H. In vivo transfection rat small intestine K-cell with pGIP/Ins plasmid by DOTAP liposome. J Drug Target. 2007;15:351-357. |