Published online Jun 28, 2008. doi: 10.3748/wjg.14.3919
Revised: May 25, 2008
Accepted: May 31, 2008
Published online: June 28, 2008
The incidence of adenocarcinoma of the esophago-gastric junction is constantly increasing. Curative treatment is no longer possible at the time of diagnosis in more than 50% of patients with esophageal carcinoma, and palliative treatment focusing on eliminating dysphagia is required. Endoscopic therapy with stent implantation is an established method of achieving this. It can be carried out quickly, with a low rate of early complications, and leads to fast symptomatic improvement, assessed using the dysphagia score. The relatively high rate of late complications such as stent migration, hemorrhage, and gastroesophageal mucosal prolapse has led to recent debate on the role of metal stents in palliative therapy. We present here a new type of stent design for transcardial application, which is intended to prevent bleeding due to mechanical mucosal lesions caused by the distal end of the stent extending into the stomach. The further intention of this case report is to force the discussion on individually designed nitinol stents in special anatomic conditions.
- Citation: Aymaz S, Dormann AJ. A new approach to endoscopic treatment of tumors of the esophagogastric junction with individually designed self-expanding metal stents. World J Gastroenterol 2008; 14(24): 3919-3921
- URL: https://www.wjgnet.com/1007-9327/full/v14/i24/3919.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3919
The incidence of adenocarcinoma of the esophago-gastric junction is constantly increasing[12]. Curative treatment is no longer possible at the time of diagnosis in more than 50% of patients with esophageal carcinoma, and palliative treatment[3] focusing on eliminating dysphagia is required. Endoscopic therapy with stent implantation is an established method of achieving this. It can be carried out quickly, with a low rate of early complications, and leads to fast symptomatic improvement, assessed using the dysphagia score[4–7]. The relatively high rate of late complications such as stent migration, hemorrhage, and gastroesophageal mucosal prolapse has led to recent debate on the role of metal stents in palliative therapy[8]. We present here a new type of stent design for transcardial application, which is intended to prevent bleeding due to mechanical mucosal lesions caused by the distal end of the stent extending into the stomach. The further intention of this case report is to force the discussion on individually designed nitinol stents in special anatomic conditions.
An 82-year-old patient presented at our department with 2-wk dysphagia, followed most recently by intermittent vomiting. He had a history of progressive prostate carcinoma (T3b Nx M0), which was treated with orchiectomy and antiandrogen therapy (bicalutamide). As there was local progression with infiltration of the urinary bladder, radiotherapy was planned. In addition, the patient had type 2 diabetes mellitus and arterial hypertension.
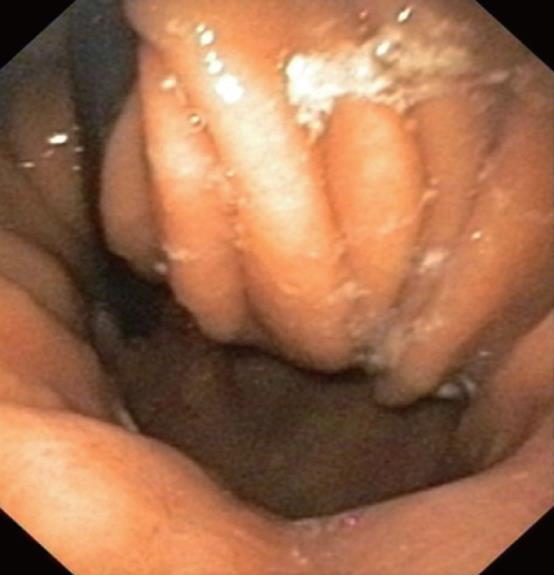
Gastroscopy showed a dilated esophagus, correspon-ding to the radiographic findings, with a high-grade stenosis 38 cm from the incisors, cranial to a 3-cm long sliding hernia (Figures 1 and 2). The stenosis was diagnosed histologically as an adenocarcinoma of the esophagogastric junction, which already developed a hepatic metastasis. Reviewing the findings, we decided to carry out palliative stent implantation to treat the stenosis. As transcardially positioned stents are associated with higher complication rates[9] and experience shows that bleeding often occurs due to stent-related mucosal lesions in the stomach, we requested individual production of an unusually shaped self-expanding nitinol stent (SEMS) (Figure 3). Decisive factors in developing this new designed SEMS included the special anatomic conditions in this patient, with a medium-sized esophageal hernia. The stent was woven from nitinol in accordance with our specifications and was 140 mm long and 24 mm wide. A 30-mm wide bulb was formed at the cranial end of the stent to prevent stent migration. The distal end of the stent was to bend cranially to ensure that the stent would fit the cardia and not extend freely into the stomach. In addition, a circular ring-like widening was added 2 cm above the distal end of the stent to prevent migration. With the exception of the uncovered proximal bulb, the stent was completely covered.
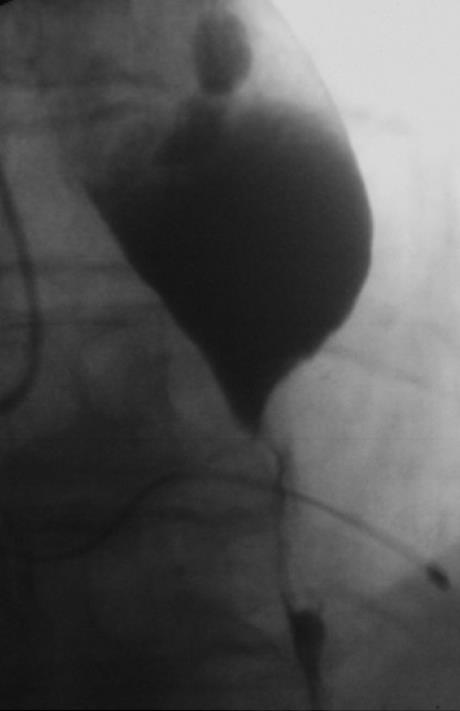
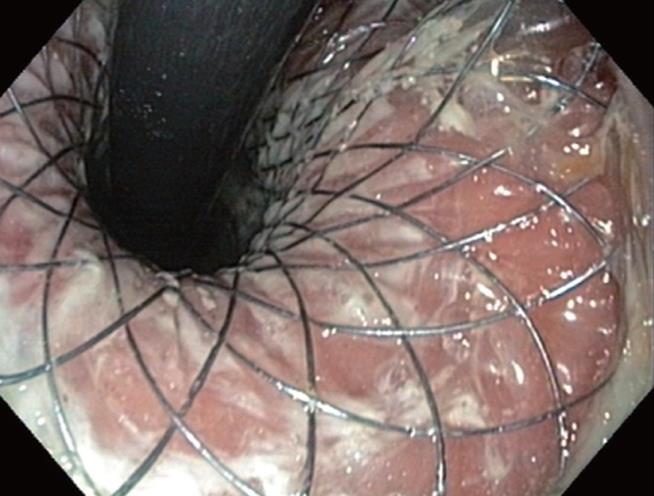
After the patient’s informed consent was obtained, the stent was placed on a carrier system with a diameter of 8 mm and released in the conventional way from the distal end by withdrawing an over tube. After the applicator with the stent was positioned fluoroscopically at the tumor level over a guide wire (Boston Scientific, Super Stiff Guidewire, 0.035 inch), the distal end of the stent was partially released. The proximally bent end of the stent was then pulled until the distal end fitted the cardia tightly. Finally, the stent was fully released by pulling and positioned without any special difficulties. The patient was able to take solid food 24 h later. A radiographic check showed that the stent was correctly positioned (Figure 4). No incidents of bleeding, vomiting as evidence of mucosal prolapse, or recurrent dysphagia occurred during a 4-mo follow-up period. An endoscopic check-up 2 mo after introduction of the stent showed that it was still correctly positioned, with no evidence of mucosal lesions (Figure 5).
Although the use of self-expanding metal stents is now an established part of palliative treatment for esophageal carcinomas, late complications are frequent. The complication rate is higher when the stent is in a transcardial position. In our experience, however, stent migrations that are frequently reported occur much less often when a partially covered stent with a large diameter is used, and previous dilation of the tumor stenosis has not been carried out[10]. Nevertheless, bleeding, particularly due to mechanical lesions caused by the distal end of the stent which extends into the stomach, and mucosal prolapse with occlusion of the stent, still continue to be major problems. The aim in this case was therefore to take advantage of an individual stent design and provide an optimal solution in the palliative situation for this patient.
The stent described above was designed and manufactured in close collaboration with the producer and distributor of the nitinol stent within 10 d in order to avoid mechanical lesions of the mucosa in this special anatomic situation. The stent was positioned without any special difficulties in conventional way. The early clinical result was good after 24 h. The radiographic, clinical and endoscopic follow-up during a 4-mo period showed that the stent was still correctly positioned and none of usual complications occurred.
We believe that the stent design presented here is particularly suitable for palliative treatment of stenotic distal esophageal carcinomas. The advantage of this stent design is particularly clear when an axial hiatus hernia is present. Further optimization of the design and studies comparing it with conventional stents are required. We believe that individual stent designing could alleviate many of the late complications associated with stent treatment.
| 1. | Blot WJ, Devesa SS, Fraumeni JF Jr. Continuing climb in rates of esophageal adenocarcinoma: an update. JAMA. 1993;270:1320. |
| 2. | Devesa SS, Blot WJ, Fraumeni JF Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049-2053. |
| 3. | Siersema PD, Marcon N, Vakil N. Metal stents for tumors of the distal esophagus and gastric cardia. Endoscopy. 2003;35:79-85. |
| 4. | Shimi SM. Self-expanding metallic stents in the management of advanced esophageal cancer: a review. Semin Laparosc Surg. 2000;7:9-21. |
| 5. | Siersema PD, Schrauwen SL, van Blankenstein M, Steyerberg EW, van der Gaast A, Tilanus HW, Dees J. Self-expanding metal stents for complicated and recurrent esophagogastric cancer. Gastrointest Endosc. 2001;54:579-586. |
| 6. | Baerlocher MO, Asch MR, Vellahottam A, Puri G, Andrews K, Myers A. Safety and efficacy of gastrointestinal stents in cancer patients at a community hospital. Can J Surg. 2008;51:130-134. |
| 7. | Nathwani RA, Kowalski T. Endoscopic stenting of esophageal cancer: the clinical impact. Curr Opin Gastroenterol. 2007;23:535-538. |
| 8. | Ross WA, Alkassab F, Lynch PM, Ayers GD, Ajani J, Lee JH, Bismar M. Evolving role of self-expanding metal stents in the treatment of malignant dysphagia and fistulas. Gastrointest Endosc. 2007;65:70-76. |
| 9. | Spinelli P, Cerrai FG, Ciuffi M, Ignomirelli O, Meroni E, Pizzetti P. Endoscopic stent placement for cancer of the lower esophagus and gastric cardia. Gastrointest Endosc. 1994;40:455-457. |