Published online Jun 28, 2008. doi: 10.3748/wjg.14.3891
Revised: April 30, 2008
Accepted: May 7, 2008
Published online: June 28, 2008
AIM: To study liver cell apoptosis caused by the toxicity of selenium and observe the alteration of choline compounds using in vitro 9.4T high resolution magnetic resonance spectroscopy.
METHODS: Twenty male Wistar rats were randomly divided into two groups. The rats in the treatment group were intraperitoneally injected with sodium selenite and the control group with distilled water. All rats were sacrificed and the livers were dissected. 1H-MRS data were collected using in vitro 9.4T high resolution magnetic resonance spectrometer. Spectra were processed using XWINNMR and MestRe-c 4.3. HE and TUNEL staining was employed to detect and confirm the change of liver cells.
RESULTS: Good 1H-MR spectra of perchloric acid extract from liver tissue of rats were obtained. The conventional metabolites were detected and assigned. Concentrations of different ingredient choline compounds in treatment group vs control group were as follows: total choline compounds, 5.08 ± 0.97 mmol/L vs 3.81 ± 1.16 mmol/L (P = 0.05); and free choline, 1.07 ± 0.23 mmol/L vs 0.65 ± 0.20 mmol/L (P = 0.00). However, there was no statistical significance between the two groups. The hepatic sinus and cellular structure of hepatic cells in treatment group were abnormal. Apoptosis of hepatic cells was confirmed by TUNEL assay.
CONCLUSION: High dose selenium compounds can cause the rat liver lesion and induce cell apoptosis in vivo. High resolution 1H-MRS in vitro can detect diversified metabolism. The changing trend for different ingredient of choline compounds is not completely the same at early period of apoptosis.
-
Citation: Cao Z, Wu LP, Li YX, Guo YB, Chen YW, Wu RH. Change of choline compounds in sodium selenite-induced apoptosis of rats used as quantitative analysis by
in vitro 9.4T MR spectroscopy. World J Gastroenterol 2008; 14(24): 3891-3896 - URL: https://www.wjgnet.com/1007-9327/full/v14/i24/3891.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3891
Apoptosis is a programmed, active, highly selective mechanism of cell death. Multicellular organisms’ apoptosis is an essential component of cellular regulation. Abnormal regulation of apoptosis can lead to disorders such as cancer[12].
The field of cell death research has undergone an explosion of new knowledge over the past decade. The methods to evaluate death of cells, especially in intact tissues, have led to the development of several techniques. However, the properties revealed in these assays are not always applicable to study of diversified metabolite of apoptosis at one time[3].
Nuclear magnetic resonance spectroscopy is a non-destructive and non-invasive technique that can provide complete structural analysis of a wide range of organic molecules in complex mixtures. It generates quantitative information, as the peak intensities can be proportional to analyze concentrations[4]. Because of their low sensitivity and small magnet gaps, the early spectrometers had limited applications, primarily to synthetic chemistry[5]. Over the past three decades, however, sensitivity has increased by orders of magnitude so that this technique can be used to detect the metabolite alteration of apoptosis[6–8].
Choline compounds are one kind of biologically interesting metabolites that can be detected by 1H-MRS. Declining of choline compounds is considered as a 1H-NMR metabolite marker of advanced stage of apoptosis[9]. Lehtimaki et al[10] consider that choline compounds stay unchanged despite reduced cell density. However, how the intensity choline compounds change when apoptosis occurs is still confused.
Our hypothesis was that there is no reason for choline compounds to stay unchanged when apoptosis of liver cell occurs. The alteration of choline compounds could be observed through detailed quantitative analysis by high-resolution 1H-MR spectroscopy. Therefore, the purpose of this study was to observe the liver cell apoptosis caused by the toxicity of selenium and the alteration of choline compounds using in vitro 9.4T high resolution magnetic resonance spectroscopy.
Twenty male Wistar rats, weighing 280-320 g, were randomly divided into two groups (n = 10). The rats in the treatment group were intraperitoneally injected with sodium selenite liquor (Na2SeO3) at a dose of 20 &mgr;mol/kg and the control group with distilled water at a dose of 1 ml/kg.The rats of both groups were fasted for 12 h but with free access to water. All the rats were sacrificed after 24 h. The livers of all rats were immediately dissected. Parts of livers were frozen in liquid nitrogen and then stored at -70°C until measured. The rest parts of livers were fixed in formalin. All animal experiments were performed according to the guidelines approved by the Ethical Committee of the Medical College of Shantou University.
Frozen liver tissue was pulverized with a pestle and mixed with a volume of 2 mmol/mL ice-cold perchloric acid. The mixture was transferred to a homogenizer and homogenized for 20 min at 4°C. The mixture was neutralized with ice-cold 3mmol/mL and 2 mmol/mL KOH and then centrifuged at 10 000 g for 30 min in order to eliminate perchlorate salts. The resulting supernatant was lyophilized and the precipitate discarded. The powder of extracts was transferred into a 5-mm NMR tube and redissolved in 500 &mgr;L D2O containing 1 mmol/L 2,2’-3,3’-tetradeutero-trimethyl-sylilpropionate (TMSP). D2O was added for locking signal. TMSP was used as an internal chemical shift reference at 0.00 ppm. Each sample was collected using in vitro 9.4T high resolution magnetic resonance spectrometer (Bruker Avance 400 MHz). Spectra of extracts were acquired with a pulse sequence with water suppression from the Bruker zgpr pulse program. Data were obtained over a 5000 Hz sweep width and digitized with 4096 data points. The number of scans was 128. Total acquisition time was 6 min. Spectra were primarily processed in the frequency domain using XWINNMR (Bruker GmBH), including fourier-transformation, phased correction and baseline correction. The chemical shift was assigned according to the internal standard TMSP and advanced analysis was then performed using MestRe-c 4.3.
The peak areas which were assigned as metabolites containing choline and TMSP and integral values were calculated separately by MestRe-c 4.3. The concentration of choline was calculated following the amended formula[11].
(metabolite) =[square(metabolite)/square(TMSP)]×[number of protons of metabolite/9] × (TMSP)
Square (metabolite) and square (TMSP) stand for the area of peaks of choline compounds and TMSP; 9 correspond to the number of protons giving rise to the TMSP peak; (TMSP) and (metabolite) represent the concentration of TMSP and metabolite.
The concentration data were put into computer and analyzed using SPSS 13.0 software. A two-sample t test was used for comparison of choline concentration of the samples in both groups. Significance level was set at P < 0.05.
Parts of livers presenting no necrosis by visual inspection underwent histopathological examination during follow-up. Formalin fixed samples were embedded in paraffin and 4-mm sections were cut. Samples were stained with hematoxylin and eosin and examined under light microscopy.
The terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay was performed using the in situ cell death detection kit according to Schrum LW[12]. Briefly, DNA ends were tagged with fluorescein-labeled dUTP using terminal deoxynucleotidyl transferase by incubating the samples at 37°C in a humidified chamber. Liver sections were then incubated with anti-fluorescein-alkaline phosphatase conjugate for 30 min in a humidified chamber. Slides were incubated with 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium for 20 min at room temperature and were counterstained with hematoxylin. The sections were observed under light microscopy.
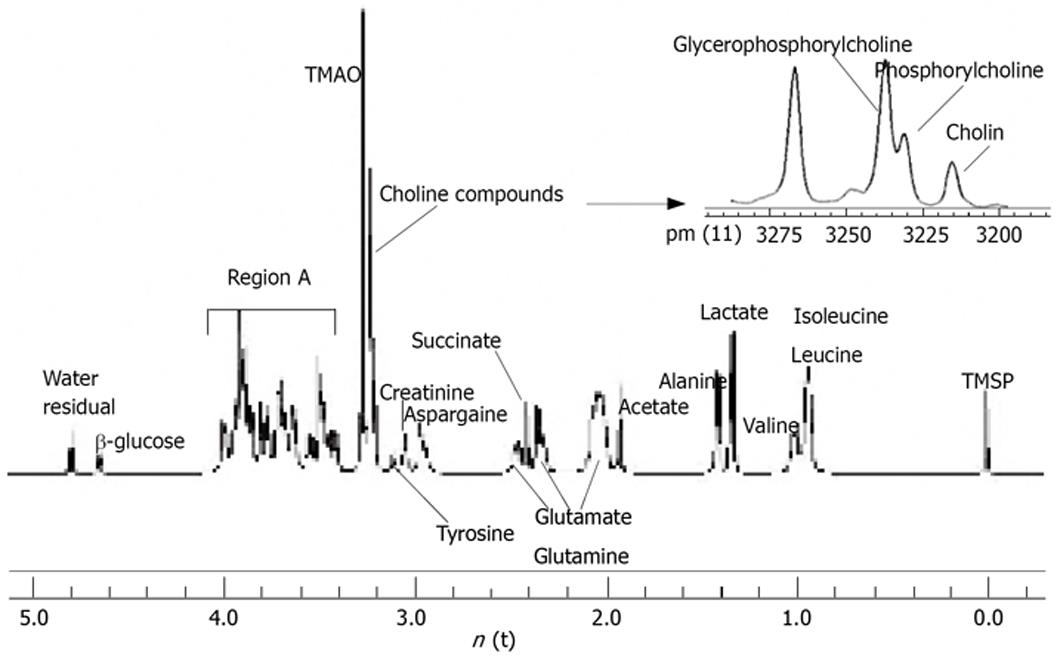
1H-MRS spectra of perchloric acid extract from rat liver tissues are shown in Figure 1. The conventional metabolites were detected and assigned. The assignments of spectra were performed following the previous studies[13–16] and presented in Table 1. The resonances of partially megascopic region 3.20-3.27 ppm were well resolved, where N(CH3)3 signals from the compounds choline, phosphorylcholine and glycerophosphorylcholine can be separated. Following the formula, the total choline compounds and free choline concentrations were calculated. The mean concentration of total choline compounds was 5.08 ± 0.97 mmol/L in control group and 3.81 ± 1.16 in treatment group and the mean concentration of free choline was 1.07 ± 0.23 mmol/L in control group and 0.65 ± 0.20 in treatment group. The differences of the two groups were statistically significant (P = 0.05 and P = 0.00, respectively). However, there were no statistical significances if we compared the concentrations of synthetical choline, including phosphorylcholine and glycerophosphorylcholin (3.71 ± 0.74 mmol/L vs 3.01 ± 0.94 mmol/L, P = 0.46) between the two groups.
| Metabolite | Chemical shift (ppm) |
| TMSP | 0 |
| Isoleucine and Leucine | 0.87 |
| Valine | 0.96 |
| Lactate | 1.32 |
| Lysine | 1.47 |
| Alanine | 1.48 |
| Acetate | 1.92 |
| Glutamate | 2.07-2.34 |
| Succinate | 2.41 |
| Glutamine | 2.13-2.45 |
| Aspargaine | 2.95 |
| Creatinine | 3.05 |
| Tyrosine | 3.11 |
| Choline compounds | 3.20-3.23 |
| TMAO | 3.27 |
| Region A | 3.41-4.0 |
| β-glucose | 4.67 |
| Water (residual) | 4.75 |
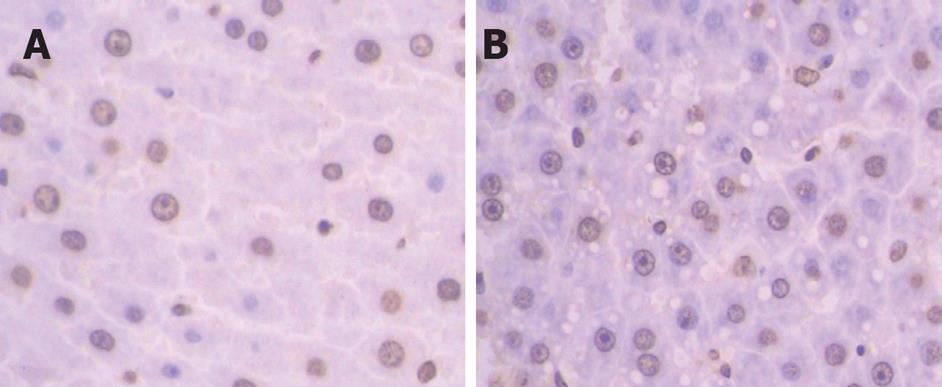
Although the structure of liver lobules was normal in both groups in the view of 100 times magnification, the hepatic sinus was wider in treatment group than in the control group. Cell shape was then inspected in 400 times magnification under light microscopy. The normal hepatic cells were like short shuttle while hepatic cells in treatment group were irregular and verge was not clear. Some of the cells were slightly bigger than the normal ones, but smaller ones were more often observed. We found that the cytoplasm was dyed redder than normal ones. Simultaneously, the condensed or diffuse chromatin remained randomly distributed, and the nuclear pores disappeared (Figure 2). No obviously inflamed cells could be detected under light microscope. Apoptosis of hepatic cells in both groups was confirmed by TUNEL assay. The brown ones in TUNEL assay were the apoptosis positive (Figure 3).
Although positive cells were detected in both groups, the number of positive cells was conspicuously more in treatment group than in control group. The positive cells were spread around in the control group but relatively assembled in the treatment group. Some of the apoptosis positive hepatocytes were vacuolated in treatment group. We found a very interesting phenomenon in our results: Few of apoptosis positive cells were detected in a rat of the treatment group. The 1H-MRS data showed that choline concentrations were also much higher than the mean concentration of the treatment group, the concentration of total choline, free choline and synthetical choline of this rat was 5.67, 0.87 and 4.04 mmol/L, respectively.
Selenium is an essential trace element for human health. The cellular effects of selenite appear quite complex and are concentration-dependent. It is demonstrated that the serum level of selenite affects cell proliferation[17]. At intermediate concentrations, selenite appears to exert its chemopreventive activity. At higher concentrations, selenite induces oxidative stress and may become toxic[18]. Many researchers focused on the induction of apoptosis by toxic concentrations of selenite. Selenocompounds have been reported to induce apoptosis in non-malignant cell lines, such as the Chang liver cells[19]. The results of our experiments also intensively suggested that high-concentration selenium was able to cause lesions in rat livers and induce apoptosis in vivo. However, we also observed that one rat liver in treatment group did not present obvious cell apoptosis. We think that this phenomenon might be related to the individual diversity. Some of the rats are not sensitive towards this remedy. Bollard et al pointed out that various intrinsic physiological factors were known to affect the metabolic composition of biological samples from healthy experimental animals. These included well-being, genetic drift, strain, hormonal differences, rate of metabolism, age, and gender[20]. It is still unclear how selenocompounds might induce apoptosis. Many potential mechanisms have been proposed, including protection against oxidative damage, modulation of metabolism of carcinogens, cytotoxicity of selenium metabolites, induction of apoptosis secondary to production of ROS, regulation of the thioredoxin redox system, regulation of the cell cycle, and inhibition of angiogenesis[21].
It has been reported that, with Se supplementation, the liver Se concentration increases disproportionately[22]. How to predict the liver lesion and inspect the efficiency and toxicity of selenocompounds is an important issue. 1H-MRS is one of suitable, low-cost, and accurate methods for this study.
MR spectroscopy possesses the sensitivity required to measure subtle biochemical changes[23]. Although MR spectroscopy detects only a fairly small number of metabolites, it can still be used to monitor the activity of many cellular activities, because so many metabolic pathways are connected[24]. Choline compounds are one group of metabolites that can be detected by 1H-MRS.
Choline is a nutrient essential for normal function of all cells[25]. It is a precursor not only for acetylcholine but also for phospholipids that are found in intracellular membranes and in the cell membrane[26]. The total choline peaks consist of glycerophosphorylcholin, phosphorylcholineand free choline, but the low resolution of in vivo spectroscopy can not identify the individual peaks from these compounds[27]. Because of the high resolution of the spectrometer which we used in this experiment, free choline is detached among the choline compounds so that we can further find out how free choline changes are when apoptosis of cells takes place separately. In our research, we found that the total choline declined when apoptosis occurred in the liver cells. This result is quite conformable with the result of Blankenberg FG and the conventional idea, but different from the results of Lehtimaki[10]. In our opinion, there are three reasons for this phenomenon: firstly, it is related to the inspected organ, which is liver but not brain. As it is known, all ingested choline and free choline generated by phospholipid metabolism enter the hepatic circulation, making the liver, where there are very active biochemical pathways for choline metabolism, a significant “sink” for choline[28]. But when a lesion arises in the liver, the liver loses the function of absorbing choline and causes choline declining. Secondly, the method used to induce liver cell apoptosis could cause this difference. One of the reasons why the selenocompounds cause cell apoptosis is that it is capable of inducing rapid superoxide generation and p53 phosphorylation[29]. This activation can initiate mitochondrial dysfunction and result in energy insufficiency. Ultimately, it may affect the exchange of the cell containing substance, including choline compounds. This is testified by Luck et al[30]. Finally, cell apoptosis period is also one of the influencing factors towards the result. In our study, the apoptosis was in its early phase. At the beginning of cell apoptosis, the total choline compounds declined because free choline decreased. This was supported by our spectroscopic and light microscopic data. It is also very interesting that the total concentration of synthetical choline, including phosphorylcholine and glycerophosphorylcholin did not have statistical difference between the two groups. Energy insufficiency and activity decline may originally cause the concentration decrease of synthetical choline when apoptosis takes place in liver cells. Because of the synthetical choline supplement by membrane dilapidation and release when the samples were mashed, the total concentration of synthetical choline finally remained fairly constant. This process of membrane perturbations is mainly the function of phospholipase A2 activity in vivo[31].
In summary, high dose selenium compounds can cause lesion of rat liver and induce cell apoptosis in vivo. In vitro high resolution 1H-MRS can detect diversified metabolism that can resolve in water. The data of the spectroscopy include quantitative and qualitative information. Moreover, it can repetitively and accurately evaluate the lesion of the organ at early stage. Thus, this method has a potential role in oncology, including detection of malignancy, grading of tumor, predicting and monitoring the treatment response, and identifying persistent or recurrent diseases[3233]. In our study, we found that the changing trend for different ingredient of choline compounds is not completely the same at early period of apoptosis. Further studies are needed to know how choline compounds change at the advanced and final stage of apoptosis.
The field of cell death research has undergone an explosion of new knowledge over the past decade. The methods to evaluate death of cells have led to the development of several techniques. However, the properties revealed in these assays are not always applicable to study of diversified metabolite of apoptosis at one time. Magnetic resonance spectroscopy is a non-destructive and non-invasive technique that can provide complete structural analysis of a wide range of organic molecules in complex mixtures. This technique used to detect the metabolite alteration of apoptosis has been reported. Choline compounds are one kind of biologically interesting metabolites that can be detected by 1H-MRS. However, how the intensity of choline compounds changes when apoptosis occurs is still confused.
The alteration of different ingredient of choline compounds could be quantitatively analyzed by in vitro 9.4T high-resolution 1H-MR spectroscopy when apoptosis of liver cell takes place because of the toxicity of selenium. The results of present article will be helpful for further studies concerning metabolism of apoptosis.
When apoptosis of liver cell takes place, the concentrations of total choline and free choline decline, whereas the total concentration of synthetical choline, including phosphorylcholine and glycerophosphorylcholine, stays unchanged.
This study is useful to explain how apoptosis of liver cell occurs. It may also play an important role in guiding the clinical diagnosis and treatment of tumors.
This is an interesting study, where the advanced technique of 1H-NMR to detect the effect of choline compounds is paralleled to TUNEL staining of liver tissues of rats injected with selenium.
| 1. | Best PJ, Hasdai D, Sangiorgi G, Schwartz RS, Holmes DR Jr, Simari RD, Lerman A. Apoptosis. Basic concepts and implications in coronary artery disease. Arterioscler Thromb Vasc Biol. 1999;19:14-22. |
| 2. | Fiedler N, Quant E, Fink L, Sun J, Schuster R, Gerlich WH, Schaefer S. Differential effects on apoptosis induction in hepatocyte lines by stable expression of hepatitis B virus X protein. World J Gastroenterol. 2006;12:4673-4682. |
| 3. | Willingham MC. Cytochemical methods for the detection of apoptosis. J Histochem Cytochem. 1999;47:1101-1110. |
| 4. | Neild GH, Foxall PJ, Lindon JC, Holmes EC, Nicholson JK. Uroscopy in the 21st century: high-field NMR spectroscopy. Nephrol Dial Transplant. 1997;12:404-417. |
| 5. | Brown CE, Battocletti JH, Johnson LF. Nuclear magnetic resonance (NMR) in clinical pathology: current trends. Clin Chem. 1984;30:606-618. |
| 6. | Shih CM, Ko WC, Yang LY, Lin CJ, Wu JS, Lo TY, Wang SH, Chen CT. Detection of apoptosis and necrosis in normal human lung cells using 1H NMR spectroscopy. Ann N Y Acad Sci. 2005;1042:488-496. |
| 7. | Griffin JL, Lehtimaki KK, Valonen PK, Grohn OH, Kettunen MI, Yla-Herttuala S, Pitkanen A, Nicholson JK, Kauppinen RA. Assignment of 1H nuclear magnetic resonance visible polyunsaturated fatty acids in BT4C gliomas undergoing ganciclovir-thymidine kinase gene therapy-induced programmed cell death. Cancer Res. 2003;63:3195-3201. |
| 8. | Blankenberg FG, Katsikis PD, Storrs RW, Beaulieu C, Spielman D, Chen JY, Naumovski L, Tait JF. Quantitative analysis of apoptotic cell death using proton nuclear magnetic resonance spectroscopy. Blood. 1997;89:3778-3786. |
| 9. | Blankenberg FG, Storrs RW, Naumovski L, Goralski T, Spielman D. Detection of apoptotic cell death by proton nuclear magnetic resonance spectroscopy. Blood. 1996;87:1951-1956. |
| 10. | Lehtimaki KK, Valonen PK, Griffin JL, Vaisanen TH, Grohn OH, Kettunen MI, Vepsalainen J, Yla-Herttuala S, Nicholson J, Kauppinen RA. Metabolite changes in BT4C rat gliomas undergoing ganciclovir-thymidine kinase gene therapy-induced programmed cell death as studied by 1H NMR spectroscopy in vivo, ex vivo, and in vitro. J Biol Chem. 2003;278:45915-45923. |
| 11. | Serres S, Bezancon E, Franconi JM, Merle M. Ex vivo analysis of lactate and glucose metabolism in the rat brain under different states of depressed activity. J Biol Chem. 2004;279:47881-47889. |
| 12. | Schrum LW, Bird MA, Salcher O, Burchardt ER, Grisham JW, Brenner DA, Rippe RA, Behrns KE. Autocrine expression of activated transforming growth factor-beta(1) induces apoptosis in normal rat liver. Am J Physiol Gastrointest Liver Physiol. 2001;280:G139-G148. |
| 13. | Sitter B, Sonnewald U, Spraul M, Fjosne HE, Gribbestad IS. High-resolution magic angle spinning MRS of breast cancer tissue. NMR Biomed. 2002;15:327-337. |
| 14. | Martinez-Granados B, Monleon D, Martinez-Bisbal MC, Rodrigo JM, del Olmo J, Lluch P, Ferrandez A, Marti-Bonmati L, Celda B. Metabolite identification in human liver needle biopsies by high-resolution magic angle spinning 1H NMR spectroscopy. NMR Biomed. 2006;19:90-100. |
| 15. | Duarte IF, Stanley EG, Holmes E, Lindon JC, Gil AM, Tang H, Ferdinand R, McKee CG, Nicholson JK, Vilca-Melendez H. Metabolic assessment of human liver transplants from biopsy samples at the donor and recipient stages using high-resolution magic angle spinning 1H NMR spectroscopy. Anal Chem. 2005;77:5570-5578. |
| 16. | Xiao YZ, Hui FW, Xiao JL, Feng KP Jia ZN. NMR Studies on the Subacute Biochemical Effects of Aristolochic Acid on Rat Serum. Chinese Chemical Letters. 2005;16:1507-1510. |
| 17. | Yoon SO, Kim MM, Park SJ, Kim D, Chung J, Chung AS. Selenite suppresses hydrogen peroxide-induced cell apoptosis through inhibition of ASK1/JNK and activation of PI3-K/Akt pathways. FASEB J. 2002;16:111-113. |
| 18. | Zhou N, Xiao H, Li TK, Nur-E-Kamal A, Liu LF. DNA damage-mediated apoptosis induced by selenium compounds. J Biol Chem. 2003;278:29532-29537. |
| 19. | Kim YS, Jhon DY, Lee KY. Involvement of ROS and JNK1 in selenite-induced apoptosis in Chang liver cells. Exp Mol Med. 2004;36:157-164. |
| 20. | Bollard ME, Stanley EG, Lindon JC, Nicholson JK, Holmes E. NMR-based metabonomic approaches for evaluating physiological influences on biofluid composition. NMR Biomed. 2005;18:143-162. |
| 21. | Zhong W, Oberley TD. Redox-mediated effects of selenium on apoptosis and cell cycle in the LNCaP human prostate cancer cell line. Cancer Res. 2001;61:7071-7078. |
| 22. | Tiwary AK, Stegelmeier BL, Panter KE, James LF, Hall JO. Comparative toxicosis of sodium selenite and selenomethionine in lambs. J Vet Diagn Invest. 2006;18:61-70. |
| 23. | Cheng LL, Anthony DC, Comite AR, Black PM, Tzika AA, Gonzalez RG. Quantification of microheterogeneity in glioblastoma multiforme with ex vivo high-resolution magic-angle spinning (HRMAS) proton magnetic resonance spectroscopy. Neuro Oncol. 2000;2:87-95. |
| 24. | Griffin JL, Shockcor JP. Metabolic profiles of cancer cells. Nat Rev Cancer. 2004;4:551-561. |
| 25. | Yen CL, Mar MH, Meeker RB, Fernandes A, Zeisel SH. Choline deficiency induces apoptosis in primary cultures of fetal neurons. FASEB J. 2001;15:1704-1710. |
| 26. | Martin K. Concentrative accumulation of choline by human erythrocytes. J Gen Physiol. 1968;51:497-516. |
| 27. | Ackerstaff E, Pflug BR, Nelson JB, Bhujwalla ZM. Detection of increased choline compounds with proton nuclear magnetic resonance spectroscopy subsequent to malignant transformation of human prostatic epithelial cells. Cancer Res. 2001;61:3599-3603. |
| 28. | Michel V, Yuan Z, Ramsubir S, Bakovic M. Choline transport for phospholipid synthesis. Exp Biol Med (Maywood). 2006;231:490-504. |
| 29. | Hu H, Jiang C, Schuster T, Li GX, Daniel PT, Lu J. Inorganic selenium sensitizes prostate cancer cells to TRAIL-induced apoptosis through superoxide/p53/Bax-mediated activation of mitochondrial pathway. Mol Cancer Ther. 2006;5:1873-1882. |
| 30. | Luck DF. Formation of mitochondria in neurospora crass. A Study Based on Mitochondrial Density Changes. J Cell Biol. 1965;24:461-470. |
| 31. | Hakumaki JM, Poptani H, Sandmair AM, Yla-Herttuala S, Kauppinen RA. 1H MRS detects polyunsaturated fatty acid accumulation during gene therapy of glioma: implications for the in vivo detection of apoptosis. Nat Med. 1999;5:1323-1327. |
| 32. | King AD, Yeung DK, Ahuja AT, Leung SF, Tse GM, van Hasselt AC. In vivo proton MR spectroscopy of primary and nodal nasopharyngeal carcinoma. AJNR Am J Neuroradiol. 2004;25:484-490. |
| 33. | Thomas EL, Brynes AE, Hamilton G, Patel N, Spong A, Goldin RD, Frost G, Bell JD, Taylor-Robinson SD. Effect of nutritional counselling on hepatic, muscle and adipose tissue fat content and distribution in non-alcoholic fatty liver disease. World J Gastroenterol. 2006;12:5813-5819. |