Published online Jun 28, 2008. doi: 10.3748/wjg.14.3866
Revised: May 26, 2007
Accepted: June 2, 2007
Published online: June 28, 2008
AIM: To investigate the changing pattern of β-catenin expression and its prognostic value in advanced colorectal cancer (CRC).
METHODS: Archival tumor samples were analyzed for β-catenin using immunohistochemistry (IHC) in 95 patients with advanced CRC.
RESULTS: Membranous β-catenin expression was found in the normal colorectal epithelium. Almost 100% of CRC cases showed membranous and cytoplasmic expression, and 55 (58%) cases showed nuclear expression. In univariate (Kaplan-Meier) survival analysis, only the nuclear index (NI) was a significant predictor of disease-free survival (DFS) (P = 0.023; n = 35), with a NI above the median associated with longer DFS (34.2 mo) than those with a NI below the median (15.5 mo) (P = 0.045, ANOVA). The other indices were not significant predictors of DFS, and none of the three tested indices (for membranous, cytoplasmic, or nuclear expression) predicted disease-specific survival (DSS). However, when dichotomized as positive or negative nuclear expression, the former was a significant predictor of more favorable DFS (P = 0.041) and DSS (P = 0.046).
CONCLUSION: Nuclear β-catenin expression provides additional information in predicting patient outcome in advanced CRC.
- Citation: Elzagheid A, Buhmeida A, Korkeila E, Collan Y, Syrjänen K, Pyrhönen S. Nuclear β-catenin expression as a prognostic factor in advanced colorectal carcinoma. World J Gastroenterol 2008; 14(24): 3866-3871
- URL: https://www.wjgnet.com/1007-9327/full/v14/i24/3866.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3866
β-catenin is a 92-kDa multifunctional protein that, in its membrane location, links the intracellular part of the E-cadherin complex to actin cytoskeleton, which is a critical step in morphogenesis and maintenance of tissue integrity[1]. Alternatively, through Wnt signaling-mediated stabilization, β-catenin may act as a down-stream transcriptional trans-activator of several target genes[2]. Alterations in β-catenin protein expression levels and genetic rearrangement located in β-catenin exon 3 have been shown to contribute to the malignant character of various carcinomas and are likely to affect both intercellular adhesion and signal transduction, which are believed to be two independent functions of β-catenin protein[3].
The rare occurrence of mutations in β-catenin exon 3 has been previously documented in ulcerative colitis-related neoplastic progression[4] and in colorectal cancer (CRC) as well[5]. Immunohohistochemical studies suggest that the observed accumulation in β-catenin protein is probably due to genomic alterations in β-catenin coding regions, particularly in exon 3.
The impact of aberrations in β-catenin expression on the clinical outcome of CRC is controversial. Some studies reported prognostic value for cytoplasmic rather than nuclear expression[6], whereas others showed that nuclear accumulation of β-catenin may be an independent marker of unfavorable prognosis[78]. The aim of this study was to evaluate the possible role of β-catenin expression as a predictor of clinical outcome in advanced CRC patients.
Ninety-five patients with advanced colorectal carcinoma (CRC), enrolled consecutively from CRC patients attending our clinic for therapeutic procedures during the late 1990s, were included in our study. Of these 95 patients, 60 had metastases at diagnosis (Stage IV disease), while the remaining 35 patients (with stage II and III disease at baseline) subsequently developed a metastatic disease during the mean follow-up (FU) time of 25.1 ± 27.8 (SD) mo. All patients were treated at the Department of Oncology and Radiotherapy, Turku University Hospital, according to the protocols in routine use for the treatment of CRC patients with stage II, III or IV disease at that time. The 95 patients included in the present study were enrolled into the study cohort between October 1998 and August 2003. All patients were prospectively followed-up until death or until their last clinical visit (March 2007), with the median FU-time of 27.6 (range 3-150) mo. The study was approved by the TUH Ethics Committee and was conducted in accordance with the Declaration of Helsinki. Samples were collected with the endorsement of the National Authority for Medico-legal Affairs.
Key clinical data for these patients are shown in Table 1. Of the 95 cases, 38 were women and 57 were men. The mean age was 61.5 (range 24-78) years. The majority (n = 39) of the tumors were localized in the left colon, followed in the order of frequency by the right colon (n = 24), rectum (n = 24), and colon transversum (n = 7). At the time of diagnosis, 15 patients were Stage II, 20 were Stage III and 60 patients were Stage IV. Accordingly, the majority (n = 63, 66.3%) had T3 tumors, and almost half of the patients had lymph node involvement at the time of diagnosis (n = 46). The patients were selected for the cohort on the basis of both the diagnosis and treatment they received, and each patient was assigned to one of two treatment arms: (1) 20 were treated with irinotecan alone, and (2) 75 received a combination of irinotecan and 5-fluorouracil (5-FU). The chemotherapy regimen the patients received was included in a previous study investigating irinotecan combined with bolus 5-fluorouracil and folinic acid[9].
| Variable | No. or value | %1 |
| Patients | 95 | |
| Male | 57 | 60.0 |
| Female | 38 | 40.0 |
| Age (yr) | ||
| Median (range) | 60.7 (24-80) | |
| Primary tumour status2 | 95 | |
| T1 | 1 | 1.1 |
| T2 | 6 | 6.3 |
| T3 | 63 | 66.3 |
| T4 | 17 | 17.9 |
| Tx | 8 | 8.5 |
| Primary nodal status2 | 95 | |
| N0 | 25 | 26.3 |
| N+ | 46 | 48.4 |
| Nx | 24 | 25.3 |
| Metastases at diagnosis2 | 95 | |
| M0 | 35 | 36.8 |
| M1 | 60 | 63.2 |
| Histological grade | 95 | |
| Gr I | 12 | 12.6 |
| Gr II | 62 | 65.3 |
| Gr III | 18 | 19.0 |
| NA | 3 | 3.2 |
| Stage | 95 | |
| Stage II | 15 | 15.8 |
| Stage III | 20 | 21.0 |
| Stage IV | 60 | 63.2 |
| Survival (mo) | ||
| From primary diagnosis Median (range) | 27.3 (3-150) | |
| From metastasis Median (range) | 21.4 (3-80) |
Formalin-fixed, paraffin-embedded primary colorectal tumor tissue was obtained from 95 patients. Sections were cut serially at 5 µm for routine haematoxylin and eosin staining and for immunohistochemical (IHC) analysis. An experienced pathologist confirmed all histological diagnoses. IHC analysis was done using an automatic system (BenchMark XT, Ventana Medical Systems, Inc. Tucson, Arizona, USA). This fully automated processing of bar code labeled slides included baking of the slides, solvent free deparaffinization, antigen retrieval in a cell conditioning buffer CC2 (Mild: 36 min conditioning, and standard: 60 min conditioning), and incubation with the monoclonal mouse β-catenin antibody (clone CAT-5H10, isotype IgG1-kappa, Zymed Laboratories, San Franscisco, CA) at a dilution 1:200 (32 min, 37°C). The dilution of the primary antibody was based on previous dilution experiments. UltraViewTM Universal DAB (a biotin-free, Multimer-based detection system for the specific and sensitive detection of mouse IgG, mouse IgM, and rabbit IgG primary antibodies) was used. UltraView DAB includes: ultraView Universal HRP, ultraView Universal DAB Inhibitor, ultraView Universal DAB Chromogen, ultraView Universal DAB H2O2, and ultraView Universal DAB Copper. Counterstaining with haematoxylin (2021) was done for 4 min, and post-counterstaining with a blueing reagent (2037) was done for 4 min as well. After staining, the sections were dehydrated in ethanol, cleared in xylene, and covered with Mountex and cover slips.
β-catenin staining was evaluated using regular light microscopy by an observer who was blind to the clinical data (AB). All membranous, cytoplasmic, and nuclear staining were evaluated separately. For cell membrane staining, four categories were used (+++, ++, +, -), starting from equivalent to normal to entirely negative[10]. The cytoplasmic staining was also graded into four categories: (0) Negative, no detectable staining, (1) Weak, but still detectable staining, (2) Moderate, clearly positive but still weak, (3) Heavy staining, intense[11]. The nuclear staining index (NI) was also graded to into four categories (+++, ++, +, -): (0) Negative, only blue staining seen, (1) Weak, blue staining clearly seen through brown staining, (2) Moderate, blue scarcely seen through brown staining, nuclei appear darker than the cytoplasm, (3) Heavy staining, no blue seen through brown staining, nuclei appear darker than the cytoplasm. Three staining indexes were calculated: the membrane index (MI), cytoplasmic index (CI), and nuclear index (NI). These indices were calculated with both the intensity of staining and the fraction of positively-stained cells taken into account using the following formula: I = 0 * f0 + 1 * f1 + 2 * f2 + 3 * f3 where I is the staining index and f0-f3 are the fractions of the cells showing a defined level of staining intensity (from 0 to 3). Theoretically, index scores could vary between 0 and 3[12]. The reproducibility of the evaluation of the β-catenin staining indices was tested by employing two observers (AE, AB), and the estimations showed good correlation and reproducibility (Pearson’s r: MI, CI, and NI, were 0.77, 0.91, and 0.90, respectively).
Statistical analyses were performed using SPSS® (SPSS, Inc., Chicago, USA) and STATA (Stata Corp., Texas, USA) software packages (SPSS for Windows, version 14.0.1 and STATA/SE 9.2). Frequency tables were analyzed using the Chi-square test, which included the likelihood ratio (LR) or Fischer's exact test to assess the significance of the correlation between the categorical variables. Odds ratios and their 95% confidence intervals (95% CI) were calculated where appropriate, using the exact method. Differences in the means of continuous variables were analyzed using non-parametric tests (Mann-Whitney or Kruskal-Wallis) for 2- and K-independent samples, respectively. ANOVA (analysis of variance) was only used for deriving the mean values (and their SD) for each individual category. Bivariate correlation (Spearman rho) and scatterplots were used to check the correlations between two continuous variables (MI, CI vs DFS, DSS), controlled by linear regression analysis (R and R2) for linearity. Univariate survival (life-table) analysis for the outcome measure (DSS, DFS) was based on Cox’s method (indices treated as continuous variables), and/or using Kaplan-Meier analysis (indices with Median as cut-off). Multivariate survival analysis was carried out using Cox’s proportional hazards model in a backward stepwise manner with the log-likelihood ratio (L-R) significance test, using the default values for enter and exclusion criteria. The assumption of proportional hazards was controlled by log-minus-log (LML) survival plots. In all tests, the values P < 0.05 were regarded statistically.
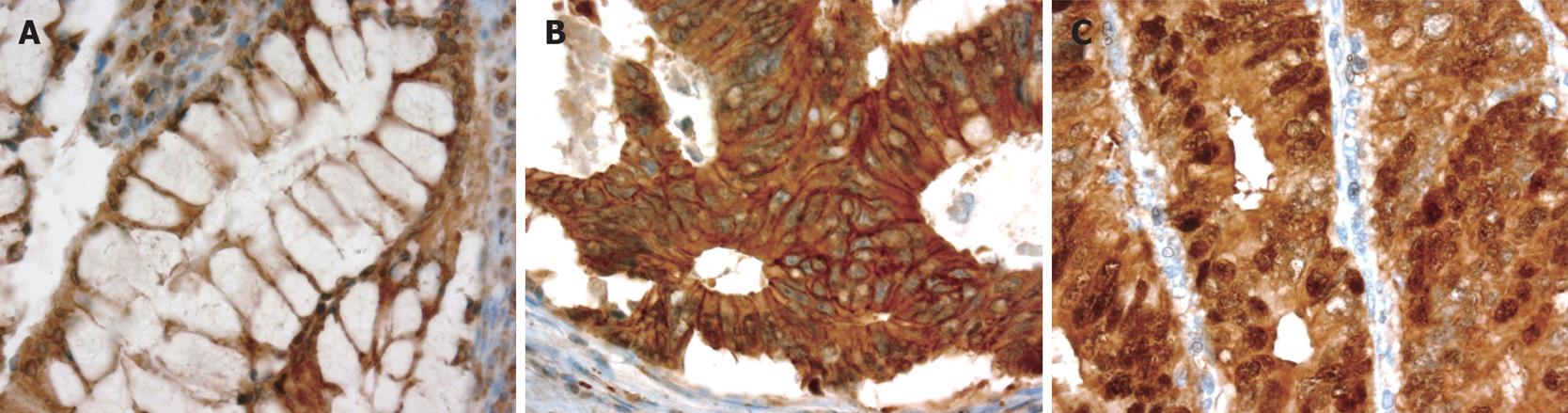
β-catenin expression patterns are illustrated in Figure 1. The expression pattern of β-catenin was predominantly membranous and weakly cytoplasmic in normal colonic epithelium but the pattern was cytoplasmic, membranous, or nuclear in the tumor tissue. Almost 100% of the cases showed membranous and cytoplasmic β-catenin expression, with nuclear expression being observed in 55 (58%) cases. The mean values of the three β-catenin staining indices (MI, CI, and NI) were 1.14, 1.26, and 0.80, respectively, and the median values were 1.20, 1.30, and 0.77, respectively.
We analyzed the three β-catenin staining indices in relation to all available clinical variables and tumor characteristics in univariate analyses. Using the median cut-off point, there was no correlation between β-catenin expression and most of the clinical variables (age, sex, stage, and grade). However, β-catenin expression (CI and NI) was borderline or significantly related (P = 0.06, P = 0.04, respectively) to the localization of the primary tumor, with expression being more intense in descending colon and rectum carcinomas than in lesions of the ascending and transverse colon.
There was also a marginal relation (P = 0.086) between NI and response to treatment in that the patients with a NI below the median had a response rate (24/48, 50%) better than those with a NI above the median (16/47, 34.0%). A direct relationship (P = 0.068) was found between the MI and response to treatment when the 75th percentile was used; patients with a MI > 75% had a higher response rate (9/16; 56.3%) than patients with a MI < 75% (31/79, 39.2%).
In univariate (Kaplan-Meier) survival analysis (calculable for 35 patients with stage II or III disease) with the median as the cut-off, only the NI was a significant predictor of more favorable disease-free survival (DFS) (P = 0.023). The patients with a NI above the median had longer DFS (34.2 mo) than those with a NI below the median (15.5 mo) (P = 0.045, ANOVA). The other indices were not significant predictors of DFS, and none of the three indices predicted disease-specific survival (DSS) in univariate analysis (Cox or Kaplan-Meier).
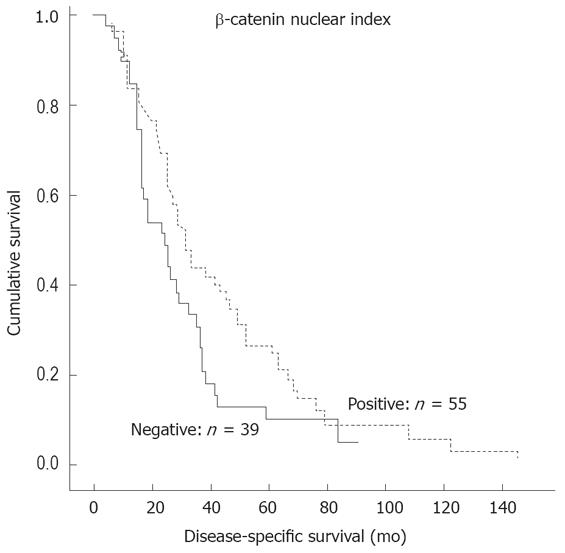
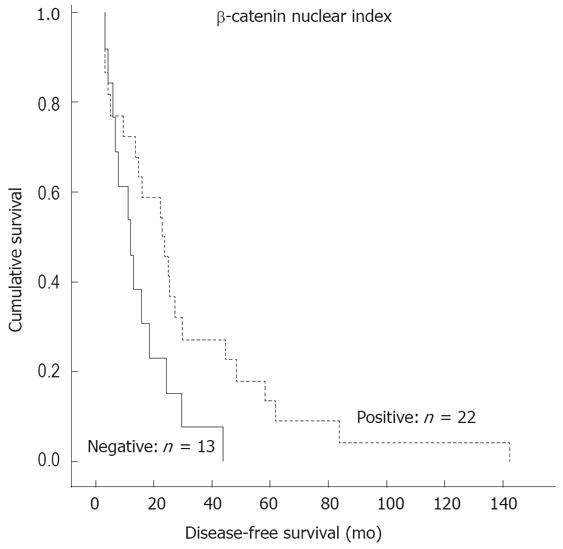
When the patients were stratified into two groups: nuclear expression positive (n = 55) and nuclear expression negative (n = 39), the former was a significant predictor of more favorable DSS (P = 0.046) (Figure 2) and DFS (P = 0.041) (Figure 3).
When β-catenin expression was analyzed jointly with E-cadherin expression[13] as a potential predictor of disease outcome, the combined (E-cadherin and β-catenin) cytoplasmic index did not provide any significant prognostic information.
We also reproduced the grading used by Ougolkov et al[7], resulting in 6/46 cases with nuclear expression at the invasive front, 20/46 cases with a diffuse nuclear pattern, and 20/46 tumors with a mixed pattern. When correlated with the treatment response and disease outcome, this grading system did not produce any results with predictive or prognostic value.
As compared with the sub-cellular distribution of β-catenin in normal colonic mucosa, neoplastic cells demonstrated a distinct shift from a membranous locali-zation to a more widespread distribution (membranous, cytoplasmic, and nuclear) in cancer lesions. This is in line with previous reports describing β-catenin expression in cancer cells with this type of altered pattern[1415]. For example, Wong et al[16] observed no nuclear β-catenin accumulation in normal tissues, whereas it was present in 8% of polyps, 92% of adenomas, and 100% of carcinomas. In the present series, nuclear expression or accumulation was observed in 48% of the cancer samples, which is in line with several other reports[717]. Interestingly, in 13% of the cases with nuclear expre-ssion, β-catenin was expressed by the tumour cells at the invasion fronts, a figure very similar to the 9% reported by Ougolkov et al[7].
We did not find significant correlations between the three β-catenin expression patterns (MI, CI, and NI) and most of the clinical variables recorded (age, sex, grade, and stage), except for tumor localization. Accordingly, β-catenin expression, both CI and NI, was more intense in carcinomas of the descending colon and rectum as compared with lesions localized in the ascending and transverse colon. Similar observations have been reported by previous studies[18–20]. There is increasing evidence to suggest that molecular mechanisms and molecular phenotypes differ in carcinomas arising in the proximal and distal segments of the large bowel[21]. The involvement of different molecular pathways in colorectal carcinogenesis is exemplified by the fact that cancers of “mutator” phenotypes preferentially occur in the proximal (right side) colon, whereas the adenoma-carcinoma sequence phenotype is characteristic of carcinomas in the distal (left side) colon and rectum[2223]. Corresponding differences have also been shown in association with other potential prognosticators[24].
Interestingly, a marginally significant relation was observed between the NI and MI and response to treatment. Accordingly, the patients who did not respond to treatment had a NI above the median, whereas patients who had a high MI responded better to treatment. The significance of these observations remains to be elucidated in a larger study. There is an obvious need to identify novel molecular targets for cancer therapeutics, and in this respect, the recent data showing that suppression of β-catenin can inhibit the neoplastic growth of APC-mutant colorectal cancer are of interest[25].
The correlation between β-catenin expression pattern and clinical outcome is a controversial subject. Some studies reported that cytoplasmic rather than nuclear accumulation of β-catenin is significantly related to metastasis-free survival in CRC[6]. The same was reported regarding the potential prognostic value of nuclear expression. There are studies reporting that positive nuclear expression at the invasive front of the tumor predicts shorter survival[719]. However, other workers failed to find any correlation between nuclear expression and survival in CRC[1726]. In contrast, our data show that nuclear expression is a significant predictor of more favorable DSS and DFS (Figures 2 and 3). We also analyzed our samples using the same system as originally described by Ougolkov et al[7]. In our study, however, this special grading system did not confirm the original observation that nuclear expression at the invasive front of the tumor predicts a shorter survival[7].
There are multiple explanations for the inconsistent and, in part, discrepant results reported in different studies[67171926]. Such potential confounding factors might include the size of tissue samples, intrinsic tumor heterogeneity, lack of standardization in the evaluation of positive and negative results, and different immunohistochemical staining and grading methods with varying degree of sensitivity. In addition, our patients represent advanced CRC, with the majority of patients having stage IV disease, as compared with the Ougolkov study, where the majority of patients had stage II CRC. Also, the type of treatment may have played a role in the detected relationships.
Cell-cell adhesion molecules are believed to participate in the processes of invasion, migration and metastasis[27–29]. In this regard, the E-cadherin and β-catenin complex plays a critical role in cell-cell adhesion. E-cadherin is a member of the cadherin family that mediates calcium-dependent adhesion to ensure the maintenance of a normal phenotype of epithelial cells[130]. β-catenin binds directly to the cytoplasmic domain of E-cadherin and to the actin microfilament network of the cellular cytoskeleton. This binding is essential for stable cell-cell adhesion[31].
It can be reasoned that altered expression of β-catenin (i.e., the shift from membranous to cytoplas-mic and nuclear sites) might compromise the integrity of the E-cadherin/β-catenin complex and result in weaker cell-cell adhesion in cancer cells. Thus, it seems feasible to assess whether altered co-expression patterns of these two markers is of any predictive value in CRC. For that purpose, we combined both the membranous and cytoplasmic E-cadherin expression with membranous and cytoplasmic β-catenin expression to compare normal (MI/MI) and abnormal (CI/CI) co-expression, respectively, of these two markers as previously analysed in CRC[13]. To our disappointment, however, neither the membranous nor the cytoplasmic E-cadherin/β-catenin index (analyzed in two different modes) provided any useful information as to DFS or DSS. Thus, no added value can be obtained with analyzing E-cadherin expression together with β-catenin expression as compared to the analysis of the latter alone (Figures 2 and 3).
Taken together, the present results confirm that β-catenin expression is markedly altered in the vast majority of colorectal cancers. This shift from normal membranous expression to the cytoplasmic (CI) and nuclear (NI) patterns seems to bear some association with the localization of the primary tumour, being most pronounced in lesions of the descending colon and rectum. Although the association of CI and NI to treatment response remains unclear, NI seems to provide some prognostic value in predicting more favourable DFS and also DSS, when dichotomized as NI+/NI- expression. Many of the issues still remain unanswered, however, and additional clinical and experimental studies are needed to fully elucidate the role of β-catenin in colorectal carcinogenesis and its potential usefulness as an independent predictor of disease outcome.
| 1. | Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci. 1994;107:3655-3663. |
| 2. | Barth AI, Nathke IS, Nelson WJ. Cadherins, catenins and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr Opin Cell Biol. 1997;9:683-690. |
| 3. | Fujimori M, Ikeda S, Shimizu Y, Okajima M, Asahara T. Accumulation of beta-catenin protein and mutations in exon 3 of beta-catenin gene in gastrointestinal carcinoid tumor. Cancer Res. 2001;61:6656-6659. |
| 4. | Nilbert M, Rambech E. Beta-catenin activation through mutation is rare in rectal cancer. Cancer Genet Cytogenet. 2001;128:43-45. |
| 5. | Aust DE, Terdiman JP, Willenbucher RF, Chang CG, Molinaro-Clark A, Baretton GB, Loehrs U, Waldman FM. The APC/beta-catenin pathway in ulcerative colitis-related colorectal carcinomas: a mutational analysis. Cancer. 2002;94:1421-1427. |
| 6. | Maruyama K, Ochiai A, Akimoto S, Nakamura S, Baba S, Moriya Y, Hirohashi S. Cytoplasmic beta-catenin accumulation as a predictor of hematogenous metastasis in human colorectal cancer. Oncology. 2000;59:302-309. |
| 7. | Ougolkov AV, Yamashita K, Mai M, Minamoto T. Oncogenic beta-catenin and MMP-7 (matrilysin) cosegregate in late-stage clinical colon cancer. Gastroenterology. 2002;122:60-71. |
| 8. | Miyamoto S, Endoh Y, Hasebe T, Ishii G, Kodama K, Goya M, Ono M, Saitoh N, Chiba T, Ochiai A. Nuclear beta-catenin accumulation as a prognostic factor in Dukes' D human colorectal cancers. Oncol Rep. 2004;12:245-251. |
| 9. | Glimelius B, Ristamaki R, Kjaer M, Pfeiffer P, Skovsgaard T, Tveit KM, Linne T, Frodin JE, Boussard B, Oulid-Aissa D. Irinotecan combined with bolus 5-fluorouracil and folinic acid Nordic schedule as first-line therapy in advanced colorectal cancer. Ann Oncol. 2002;13:1868-1873. |
| 10. | Elzagheid A, Kuopio T, Ilmen M, Collan Y. Prognostication of invasive ductal breast cancer by quantification of E-cadherin immunostaining: the methodology and clinical relevance. Histopathology. 2002;41:127-133. |
| 11. | Elzagheid A, Algars A, Bendardaf R, Lamlum H, Ristamaki R, Collan Y, Syrjanen K, Pyrhonen S. E-cadherin expression pattern in primary colorectal carcinomas and their metastases reflects disease outcome. World J Gastroenterol. 2006;12:4304-4309. |
| 12. | Lipponen P, Collan Y. Simple quantitation of immuno-histochemical staining positivity in microscopy. Acta Stereol. 1992;11:125-132. |
| 13. | Bendardaf R, Elzagheid A, Lamlum H, Ristamaki R, Collan Y, Pyrhonen S. E-cadherin, CD44s and CD44v6 correlate with tumour differentiation in colorectal cancer. Oncol Rep. 2005;13:831-835. |
| 14. | Mikami T, Mitomi H, Hara A, Yanagisawa N, Yoshida T, Tsuruta O, Okayasu I. Decreased expression of CD44, alpha-catenin, and deleted colon carcinoma and altered expression of beta-catenin in ulcerative colitis-associated dysplasia and carcinoma, as compared with sporadic colon neoplasms. Cancer. 2000;89:733-740. |
| 15. | Horkko TT, Klintrup K, Makinen JM, Napankangas JB, Tuominen HJ, Makela J, Karttunen TJ, Makinen MJ. Budding invasive margin and prognosis in colorectal cancer--no direct association with beta-catenin expression. Eur J Cancer. 2006;42:964-971. |
| 16. | Wong SC, Lo ES, Lee KC, Chan JK, Hsiao WL. Prognostic and diagnostic significance of beta-catenin nuclear immunostaining in colorectal cancer. Clin Cancer Res. 2004;10:1401-1408. |
| 17. | Roca F, Mauro LV, Morandi A, Bonadeo F, Vaccaro C, Quintana GO, Specterman S, de Kier Joffe EB, Pallotta MG, Puricelli LI. Prognostic value of E-cadherin, beta-catenin, MMPs (7 and 9), and TIMPs (1 and 2) in patients with colorectal carcinoma. J Surg Oncol. 2006;93:151-160. |
| 18. | Zhang B, Ougolkov A, Yamashita K, Takahashi Y, Mai M, Minamoto T. beta-Catenin and ras oncogenes detect most human colorectal cancer. Clin Cancer Res. 2003;9:3073-3079. |
| 19. | Baldus SE, Monig SP, Huxel S, Landsberg S, Hanisch FG, Engelmann K, Schneider PM, Thiele J, Holscher AH, Dienes HP. MUC1 and nuclear beta-catenin are coexpressed at the invasion front of colorectal carcinomas and are both correlated with tumor prognosis. Clin Cancer Res. 2004;10:2790-2796. |
| 20. | Feng Han Q, Zhao W, Bentel J, Shearwood AM, Zeps N, Joseph D, Iacopetta B, Dharmarajan A. Expression of sFRP-4 and beta-catenin in human colorectal carcinoma. Cancer Lett. 2006;231:129-137. |
| 21. | Chung DC. The genetic basis of colorectal cancer: insights into critical pathways of tumorigenesis. Gastroenterology. 2000;119:854-865. |
| 22. | Loeb LA. A mutator phenotype in cancer. Cancer Res. 2001;61:3230-3239. |
| 23. | Yashiro M, Carethers JM, Laghi L, Saito K, Slezak P, Jaramillo E, Rubio C, Koizumi K, Hirakawa K, Boland CR. Genetic pathways in the evolution of morphologically distinct colorectal neoplasms. Cancer Res. 2001;61:2676-2683. |
| 24. | Hilska M, Roberts PJ, Collan YU, Laine VJ, Kossi J, Hirsimaki P, Rahkonen O, Laato M. Prognostic significance of matrix metalloproteinases-1, -2, -7 and -13 and tissue inhibitors of metalloproteinases-1, -2, -3 and -4 in colorectal cancer. Int J Cancer. 2007;121:714-723. |
| 25. | Green DW, Roh H, Pippin JA, Drebin JA. Beta-catenin antisense treatment decreases beta-catenin expression and tumor growth rate in colon carcinoma xenografts. J Surg Res. 2001;101:16-20. |
| 26. | Chung GG, Provost E, Kielhorn EP, Charette LA, Smith BL, Rimm DL. Tissue microarray analysis of beta-catenin in colorectal cancer shows nuclear phospho-beta-catenin is associated with a better prognosis. Clin Cancer Res. 2001;7:4013-4020. |
| 27. | Mareel M, Boterberg T, Noc V, Van Hoorde L, Vermeulen S, Bruyneel E, Bracke M. E-cadherin/catenin/cytoskeleton complex: a regulator of cancer invasion. J Cell Physiol. 1997;173:271-274. |
| 28. | Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483-1487. |
| 29. | Bonitsis N, Batistatou A, Karantima S, Charalabopoulos K. The role of cadherin/catenin complex in malignant melanoma. Exp Oncol. 2006;28:187-193. |
| 30. | Munro SB, Blaschuk OW. A comprehensive survey of the cadherins expressed in the testes of fetal, immature, and adult mice utilizing the polymerase chain reaction. Biol Reprod. 1996;55:822-827. |