Published online Jul 7, 2008. doi: 10.3748/wjg.14.3990
Revised: May 30, 2008
Accepted: June 6, 2008
Published online: July 7, 2008
AIM: To investigate the anti-tumor effect of Chinese medicine Gecko on human esophageal carcinoma cell lines and xenografted sarcoma 180 in Kunming mice and its mechanism.
METHODS: The serum pharmacological method was used in vitro. The growth rates of the human esophageal carcinoma cells (EC9706 or EC1) were measured by a modified 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The transplanted tumor model of the mouse S180 sarcoma was established. Fifty mice were randomly divided into five groups (n = 10). Three Gecko groups were treated respectively with oral administration of Gecko powder at a daily dose of 13.5 g/kg, 9 g/kg, and 4.5 g/kg. The negative group (NS group) was treated with oral administration of an equal volume of saline and the positive group (CTX group) was treated with 100 mg/kg Cytoxan by intraperitoneal injection at the first day. After 2 wk of treatment, the anti-tumor activity was evaluated by tumor tissue weighing. The impact on immune organ was detected based on the thymus index, spleen index, phagocytic rate and phagocytic index. The protein expression of vascular endothelin growth factor (VEGF) and basic fibroblast growth factor (bFGF) were detected by immunohistochemistry. The cell apoptotic rate was detected by terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end labeling (TUNEL) assay.
RESULTS: The A value in each group treated with Gecko after 72 h was reduced significantly in EC9706 and in EC1. The tumor weight in each group of Gecko was decreased significantly (1.087 ± 0.249 vs 2.167 ± 0.592; 1.021 ± 0.288 vs 2.167 ± 0.592; 1.234 ± 0.331 vs 2.167 ± 0.592; P < 0.01, respectively). However, the thymus index and Spleen index of mice in Gecko groups had no significant difference compared with the NS group. The immunoreactive score of VEGF and bFGF protein expression of each Gecko group by immunohistochemical staining were lowered significantly. The apoptosis index (AI) of each group was increased progressively with increase of dose of Gecko by TUNEL.
CONCLUSION: Gecko has anti-tumor effects in vitro and in vivo; induction of tumor cell apoptosis and the down-regulation of protein expression of VEGF and bFGF may be contributed to anti-tumor effects of Gecko.
-
Citation: Liu F, Wang JG, Wang SY, Li Y, Wu YP, Xi SM. Antitumor effect and mechanism of
Gecko on human esophageal carcinoma cell linesin vitro and xenografted sarcoma 180 in Kunming mice. World J Gastroenterol 2008; 14(25): 3990-3996 - URL: https://www.wjgnet.com/1007-9327/full/v14/i25/3990.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3990
Malignant tumor is always a great menace to human health. The incidence and mortality of tumor keep ascending all over the world. Anti-tumor therapies contain surgery, radiotherapy and chemotherapy, and it depends on synthetic agents to a great extent in chemotherapy. Although chemical anti-neoplastics have definite effect; they cause severe adverse effects. Moreover, many chemotherapy drugs bring about multiple drug resistance. Great efforts have been made to develop new anti-cancer pharmaceuticals from Chinese herbal medicine[1–4].
Gecko is a traditional Chinese medicine found to restrain inflammation and allergic response, detumescence and alimentation. Gecko is also called Tian Long, Shou Gong. It contains Gekko swinhonis Gunther, Gekko japonicus, etc.. It is cold in nature and flavor. The dosage forms are pill, powder and mastic[5]. Although the Gecko was not recorded in a medical dictionary, there have been reports that Gecko and its compound prescriptions could treat malignant tumors, tuberculosis, osteomyelitis and syrinx. In clinical practice, it has definite effect against malignant tumors, especially digestive system tumors, such as esophagus cancer, gastric cancer and liver cancer[6–8]. Research on Gecko mainly focused on the anti-tumor compound prescriptions, but there have been few pharmacological studies of Gecko and its mechanisms of anti-tumor action.
The present study was to observe the anti-tumor activities of Gecko in vivo and in vitro using the transplanted tumor model of the mouse S180 sarcoma on mice and the human esophageal carcinoma cells and investigate the effects of Gecko on cell apoptosis and the expression of (vascular endothelin growth factor) VEGF and (basic fibroblast growth factor) bFGF of S180 mice.
Chinese medicine Gecko was purchased from Anhui Bozhou Yonggang Co. Ltd. They were identified Gekko japonicus. The whole dry Gecko were ground to fine powder and diluted in suspension using 0.2% carboxymethyl cellulose (CMC). Cytoxan (CTX) was purchased from Jiangsu Hengrui Medicine Co. Ltd. (Batch No. H32020857). Fifty Kunming mice and ten SD rats were provided by the Medical Experimental Animal Center, Henan Province. All were female. The code number of the animals was 0001350. The human esophageal carcinoma EC9706 cell line was of esophageal carcinoma of fungating type which is well-differentiated squamous cell carcinoma, EC1 cell line was isolated from esophageal carcinoma cell line EC9706 and one subline, and the S180 mouse sarcoma cell line was kindly provided by the Medical College, Zhengzhou University.
The cells were grown in a monolayer culture containing humidified 5% CO2 in air at 37°C. They were cultured in RPMI-1640 (Sigma, USA) medium supplemented with 10% fetal calf serum, 100 U/mL and penicillin and 100 mg/L streptomycin. The S180 model was established by subcutaneous injection as previously described[9]. Briefly, the S180 mouse sarcoma cells with ascites were harvested, diluted with sterilized saline at a ratio of 1:8 (cell concentration was adjusted to 1.0 × 106/mL), and inoculated subcutaneously into the right armpit region of Kunming mice.
Ten SD rats (aged 10-12 wk and weighing 280 ± 30 g) were randomly divided into five groups: the negative group (NS group), the positive group (CTX group) and three Gecko groups. The CTX group received 50 mg/kg intraperitoneally once a day. Three Gecko groups were treated respectively with oral administration of Gecko at a dose of 13.5 g/kg, 9 g/kg and 4.5 g/kg, twice a day. The NS group received the equivalent amounts of normal saline in the same way. After 1 wk of treatment, according to the method principle of Li Yikui[1011], blood was collected aseptically and serum was collected by centrifugation. It was deactivated at 56°C for 30 min and filtrated for sterilization. Medical serum was stored at -20°C for use.
Cell growth was measured by a modified MTT assay[1213]. The logarithmic cells (EC9706, EC1) were dispersed with 0.02% (w/v) EDTA to prepare 1 × 104/mL cell suspension, and partitioned into 96-well plates at 180 &mgr;L/well for 24 h culture in a 5% CO2 incubator under 37°C. Five groups were divided, the negative group, the CTX group and the three Gecko groups. Each group had 6 wells. Each well was treated respectively with 20 &mgr;L medical serum prepared as above. Cells were incubated for 24, 48 and 72 h. Then 20 &mgr;L MTT (Sigma, USA) was added to each well and the cells were further incubated at 37°C for 4 h. The supernatant was removed and 200 &mgr;L DMSO (Sigma, USA) was added into each well to solubilize the formazan product. The absorbance also known as A value at wavelength of 470 nm was measured by a microplate reader (Sigma, USA). Triplicate experiments were performed in a parallel manner for each concentration point and the results were presented as mean ± SD. Cell inhibitory rate was calculated by the following formula: inhibitory rate (%) = (1-A treated/A control) × 100%.
EC9706, EC1 cells (1 × 104/mL cell suspension) were plated onto 96-well plates at 180 &mgr;L/well. Each well was treated respectively with 20 &mgr;L medical serum prepared as above and cultured for 7 d. Viable cells were counted under the inverted light microscope by trypan blue dye (Sigma, USA) exclusion method and growth curves were drawn.
Twenty-four hours after establishment of S180 model, the fifty transplanted mice (aged 8-10 wk and weighing 26 ± 2 g) were randomly divided into five groups: the NS group, the CTX group (CTX was proved to have definite effect against mouse S180 sarcoma and frequently used in experiment), and the three Gecko groups. They were treated respectively with oral administration of saline once a day, intraperitoneal injection of CTX 100 mg/kg only once, and oral administration of Gecko at a dose of 13.5 g/kg, 9 g/kg and 4.5 g/kg once a day. After 2 wk of treatment, the anti-tumor activity was evaluated by tumor tissue weighing. The following formula was used: Tumor inhibitory rate (%) = (1-average tumor weighing of administration team/average tumor weighing of the control) ×100%[9].
According to the above-mentioned methods, at the 14th day, 5% chicken-red cells were injected intraperitoneally into each group. After 12 h, the mice were killed and 1 mL peritoneal fluid was drawn for glass slide. After incubated for 30 min, peritoneal fluid was fixed with the mixture of acetone/methanol (1:1, v/v) and dyed with 4% Giemsa stain. Peritoneal macrophages were counted under microscope. The effect of Gecko on phagocytosis of enterocoelia macrophage was evaluated by the chicken-red cell phagocytic index and phagocytic rate[14]. At the same time, thymus and spleen were taken from mice. The impact on immune organ was evaluated based on the thymus index and spleen index[15].
The tumor tissues were fixed with 10% neutral formalin at room temperature for 24 h. The paraffin-embedded specimens were cut into sections with a thickness of 5 &mgr;m. The detection procedure was done as described in Kit protocol (Wuhan Boster Biological Technology Co. Ltd). PBS instead of the first antibodies was used in the negative control. The VEGF and bFGF positive cells were defined when there was an aggregation of brown particles in the cytoplasm of the tumor cells. The immunoreactive score was determined by the sum of extension and intensity as reported previously[16].The intensity of staining was scored on a scale of 0 to 3 (0 = negative staining, 1 = weakly positive staining, 2 = moderately positive staining, and 3 = strongly positive staining). The extent of positivity (“extent of distribution” of positive cells) was estimated on a scale of 0 to 4 (0 = negative, 1 = positive staining in 1%-25% of cells, 2 = positive staining in 26%-50%; 3 = positive staining in 51%-75%; and 4 = positive staining in 76%-100%). The combined staining score (extension + intensity) was considered as positive staining.
Apoptotic cells were detected in situ using TUNEL method according to the manufacturer’s instructions[17]. Slices were treated with proteinase K and 0.3% H2O2, labeled with fluorescein dUTP (Wuhan Boster Biological Technology Co.Ltd) in a humid box for 1 h at 37°C, then combined with POD-horseradish peroxidase, stained with DAB and counterstained with methyl green. Controls received the same management except the labeling fluorescein dUTP. Dark brown nucleus represented the positive apoptotic cells. In each section, 5 high -power fields were chosen and the apoptosis index (AI), the percentage of positive cells in the total cells, was calculated. The AI was calculated as follows: AI = number of apoptotic cells/total number × 100%.
Data were presented as mean ± SD and analyzed with SPSS 11.0 software. The one-way ANOVA or Student’s t test was used to analyze data. All comparisons were made with untreated controls and significance of difference is indicated as P < 0.05 and P < 0.01.
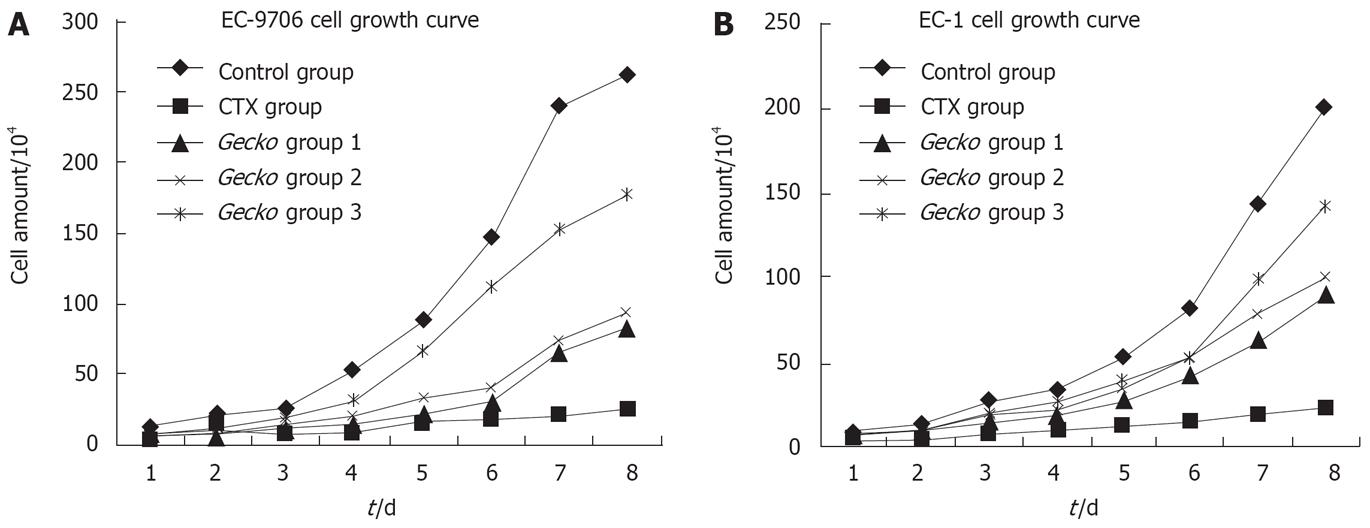
As shown in Tables 1 and 2, compared with control group, the growth of cells treated with serum of different concentrations of mice with medicine Gecko was inhibited significantly in a concentration and time-dependent manner. Seventy-two h after treatment, the inhibitory rates were 22.3%-39.1% (EC9706) and 18%-34.1% (EC1). Compared with the NS group, the differences were significant among the groups of Gecko (P < 0.05).
| Groups | 24 h | 48 h | 72 h | |||
| A | Inhibition rate (%) | A | Inhibition rate (%) | A | Inhibition rate (%) | |
| NS | 0.472 ± 0.05 | 0.97 ± 0.118 | 1.63 ± 0.224 | |||
| CTX | 0.286 ± 0.06a | 39.1 | 0.55 ± 0.09a | 42 | 0.82 ± 0.163a | 49.6 |
| Gecko 1 | 0.355 ± 0.04a | 24.8 | 0.65 ± 0.136a | 32.5 | 0.99 ± 0.154a | 39.1 |
| Gecko 2 | 0.372 ± 0.07a | 21 | 0.721 ± 0.09a | 25.1 | 1.15 ± 0.925a | 29.8 |
| Gecko 3 | 0.393 ± 0.03a | 16.4 | 0.756 ± 0.07a | 22.4 | 1.27 ± 0.07a | 22.3 |
| Experiment | 24 h | 48 h | 72 h | |||
| A | Inhibition rate (%) | A | Inhibition rate (%) | A | Inhibition rate (%) | |
| NS | 0.282 ± 0.09 | 0.506 ± 0.05 | 1.329 ± 0.73 | |||
| CTX | 0.19 ± 0.03a | 32.6 | 0.298 ± 0.124a | 41.1 | 0.673 ± 0.08a | 48.7 |
| Gecko 1 | 0.215 ± 0.08a | 24.8 | 0.368 ± 0.03a | 27.2 | 0.865 ± 0.08a | 34.1 |
| Gecko 2 | 0.234 ± 0.04a | 16.6 | 0.378 ± 0.03a | 25.1 | 0.94 ± 0.112a | 30.5 |
| Gecko 3 | 0.255 ± 0.05a | 9.5 | 0.426 ± 0.08a | 15.8 | 1.075 ± 0.132a | 18 |
To investigate anti-tumor activity of the Gecko in vitro, tumor cells treated with serum medicine were cultured for 7 d, and then the cell growth curve was drawn. Under the inverted light microscope, obvious difference was observed in the cell morphology among the five groups of cells. Compared with the control group, growth curves of the three Gecko groups gradually moved down in a dose-dependent manner (Figure 1). These results indicated that the serum with medicine Gecko could inhibit EC9706 and EC1 growth and proliferation in vitro.
As shown in Table 3, compared with the NS group, the tumors of the Gecko and CTX groups shrank significantly (P < 0.01), but the tumor weight of three Gecko groups had no difference statistically compared with the CTX group (P > 0.05). The tumor inhibitory rates of the CTX group and Gecko groups were 74.6%, 49.8%, 52.8% and 43.1%, respectively. After experiment, there no significant difference was found in mouse weight between the Gecko groups and the NS group (P > 0.05). Compared with the NS groups, the weight of mice in the CTX group decreased significantly (P < 0.05).
| Groups | Dose (g/kg) | Weight(g) | Tumor weight (g) | Inhibitory rate (%) | |
| Pre-treatment | Post-treatment | ||||
| NS | 26.1 ± 2.57 | 28.1 ± 2.64 | 2.167 ± 0.592 | ||
| CTX | 0.1 | 26.2 ± 2.13 | 24.3 ± 2.91a | 0.548 ± 0.135b | 74.6 |
| Gecko 1 | 13.5 | 26.5 ± 2.44 | 28.8 ± 3.01 | 1.087 ± 0.249b | 49.8 |
| Gecko 2 | 9.0 | 26.1 ± 2.11 | 26.6 ± 2.18 | 1.021 ± 0.288b | 52.8 |
| Gecko 3 | 4.5 | 25.4 ± 1.90 | 27.8 ± 3.74 | 1.234 ± 0.331b | 43.1 |
As shown in Table 4, compared with the NS group, the thymus index and Spleen index of mice in the CTX group decreased significantly (P < 0.05). However, there was no significant difference between the Gecko groups and the NS group; CTX could decrease the phagocytic index and phagocytic rate very significantly (P < 0.01); Gecko could decrease the phagocytic index and phagocytic rate significantly (P < 0.05). These results indicated that Gecko could not enhance the immune functions.
| Groups | Dose (g/kg) | Thymus index (× 10-3) | Spleen index (× 10-3) | Phagocytic rate(%) | Phagocytic index |
| NS | 2.662 ± 0.131 | 8.143 ± 0.294 | 30.2 | 0.414 | |
| CTX | 0.1 | 1.939 ± 0.981a | 5.376 ± 0.570a | 7.0b | 0.085b |
| Gecko 1 | 13.5 | 2.260 ± 0.092 | 6.943 ± 0.306 | 16.8a | 0.206a |
| Gecko 2 | 9.0 | 2.680 ± 0.064 | 7.418 ± 0.209 | 19.0a | 0.218a |
| Gecko 3 | 4.5 | 2.376 ± 0.051 | 7.354 ± 0.236 | 20.5a | 0.231a |
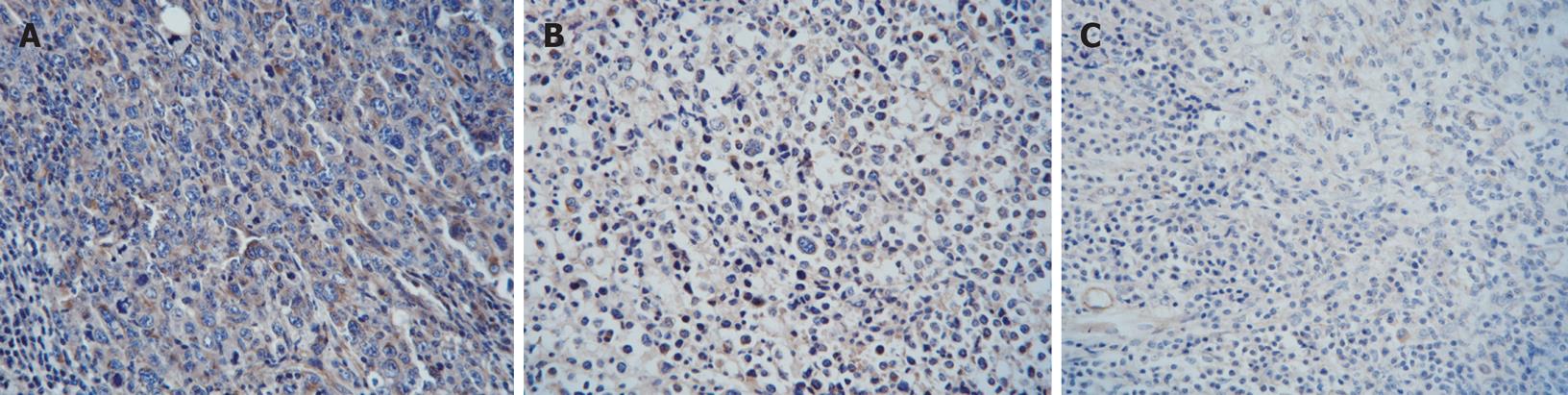
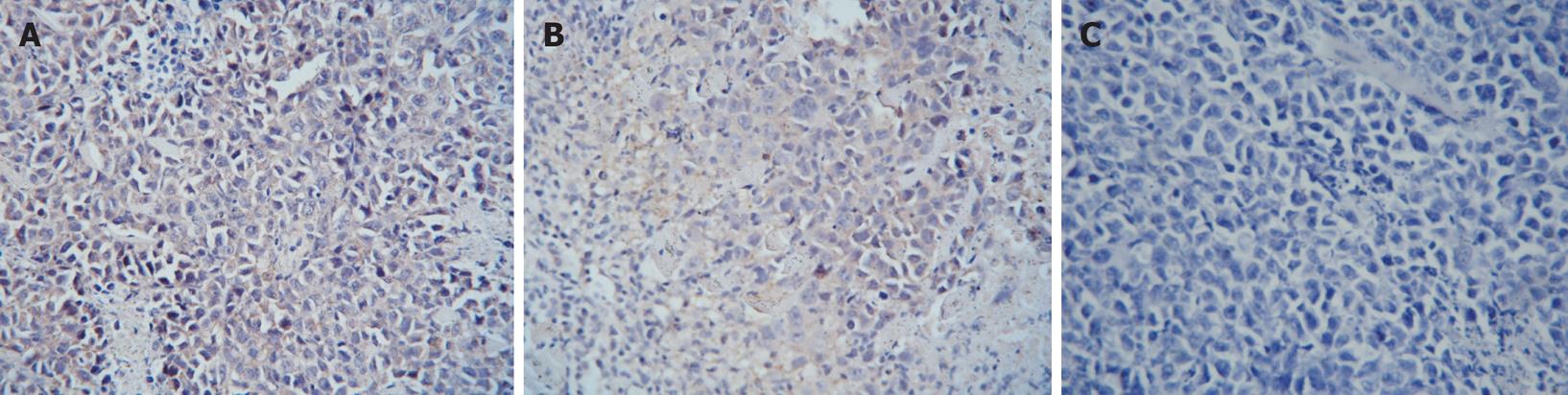
As shown in Figures 2 and 3, the VEGF and bFGF positive cells were defined when there was an aggregation of brown particles in the cytoplasm of the tumor cells. The proteins of VEGF and bFGF had high expression in the tissue of transplanted sarcoma 180 in mice. Compared with the NS group, the immunoreactive score of expression VEGF and bFGF expression of CTX group and Gecko groups decreased significantly (Table 5), (P < 0.05). It indicated Gecko could decrease VEGF and bFGF protein expression in the tissue of transplanted sarcoma 180 in mice.
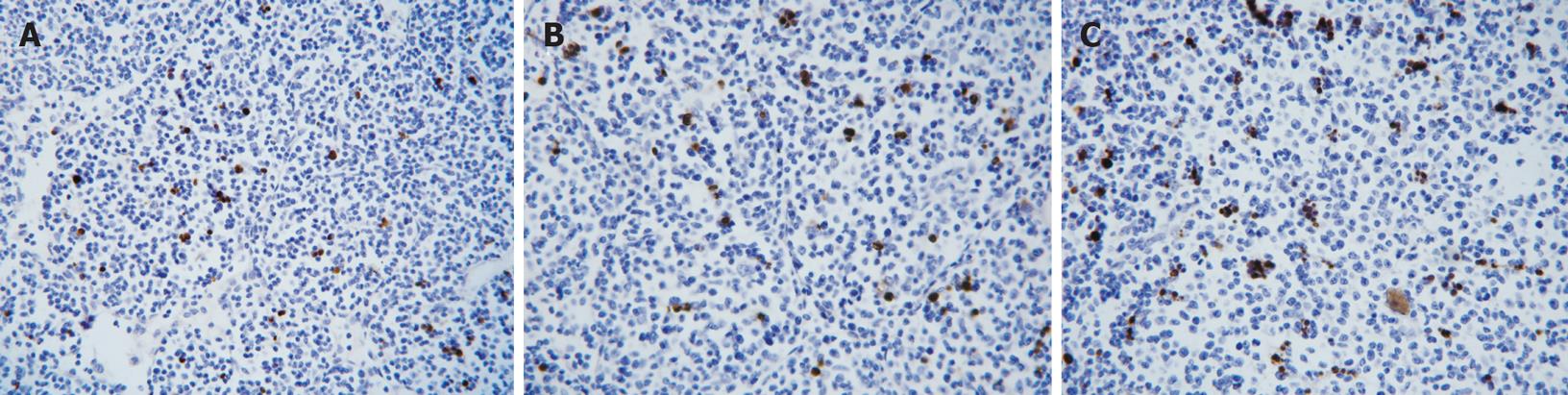
As shown in Figure 4, dark brown nucleus represented the positive apoptotic cells on the tissue of transplanted sarcoma 180 in mice. As shown in Table 5, compared with the NS group, AI of the CTX group and the Gecko groups increased very significantly (P < 0.01), indicating that Gecko could increase AI in the tissue of transplanted sarcoma 180 in mice.
The processing of Gecko introduced in the “Holy Benevolent Prescription” is to “grind it into powder”, while according to “The Medical Science Outline”, the processing is “drying by baking”[18]. Basically two processing methods, living and drying, are used in clinical practice. For fresh Gecko, there are a few studies describing the mechanisms of lyophilized powder of fresh Gecko in inhibiting H22 hepatocarcinoma and C6 glioma cells[19–22], but there has been no experimental study on anti-tumor of drying Gecko. We therefore, selected drying Gecko as a study object.
Serum pharmacological method was first introduced by Japanese authors. It is a good approach to study traditional Chinese medicine(TCM). The serum pharmacology points out that the serum collected from animals treated with traditional Chinese medicine can be used in experiments in vitro. This kind of experimental methods can expel various interference of traditional Chinese medicine and they are close to the true process of the pharmacological effect inside the body[101123]. So we used the method of serum pharmacology in vitro experiments and observed the effect of Gecko-contained serum on growth of the tumor cells. In this study, MTT showed the growth of EC9706, EC1 cells treated with different concentrations of the Gecko-contained serum was inhibited significantly in a concentration and time-dependent manner. Growth curves of the three Gecko groups gradually moved down, being obviously dose dependent. These results indicated that the Gecko-contained serum could inhibit the growth and proliferation of human esophageal carcinoma cells. In vivo, the transplanted tumor model of the mouse S180 sarcoma was established. In the Gecko groups at dosage of 13.5, 9 and 4.5 g/kg, the inhibitory rate was 49.8%, 52.8% and 43.1%, respectively. The differences of the groups of Gecko were very significant from the control group (P < 0.01). These results indicated that Gecko could inhibit growth of solid tumor of S180 mice.
The effect on anti-tumor of TCM is related to pathways and targets. Most studies on anti-tumor mechanisms of TCM showed that TCM could inhibit tumors though supporting the healthy energy and strengthening the body resistance[12]. In our study, the thymus index, Spleen index, phagocytic index and phagocytic rate showed that Gecko could not reinforce immunity of organism. These results indicated the anti-tumor mechanism of TCM might not be related to reinforcement of immune organs.
With the development of modern molecular technology, the anti-angiogenic effect and inducing apoptosis by TCM has become a new area for the research and development of TCM. Apoptosis is a fundamental cellular activity to maintain the physiological balance of the organism and plays a necessary role as a protective mechanism against carcinogenesis by eliminating damaged cells or cells that proliferate excessively[24]. Furthermore, the apoptotic cell death plays an important role in the regulation of pathological conditions as well, such as development and progression of malignant tumors[2526]. Among the methods that are used to identify cells undergoing the apoptotic process, the TUNEL technique is one of the most successfully utilized[26]. Angiogenesis, the growth of new blood vessels from pre-existing capillaries, is necessary for solid tumor growth and metastasis. Angiogenesis is initiated by the release of certain angiogenic factors from tumor cells. VEGF and bFGF which have been shown to be the most potent angiogenic factors are associated with tumor-induced angiogenesis[2728]. To investigate the mechanisms of anti-tumor action of Gecko, we detected the protein expression of VEGF and bFGF using immunohistochemical method and detected apoptotic index by the TUNEL. The results indicate Gecko can decrease VEGF and bFGF protein expression in tumor tissues and induce tumor cell apoptosis.
In conclusion, this study has demonstrated that traditional Chinese medicine Gecko has anti-tumor activity in vitro and in vivo; its mechanism might be related to the induction of tumor cell apoptosis and down-regulation of protein expression of VEGF and bFGF.
Malignant tumor is always a great menace to human health. Chemical anti-neoplastics have definite effect, but they cause much severe adverse effects. Gecko is a traditional Chinese medicine. It could treat malignant tumors, especially in the tumor of digestive tract. Research on Gecko mainly focuses on anti-tumor compound prescriptions, but the pharmacological studies of Gecko and its mechanisms of anti-tumor action are rare.
Basically, two processing methods of Gecko, living and drying, are used in clinical practice. Questions still remain to be answered such as what is the difference of anti-tumor effect between fresh Gecko and dry Gecko? What kind of tumors can Gecko treat? What is the mechanism of Gecko in treating tumors?
This article describes the effect of the Gecko on esophageal carcinoma cell lines (EC9706 and EC1) in vitro and xenografted sarcoma 180 in vivo. Serum pharmacological method in vitro was used to study the anti-tumor mechanism of Gecko in the aspect of the anti-angiogenic and apoptosis inducing effect and investigate if Gecko had impact on immune organs.
The incidence and mortality of tumors keep ascending all over the world. The study on anti tumor effect and mechanisms of anti-tumor action of Gecko can provide the theoretical evidence in clinical practice. Furthermore, the results may lay a foundation for further research in Gecko’s effective constituent.
Serum pharmacological method is a good approach to study traditional Chinese medicine in vitro. It can expel various interference of traditional Chinese medicine as it is close to the true process of the pharmacological effect inside the body
This is a well-conducted study. This manuscript describes the effect of the Chinese medicine Gecko on esophageal carcinoma cell lines (EC9706 and EC1) and in xenografted sarcoma 180 Kunming mice. The studies have provided data that support the conclusions of the authors. This is an interesting study.
| 1. | Zhao F, Liu PX. [Progress of study on action mechanisms of TCM in anti-tumor and preventing metastasis of tumor]. Zhongguo Zhongxiyi Jiehe Zazhi. 2007;27:178-181. |
| 2. | He YH. General survey of traditional Chinese medicine and Western medicine researches on tumor metastasis. Chin J Integr Med. 2006;12:75-80. |
| 3. | Zhang SF, Chen Z, Li B. [Progress on research and application of traditional Chinese medicine in intervention treatment of primary liver carcinoma]. Zhongguo Zhongxiyi Jiehe Zazhi. 2006;26:759-763. |
| 4. | Yang JX, Wang XM. [Progress in studies on anti-hepatoma effect of traditional Chinese medicine by adjusting immune function]. Zhongguo Zhongyao Zazhi. 2007;32:281-284. |
| 5. | Chen M, Huang JH. Present Status of Study on Gecko Used as Traditional Chinese Medicine. Shijie Kexue Jishu-Zhongyiyao Xiandaihua. 2001;53-56 Available from: URL: http://www2.chkd.cnki.net/kns50/detail.aspx?QueryID=30&CurRec=1. |
| 6. | Yang JX, Wang XM. Progress study and research on treating tumor of Gecko. Shijie Huaren Xiaohua Zazhi. 2006;2428-2431 Available from: http://www2.chkd.cnki.net/kns50/detail.aspx?QueryID=113&CurRec=1. |
| 7. | Wu BD. Treatment of 105 cases of esophagus tumor by compound recipe of Gecko. Zhongguo Zhongxiyi Jiehe Zazhi. 1999;502 Available from: URL: http://www2.chkd.cnki.net/kns50/detail.aspx?QueryID=180&CurRec=1. |
| 8. | Song P, Wang XM, Xie S. [Experimental study on mechanisms of lyophilized powder of fresh gekko Chinenis in inhibiting H22 hepatocarcinoma angiogenesis]. Zhongguo Zhongxiyi Jiehe Zazhi. 2006;26:58-62. |
| 9. | Liu JW. Pharmaco-empirical method technology, new technology and new methods. 1st ed. Beijing: Chemical Industry Pub 2003; 23. |
| 10. | Wang SJ, Zhang XY, Lu LB. Research and application of the pharmacological testing method using serum with Chinese herbal medicine. Zhongguo Shouyao Zazhi. 2004;35-37 Available from: URL: http://www2.chkd.cnki.net/kns50/detail.aspx?QueryID=246&CurRec=1. |
| 11. | Xue J, Xie ML. Advances in studies on methodology in serum pharmacology of Chinese materia medica. Zhongcaoyao. 2003;9-11 Available from: URL: http://www3.chkd.cnki.net/kns50/detail.aspx?QueryID=10&CurRec=17. |
| 12. | Liu J, Xu WF, Cui SX, Zhou Y, Yuan YX, Chen MH, Wang RH, Gai RY, Makuuchi M, Tang W. Inhibition of human gastric carcinoma cell growth by atofluding derivative N3-o-toluyl-fluorouracil. World J Gastroenterol. 2006;12:6766-6770. |
| 13. | Liu Y, Liu SJ, Zhang X, Lin JM. [Antitumor effect of GPI-CD80 fusion protein in nude mice]. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27:1027-1029. |
| 14. | Zheng II, Xu JQ. Research Progress on Traditional Chinese Medicine in the Immune Mechanism. Dongwu Yixue Jinzhan. 2004;32-34 Available from: http://www2.chkd.cnki.net/kns50/detail.aspx?QueryID=936&CurRec=63. |
| 15. | Chen Q. Pharmaco-study and research technology of traditional Chinese medicine. 2nd ed. Beijing: The People Public Health Pub 2006; 704. |
| 16. | Rahman MA, Dhar DK, Yamaguchi E, Maruyama S, Sato T, Hayashi H, Ono T, Yamanoi A, Kohno H, Nagasue N. Coexpression of inducible nitric oxide synthase and COX-2 in hepatocellular carcinoma and surrounding liver: possible involvement of COX-2 in the angiogenesis of hepatitis C virus-positive cases. Clin Cancer Res. 2001;7:1325-1332. |
| 17. | Saji K, Fukumoto Y, Suzuki J, Fukui S, Nawata J, Shimokawa H. Colchicine, a microtubule depolymerizing agent, inhibits myocardial apoptosis in rats. Tohoku J Exp Med. 2007;213:139-148. |
| 18. | Luo HD. processing of Gecko. Zhongguo Zhongyao Zazhi. 1996;27 Available from: URL: http://www2.chkd.cnki.net/kns50/detail.aspx?QueryID=863&CurRec=1. |
| 19. | Kang JG, Zhang SZ, Li YH, Qu LT. Experimental study of the effects of fresh Gekko swinhonis anti-neoplasm active component on inhibiting CT-26 tumor growth. Zhongguo Yiyuan Yaoxue Zazhi. 2007;441-444 Available from: URL: http://www2.chkd.cnki.net/kns50/detail.aspx?QueryID=776&CurRec=9. |
| 20. | Song P, Wang XM, Xie S, Pu D, Fu H, Liu G. [Study on sero-pharmacology of fresh Gecko Swinhonis Gunther freeze-dried powder in inducing cell apoptosis of C6 glioma cells in mice]. Zhongguo Zhongxiyi Jiehe Zazhi. 2004;24:919-921. |
| 21. | Song P, Wang XM, Xie S. Effects of natural extraction of gecko in inducing apoptosis and antiproliferation of C6 glioma cells. Zhongliu Fangzhi Yanjiu. 2003;458-461 Available from: URL: http://www2.chkd.cnki.net/kns50/detail.aspx?QueryID=708&CurRec=1. |
| 22. | Yang JX, Yang GS, Zhu W, Fu H, Liu GX, Wang XM. [Study on anti-tumor effect of dry and fresh Gekko swinhonis freeze-dried powders on mice sarcoma S180 and acute toxicity testing of two powders]. Zhongguo Zhongyao Zazhi. 2007;32:238-241. |
| 23. | Iwama H, Amagaya S, Ogihara Y. Effect of shosaikoto, a Japanese and Chinese traditional herbal medicinal mixture, on the mitogenic activity of lipopolysaccharide: a new pharmacological testing method. J Ethnopharmacol. 1987;21:45-53. |
| 25. | Yuasa Y. Control of gut differentiation and intestinal-type gastric carcinogenesis. Nat Rev Cancer. 2003;3:592-600. |
| 26. | Kasagi N, Gomyo Y, Shirai H, Tsujitani S, Ito H. Apoptotic cell death in human gastric carcinoma: analysis by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling. Jpn J Cancer Res. 1994;85:939-945. |
| 27. | Xiao YF, Liu SX, Wu DD, Chen X, Ren LF. Inhibitory effect of arsenic trioxide on angiogenesis and expression of vascular endothelial growth factor in gastric cancer. World J Gastroenterol. 2006;12:5780-5786. |