INTRODUCTION
The liver is the largest organ in the abdominal cavity and the main region of primary tumor and distant metastasis of malignant tumors. Detection of tumor nodules in the liver is of major importance for formulating therapeutic strategies and predicting the prognosis in malignant tumors[1].
Non-ionizing radiation, portable and noninvasive real-time imaging[23], ultrasonography (US) are the most commonly used imaging techniques. Introduction of microbubbles as contrast agents for ultrasound has improved the image quality and diagnostic value[4–8]. Contrast-enhanced ultrasonographic imaging enables noninvasive measurements of microvascular perfusion in the heart, brain, kidney, skeletal muscle, skin grafts and solid tumors[9] and provides functional images of angiogenesis in animals and humans.
At present, contrast-enhanced ultrasonographic imaging research has mainly focused on angiogenic blood vessels, blood vessel function and efficacy of angiogenesis inhibitors. Recently, lymphangiogenesis has become a new research frontier[10]. Tumor lymphangiogenesis is the process of forming new lymph vessels in tumors and closely related to tumor development and progression. It is necessary to find noninvasive methods for evaluating lymphangiogenesis in situ. However, little is known about the contrast-enhanced ultrasonographic imaging used to detect tumor lymphangiogenesis. Recently, lymphosonography after interstitial injection of microbubble-based contrast agents can trace the lymphatic channels from the injection site up to the draining sentinel lymph nodes[11–15]. However, no report is available on lymphosonography for tumor lymphangiogenesis.
The aim of the present study was to evaluate the feasibility and efficacy of PTL with a small volume of SonoVue® as a novel method for the detection of tumor lymphangiogenesis of hepatic VX2 in rabbits and to evaluate the combined PTL and contrast-enhanced ultrasonographic imaging in the diagnosis of liver cancer.
MATERIALS AND METHODS
Animal model
Ten male health New Zealand rabbits, weighing 2.5-3.0 kg, were included in this study and housed in an approved facility with free access to water and standard diet throughout the study. The study, approved by the Institutional Review Board for Animal Research, was performed following the Guidelines for the Care and Use of Laboratory Animals[16].
An undifferentiated VX2 carcinoma growing rapidly in rabbits served as the experimental tumor. Two VX2 tumors were implanted into the right and left lobes of liver, respectively. In brief, rabbits were anesthetized with ketamine hydrochloride (40 mg/kg) and xylazine hydrochloride (5 mg/kg) intramuscularly. The rabbits were intermittently given small supplementary doses of sodium pentobarbital (ranging from 3.1 to 6.5 mg/kg) during the experiment to maintain adequate sedation, fixed in a supine position on a rigid board of paper. Hair on the abdominal skin was shaved after the animals became stable. Diagnostic US was performed to assess the implantation site. Cryoconserved tumor material, implanted in the lower leg muscles of an additional animal and harvested after it reached a size of 1.5 cm, was placed into a saline solution and cut into sections measuring 1 mm × 1 mm × 1 mm.
The implantation method used has been described elsewhere[17]. Only part of tumor tissue showing no macroscopic signs of necrosis was used. A 16-gauge intravenous cannula was placed into the left and right liver lobe respectively under US guidance, and the prepared tumor tissue sections were pushed through the cannula and placed at the preselected position. The same procedure was performed on each animal. The rabbits were permitted to recover and followed up sonographically (Sequia 512, Siemens, Germany) weekly until a localized, avascular carcinoma-like mass developed at the injection site after 10-15 d.
Equipment
Sequia 512 US image system was purchased from Siemens, Germany, with a L15-8 probe equipped for Cadence CPS software. Its acoustic output was carefully controlled by the operator. MI was set at 0.1-0.3 in order to avoid considerable bubble destruction and reduction of the contrast effect. Cadence CPS is a real-time, non-linear imaging technique specific for the second echo-contrast agent examination. Cadence CPS processing utilizes all non-linear responses, fundamental and higher order harmonics, to produce high sensitivity contrast agent images with excellent agent-to-tissue specificity at a very low MI. Images and/or movie clips were stored during PTL and contrast-enhanced ultrasonographic imaging.
Contrast agent
Contrast agent used in this study was SonoVue® (Bracco, Milan, Italy). Microbubbles are sulfur hexafluoride stabilized in a phospholipid shell, 1-10 &mgr;m in diameter, averaging about 2.5 &mgr;m. The SonoVue® preparation was reconstituted just before administration by adding 5 mL sterile saline to the freeze-dried powder, so that sulfur hexafluoride had a concentration of 45 &mgr;g/mL in the suspension.
SonoVue® injection
SonoVue (0.1 mL/kg) was injected via an ear vein as a rapidly injected bolus, followed by a 1.5 mL saline flush for routinely contrast-enhanced ultrasonographic imaging.
SononVue (0.5 mL) was injected into the normal liver parenchyma near the VX2 tumors as a rapidly injected bolus using a tuberculin syringe and a 26-gauge needle for PTL. The absorption of the contrast agent and its flow were observed in lymphatic channels of the VX2 tumors.
Statistical analysis
For quantitative analysis, videodensities of the appropriate regions of interest (ROI), including perineoplastic liver parenchyma, boundaries of the tumor and tumor parenchyma were recorded during PTL. Respective evaluations were made for PTL. Data analysis was carried out using SPSS 16 statistical software. All videodensity data were expressed as mean ± SD. Parameters were tested using paired t test. Statistical analysis was performed using one-way analysis of variance and Dunnett’s multiple comparison tests. P < 0.05 was considered statistically significant.
RESULTS
The VX2 tumor in liver of rabbits ranging 5-19 mm was found to be a low echoic mass. However, because the VX2 tumor was almost isoechoic with the normal tissue and boundaries of the masses were unclear, detection and delineation of the lesion were difficult before SonoVue® injection (Figure 1A).
Figure 1 Liver of a VX2 tumor-bearing rabbit imaged in the conventional mode before (A), immediately after 18 s (B) and 96 s (C) of injection of 0.
1 mL sonazoid microbubbles/kg. Arrows indicate VX2 tumor.
Since the typical enhancement pattern of VX2 tumor detected by routine contrast-enhanced ultrasonographic imaging was hyperechoic and hypoechoic to liver parenchyma during the early and later phase, respectively, a much more rapid wash-in and -out of ultrasonographic contrast agent was observed compared to the normal liver parenchyma (Figure 1B and C).
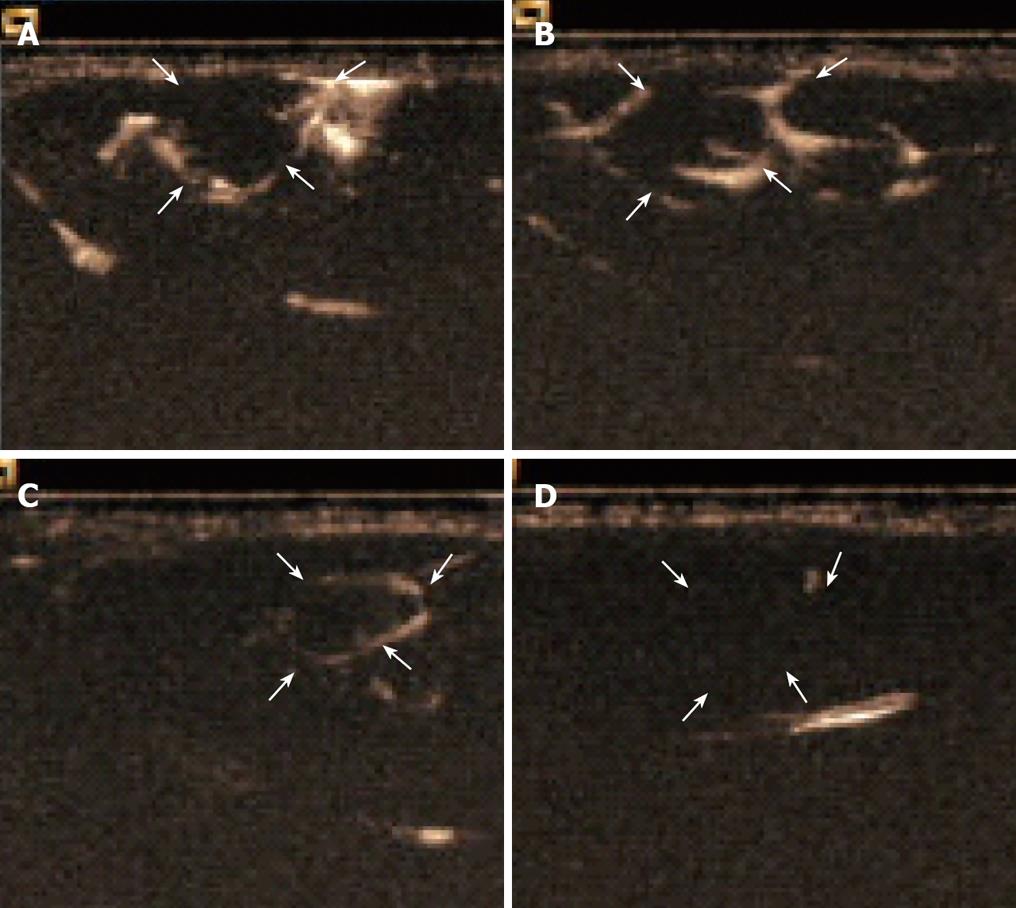
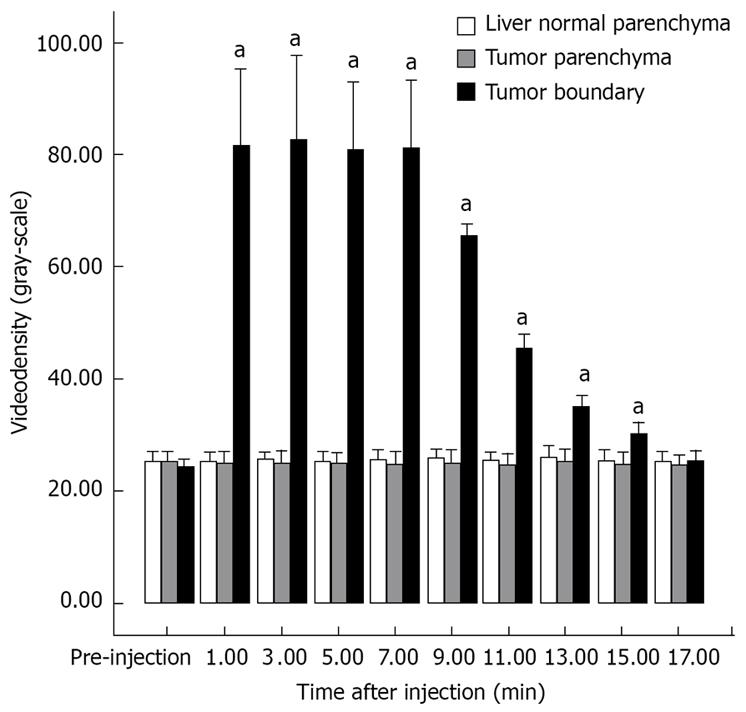
The enhancement pattern of VX2 tumors detected by PTL was significantly different from the typical enhancement pattern of VX2 tumors detected by routine contrast-enhanced ultrasonographic imaging. The hepatic lymph vessels were visualized immediately and continuously during PTL. SonoVue® was deposited in the parenchyma relatively quickly in winding channels. At the same time, the boundaries of VX2 tumors were hyperechoic to liver parenchyma and the tumors. The hyperechoic boundaries clearly delineated VX2 tumors compared with the normal liver and tumor parenchyma (Figure 2A-C). The difference in the videodensitometric measurements of the boundaries of VX2 tumors was significantly higher than the baseline (Figure 3). Conversely, videodensity in the normal liver and tumor parenchyma had no signal enhancement compared with the baseline (Figure 3). There was a significant difference in the boundaries of VX2 tumors compared with the baseline as well as the normal liver and tumor parenchyma (Figure 3).
Figure 2 Hepatic lymph vessels visualized 36 s (A), 4 min (B), 7 min (C) 18 min (D) after injection of contrast agent and continuously visualized with SonoVue® during PTL with hyperechoic boundaries of VX2 tumors to liver parenchyma and the tumor.
Figure 3 Videodensitometric measurements of liver normal parenchyma (white), tumor parenchyma (gray) and tumor boundary (black) before and after percutaneous transhepatic injection of contrast agent SonoVue® into the normal liver parenchyma near the VX2 tumors during PTL.
aP < 0.05 vs respective pre-injection values (Dunnett).
DISCUSSION
Ultrasound is an important and useful imaging method for the detection of tumors. Ultrasound contrast agents containing encapsulated microbubbles are mainly used to increase the diagnostic imaging of tumors. McCarville et al[18] showed that gray-scale US measurements of microbubble contrast agent flow can be used to detect the functional consequences of antiangiogenic therapy for tumors and to assess angiogenesis inhibitors that act through different mechanisms[19–23].
Recently, lymphangiogenesis has become a new research frontier[10]. The important functions of the lymphatic system are to remove damaged cells from the body and to prevent the spread of infection and cancer for the maintenance of normal tissue fluid balance and immune surveillance. In spite of its important functions in physiological and pathological conditions, including tumor metastasis, lymphoedema and inflammation, lymphatic vessels have not received as much attention as blood vessels, and the mechanisms regulating their development and growth have been poorly understood[24]. Lymphangiogenesis is associated with increased tumor cells in lymphatics and lymph nodes, served as an independent prognostic factor and a potential target in the development of new therapies for hilar cholangiocarcinoma[25]. At present, neovessel formation, including lymphangiogenesis, represents the key event in tumor progression. Inhibition of metastatic spread may be achieved by restriction of lymphatic vessel growth with novel therapeutic strategies for anti-lymphangiogenic therapies[26].
Currently, histologic determination of the mean intratumoral or peritumoral lymphatic vessels is the most commonly used method for assessing lymphangiogenesis. However, obtaining tissue for histologic evaluation may require an invasive procedure that cannot be normally accepted by patients. Furthermore, determination of the lymphatic microvessel density does not provide an accurate assessment of the functionality of tumor lymphatic vessels because many poorly functioning or collapsed lymphatic vessels have endothelial cells that are stained and counted. Therefore, the lymphatic microvessel density in vivo may be a potentially useful marker for assessing lymphangiogenesis in tumors at diagnosis, and accurately reflects the effectiveness of antitumor therapy.
Ultrasound lymphography with subcutaneous injection of ultrasound contrast material enables direct visualization of the lymphatic drainage pathways and sentinel lymph nodes of breast diseases, melanoma, etc [11–15].
In the present study, the traditional percutaneous hepatic injection method was used to deliver SonoVue® microbubbles into the liver under US guidance to investigate tumor lymphangiogenesis. To the best of our knowledge, lymphosonography for the detection of tumor lymphangiogenesis has not been reported before. Hepatic lymph vessels were visualized immediately after injection of contrast agent and opacified with SonoVue® during PTL, whereas liver parenchyma was not enhanced by SonoVue®. SonoVue® was deposited in the parenchyma relatively quickly in winding lymph vessels. At the same time, the boundaries of VX2 tumors were hyperechoic to liver parenchyma and the tumors, indicating that hyperechoic boundaries clearly delineate the peritumoral lympathatic vessels of VX2 tumors. Compared with the hyperechoic boundaries of VX2 tumors, the videodensity in the tumor parenchyma had no signal enhancement compared with the baseline. This is consistent with the findings in a previous study[27]. It was reported that three-dimensional changes of lymphatic architecture in rabbit VX2 tongue cancer, dynamics of its adjacent lymphatic architecture, especially the increased number of capillaries in preexisting lymphatic vessels outside the tumor margin, are associated with lymph node metastasis[2829]. The morphological features of lymphatic vessels during PTL may be important predictive markers for evaluating lymphatic metastasis and prognosis of tumors. The lymphatic drainage paths and lymphatic distribution pattern in hepatic tissue have been found to be very constant, showing that angiogenesis is a critical factor for tumor growth and metastasis[23]. In this study, the typical enhancement pattern of VX2 tumors detected by routine contrast-enhanced ultrasonographic imaging was hyperechoic and hypoechoic to the liver parenchyma at the early and later phases, respectively, confirming that routine contrast-enhanced ultrasonographic imaging can assess tumor vascularity and reveal the microvascular perfusion and function[233031].
The specific mechanism by which the contrast agents used in this study enter the lymphatic system is unclear. SonoVue® microbubbles have a mean diameter of 2.5 &mgr;m with 99% smaller than 11 &mgr;m, allowing a free passage of capillaries, but keeping within the vascular lumen. This means that SonoVue® microbubbles in the hepatic inter-space cannot come into blood vessels. Although the optimal particle diameter for lymphatic uptake is 10-50 nm, particles up to hundreds of nanometers in diameter appear to be able to cross the lymphatic endothelium[32–34]. Due to the flexibility of microbubbles, phospholipidic shell and poor solubility and diffusivity of SF6, SonoVue® is highly resistant to pressure. This means the microbubbles may more easily distort and traverse lymphatic wall fenestrations into lymph capillaries.
Due to the different membranes, 99% of Sonazoid and Optison are phagocytosed by Kupffer cells, whereas only 7.3% of SonoVue® is phagocytosed by Kupffer cells[35]. This means that the SonVue® microbubbles are not easily phagocytosised by macrophages. Tracing the SonVue® microbubble flowing in the lymph vessels can improve the pathologic staging of the disease and its treatment.
At the same time, microbubbles are used not only for contrast enhancement of ultrasound images and improvement of diagnosis, but also for delivery of drugs and genes[36–40]. The ability to localize lymphatic vessels in tumors may be of value for a new route to the administration of drugs, gene and immunotherapy, etc. Drugs/genes containing vesicles may be injected simultaneously with microbubbles or microbubbles in combination with microbubble-forming vesicle aggregates. Using microbubbles oscillation and cavitation under US guidance might assist in delivering drugs/genes from vesicles to the interstitial tissue, which may be an effective treatment for some diseases.
Since few studies about hepatic lymphography are available at present, it is difficult to find microbubbles in lymphatic vessels. Due to this reason, the study only limited to the ultrasound characteristic aspects of PTL, which were not compared with the histopathologically aspects of rabbit VX2 tumors.
In conclusion, PTL with a small volume of SonVue microbubbles is a novel method for the detection of tumor lymphangiogenesis of hepatic VX2 in rabbits. Combined PTL and contrast-enhanced ultrasonographic imaging can improve the diagnosis of liver cancer. Additional research is needed to determine the potential advantages of PTL and to determine if PTL can be used in clinical practice.
COMMENTS
Background
Ultrasonography (US) is one of the most commonly used imaging techniques. Lymphangiogenesis has become a new research frontier. Tumor lymphangiogenesis is the process of generating new lymph vessels within and surrounding tumors, which is closely related to tumor development and progression. It is neccessary to develop noninvasive methods for evaluating lymphangiogenesis in situ. However, to the best of our knowledge, lymphosonography showing tumor lymphangiogenesis with percutaneous hepatic injection of ultrasound contrast material has not been reported before.
Research frontiers
This study investigated tumor angiogenesis and lymphangiogenesis with combined percutaneous transhepatic lymphosonography (PTL) and contrast-enhanced ultrasonographic imaging for hepatic VX2 in rabbit liver.
Innovations and breakthroughs
Contrast-enhanced ultrasonographic imaging enables noninvasive measurements of microvascular perfusion in the heart, brain, kidney, skeletal muscle, skin grafts and solid tumors in animals and humans. It was recently reported that lymphosonography after interstitial injection of microbubble-based contrast agents can trace lymphatic channels from the injection site up to the draining sentinel lymph nodes. This is the first study to evaluate the feasibility and efficacy of PTL with a small volume of SonoVue® as a novel method for the detection of tumor lymphangiogenesis of hepatic VX2 in rabbits and to evaluate the role of combined PTL and contrast-enhanced ultrasonographic imaging in improving the diagnosis of liver cancer.
Applications
PTL with a small volume of SonVue microbubbles is a novel method for the detection of tumor lymph angiogenesis of hepatic VX2 in rabbits. Combined PTL and contrast-enhanced ultrasonographic imaging can improve the diagnosis of liver cancer. Additional research is needed to determine the potential advantages of PTL and to determine if PTL can be used in clinical practice.
Peer review
PTL is a new tool for the diagnosis of liver cancer. The study is well designed and interesting.