Published online Jun 7, 2008. doi: 10.3748/wjg.14.3425
Revised: April 7, 2008
Accepted: April 14, 2008
Published online: June 7, 2008
AIM: To characterize the factors of the improved survival following combined pancreaticoduodenectomy (PD) and gastrectomy for the treatment of advanced gastric cancer with pancreaticoduodenal region involvement.
METHODS: From 1995 to 2004, 53 patients with primary gastric cancer were diagnosed with synchronous (n = 44) or metachronous (n = 9) pancreaticoduodenal region involvement. Of these, 17 patients (32%) underwent total gastrectomy (TG) or distal subtotal gastrectomy (SG) combined with PD simultaneously. The preoperative demographic, clinical information, clinicopathologic features and the surgical results of these 17 patients were considered as factors influencing survival and were analyzed by the Kaplan-Meier method with log-rank comparison.
RESULTS: The actual 1- and 3-year survival rates of these 17 patients after resection were 77% and 34%, respectively, and three patients survived for more than 5 years after surgery. The tumor-free resection margin (P = 0.0174) and a well-differentiated histologic type (P = 0.0011) were significant prognostic factors on univariate analysis. No mortality occurred within one mo after operation, postoperative weight loss of different degree was present in all the patients with TG and 12 cases had other complications. There were 9 (53%) cases of recurrence in 5-48 mo after operation. The survival rate in the palliative and explorative group was significantly (P = 0.0064) lower than in the combined PD group.
CONCLUSION: Judicious use of en bloc PD and gastrectomy and strictly preventing postoperative complications may improve the long-term survival for advanced gastric cancer patients with pancreati-coduodenal region involvement. Well-differentiated histology and negative resection margin are the most important predictors of long survival.
- Citation: Wang XB, Yang LT, Zhang ZW, Guo JM, Cheng XD. Pancreaticoduodenectomy for advanced gastric cancer with pancreaticoduodenal region involvement. World J Gastroenterol 2008; 14(21): 3425-3429
- URL: https://www.wjgnet.com/1007-9327/full/v14/i21/3425.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3425
Because of earlier diagnosis, more accurate staging and safer operations, outcomes after treatment of gastric cancer are being improved, especially for early gastric cancer the results have become satisfactory[1]. And in the treatment of advanced carcinoma of the stomach, gastrectomy with extensive lymph node dissection has been reported to acquire a substantial improvement in survival[2–4]. In spite of these advances, there were still some problems as for areas of resection for advanced gastric cancer involving local organs. Arguments against enlarged resection are based on the observed increase in the morbidity and mortality rates, with little objective benefit in survival[5]. And some surgeons still consider the invasion of adjacent organs by the carcinoma of stomach as a sign of incurable disease. But others believe patients with T4 gastric cancer will benefit from extended en bloc surgical resection[6–8].
For the anatomic reason, pancreaticoduodenal region involved in advanced gastric cancer was not scarce. But few articles about its surgical treatment were available. The aim of this study was to report our experience in undergoing pancreaticoduodenectomy (PD) with gastrectomy for advanced gastric cancer with pancreaticoduodenal region involvement.
From January 1995 to January 2004, 916 patients with gastric carcinoma underwent surgical treatment in the Department of Hepatobiliary-Pancreatic-Gastric Surgery, Zhejiang Cancer Hospital. Of the 916 cases, 44 were found to have synchronous pancreaticoduodenal region involved and 9 metachronous pancreaticoduodenal region invaded or involved. Among the 53 patients, palliative gastrectomy was performed in 6 patients, bypass through exploration performed in 14, explorations in 7 and surgical treatment was given up in 9 because of additional organs metastasis or poor physical state. And 17 patients who underwent PD with gastrectomy were selected for this study, including 11 men and 6 women with a mean age of 56 years (range from 38 to 71 years). Overall radical resectability was 32.1% (17/53) for the 53 patients, 32% (14/44) for synchronous metastases, and 33.3% (3/9) for metachronous lesions. Follow-up period ranged from 2 to 72 mo (median 38 mo).
PD was indicated for patients with visibly synchronous pancreaticoduodenal region involved lesions who did not have peritoneal dissemination or any other distant metastasis, or for patients with metachronous pancreaticoduodenal region involved who did not have any other recurrent lesions. Three patients who had a desmoplastic reaction at the site of presumed tumor invasion were not included in this study. Confirmation of cancerous invasion of pancreaticoduodenal region was established histologically in operation. In addition to PD, TG was done in 11 (64.4%) and SG in 6 (35.6%) patients, depending on the location of the primary gastric lesion. En bloc surgical resection and D2 lymphadenectomy were performed as the standard radical gastrectomy for these 17 patients. As for reconstruction of digestive tract, binding pancreaticojejunostomy[9] and Roux-en-Y anastomosis were adopted for all the cases. In this group, two patients underwent right hemicolectomy and one patient underwent cholangiocystectomy additionally at the same time. All patients were treated with postoperative adjuvant chemotherapy using the same chemotherapy regimens (ELF regimens: etoposide + leucovorin + fluorouracil)[1011].
The preoperative demographic and clinical information was obtained from the patient records: age, gender, interval between gastrectomy and PD, surgical procedure and recurrence. The number and size of the tumors, extent of lymph node metastasis and surgical margin of the tumors were also recorded. The pathologic diagnosis and classification of the tumors were performed by a minimum of two pathologists.
All data were treated with statistic software kit of SPSS 12.0. Parameters influencing survival were compared using the Kaplan-Meier method with log-rank comparison. P < 0 .05 was considered significant differences.
No patient died during the initial hospital stay or within 1 mo after surgery. The median diameter of the tumors was 4.0 cm (range 2.8-9.5 cm). Only four patients had well differentiated tumors and the other 13 patients had moderately or poorly differentiated tumors. According to Borrmanm Type, six patients were Borrmann III and 11 were Borrmann IV. Ten patients had positive lymph node metastasis and seven had negative lymph node metastasis. Resection margins in five patients were tumor-positive and 12 were tumor-free. The actual 1- and 3-year survival rates after PD with gastrectomy were 77% and 34%, respectively. The results of the analysis of the prognostic factors are given in Table 1. Tumor-free (negative) resection margin (P = 0.0174) was significant determinants for a favorable prognosis after PD. In terms of pathologic features, a well-differentiated type of metastases (P = 0.0011) was a significant prognostic factor. Factors associated with the primary lesion and surgical procedures were not significant prognostic determinants. Cancer recurred in 11 (59%) of the 17 patients between 5 mo and 48 mo after PD resection. The site of initial recurrence after PD and gastrectomy was the gastric and pancreaticoduodenal bed in 6 patients, the liver in 3, and the retroperitoneal lymph nodes in 2, which were diagnosed by image methods (CT, MRI or US). Three patients survived more than 5 years after PD. No mortality occurred in one mo after operation, postoperative weight loss of different degree was present in all the patients with TG. Twelve had other complications among the 17 patients, including intra-abdominal abscesses 5 (41.7%) and anastomotic leak 3 (gastrointestinal leak 1 and bile leak 2) (25%), pneumonia 2 (16.7%), returned esophagitis 1 (8.3%) and acute renal failure 1 (8.3%). All the complications were cured by operative or conservative treatment.
| Factors | Number of patients | P |
| Age (yr) | 0.1405 | |
| < 56 | 7 | |
| ≥ 56 | 10 | |
| Gender | 0.4412 | |
| Male | 11 | |
| Female | 6 | |
| Metastases | 0.2010 | |
| Synchronous | 14 | |
| Metachronous | 3 | |
| Tumor size | 0.9837 | |
| < 4 cm | 8 | |
| ≥ 4 cm | 9 | |
| Histologic differentiation | 0.0011 | |
| Well | 4 | |
| Moderate or poor | 13 | |
| Gastric carcinoma depth of invasion | 0.0610 | |
| Borrmann III | 6 | |
| Borrmann IV | 11 | 0.0516 |
| Lymph node metastasis | ||
| Positive | 10 | |
| Negative | 7 | 0.7948 |
| Gastrectomy pattern | ||
| Total gastrectomy | 11 | |
| Subtotal gastrectomy | 6 | 0.0174 |
| Resection margins | ||
| Positive | 5 | |
| Negative | 12 | 0.1486 |
| Combined other organs | ||
| No | 14 | |
| Yes | 3 |
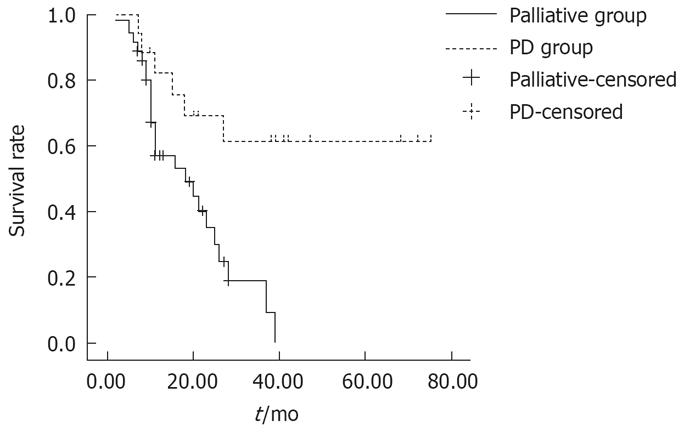
Of the 36 patients with pancreaticoduodenal region involvement who did not undergo radical resection, the actual 1- and 3-year survival rates were 41.7%(15/36) and 5.6%(2/36), respectively. The survival rate of these 36 patients (palliative group) was significantly (P = 0.0064) lower than that of PD group (Figure 1).
Surgery remains the only method of treatment that offers the potential for cure of gastric cancer[12]. But gastric cancer is usually diagnosed at an advanced stage because of its vague and nonspecific symptoms. And poor survival will be followed by late stage, especially when tumors invade the serosa or other organs. There is a report that with serosal involvement alone, less than 30% of the patients are living five years after surgery, and with involvement of both serosa and lymph nodes, the 5-year survival is less than 15%[13]. At the same time, opinions about extended surgical resections for advanced gastric cancer remains controversial. Takeuchi et al[12] retrospectively studied 65 patients without distant metastasis who underwent TG with pancreaticosplenectomy (PS) and 98 patients without distant metastasis who underwent TG alone (the TG alone group) by essentially the same technique, and concluded that combined PS with TG should never be performed as the standard surgical procedure for every stage of gastric cancer, especially stage II. But we think that in his report the PS was performed with TG to facilitate dissection of the lymph nodes around the splenic artery and splenic hilus, but not performed to resect the involved lesions of spleen or pancreas tail.
On the other hand, some authors agreed to extend gastric resection in patients with adjacent organs involved. In the report by Kodama et al[14], 77 patients with carcinoma of the stomach directly invading adjacent organs or structures were analyzed retrospectively to investigate the efficacy of en bloc resection. Forty-one patients underwent gastrectomy combined with resection of one or more invaded organs (combined resection group), while the other 36 patients underwent gastrectomy with palliative abrasion between the primary tumor and the invasion site (non-combined resection group). The results demonstrated that the five-year survival rate was 23% in the combined resection group and 0% in the non-combined resection group.They thought that an en bloc combined resection would be worth trying. Iriyama et al[15] reported the highest 5-year survival rate of 46% after extended gastric resection with adjacent organs in patients with T4 gastric cancer. And Korenaga et al[16] reported that the 5-year survival was 36.7% for those who underwent radical resection of adjacent organs and 17.4% for those who underwent palliative resection of adjacent involved organs. Ozaki et al[17] and others[16] have found that an aggressive approach to resection of the stomach with the body and tail of the pancreas or PD and right hemicolectomy can lead to an acceptable 5-year survival rate of 29%. Yonemura et al[18] have performed 26 SG with PD in combination with right hemicolectomy without any operative mortality and a 5-year survival of 33% even for patients with N3 metastases (e.g. retropancreatic nodes or superior mesenteric nodes). Cho et al[19] reported their 15-year experience of extended gastrectomy for advanced gastric cancer. The median survival time of the positive margin group was 34 mo. The negative margin group had a significantly longer median survival of 69 mo (P = 0.025). When both groups of patients were stratified according to nodal stage, a positive resection margin determined a worse prognosis only in patients with node-negative disease (174 mo vs 37 mo, P = 0.0001). In patients with nodal metastasis, the median survival time was similar in both groups. Their results suggested that a positive microscopic margin was associated with a worse outcome in patients with node-negative disease. Therefore, a more aggressive treatment, such as reoperation, was needed in node-negative patients with a positive microscopic disease. Our results have shown that three patients survived more than five years in radical resection group (PD group) and no patient survived more than five years in the palliative group. And the actual survival of these two groups is statistically different. Although factors associated with the primary lesion, patient demographic data and surgical procedures were not significant prognostic determinants, but negative resection margin was very important for the higher survival rate. And when pancreaticoduodenal region is involved by advanced gastric cancer, combined PD and gastrectomy will bring the chance of tumor-free resection margin. And curative (R0) resection improves prognosis[1920]. PD with gastrectomy will benefit the lymphadenectomy of No. 7, 8, 9 and 11. Sometimes, it is helpful for lymphadenectomy of No. 16 (Figure 2), though lymph nodes of the No.16 were not resected routinely in our group. We proposed the indication of PD with gastrectomy as follows: (1) head of pancreas was invaded by gastric cancer, (2) metastasis of lymph nodes of No.6 and head of pancreas was infiltrated, (3) duodenum below pylorus 2 cm was invaded by gastric cancer, and (4) the inferior segment of common bile duct was invaded by gastric cancer. Occasionally, the above condition was not found by preoperative examination. In our group, three cases were not found till during operations. So it is necessary to check the head of pancreas, the inferior segment of common bile duct and the superior segment of duodenum below pylorus during operations when tumor was near the pancreaticoduodenal region. As for the pattern of gastrectomy, we adopted TG or SG according to the different locations of the cancer within the stomach, its pattern of growth, and the level of local spread. Subtotal gastrectomies were reserved for exophytic and small infiltrative tumors located in the lower third of the stomach. Total gastrectomy was used for tumors located in the middle and upper third of the stomach, or tumors with an infiltrative growth pattern. Our results manifested that tumor-free resection margins will benefit the survival. And we emphasize that frozen-histologic examination during operation should benefit both the definition of resection margins and the definition of tumor invasion on pancreaticoduodenal region. Large inflammatory perigasrtic lymph nodes or desmoplastic reaction around gastric cancer can be inaccurately presumed to be tumor invasion and adopt extended gastric resection mistakenly. Three patients who had desmoplastic reaction at the site of presumed tumor invasion were found in our hospital during operation, the pancreas and duodenums were reserved. And no inflammatory perigasrtic lymph nodes or desmoplastic reaction around gastric cancer were found during postoperative histologic examination in our data.
Upon decision of extended resection, as the procedure of PD and gastrectomy was relatively complicated and status of most patients was poor, a high complication rate could not be ignored. The complication rates of additional organ resection with gastrectomy have been consistently reported to be higher when compared with the patients undergoing gastrectomy alone[21–23]. And the increasing overall complications and infectious complications have been found to be factors for the decrease in the survival of the patients. So, reinforcing perioperative management is a key point to prevent postoperative complications. Our data demonstrate that the overall complication rate was very high, and postoperative weight loss of different degree was present in all the patients with TG. Judicious use of additional PD with gastrectomy is also important for reducing postoperative complications. If a patient could not tolerate a prolonged operation, we would take simple operative method such as bypass operation. And simple gastroenterostomy or esophagojejunostomy should be adopted to reduce postoperative complications and postoperative nutritional support would improve the state of weight loss.
We adopted total parenteral nutrition (TPN) at the early stage after TG to spur positive nitrogen balance and reduce weight loss, and after an interval we adopted suitable enteral nutrition to reduce complications. A leak or fistula from the pancreatic anastomosis is the leading cause of morbidity and mortality after PD, but no pancreatioenteral anastomosis leak occurred in our study, as we adopted binding pancreaticojejunostomy[9], by which 3 cm of the serosa-muscular sheath of the jejunum was bound to the pancreatic remnant and could effectively prevent the development of pancreatic leak or fistula. It is a safe, simple and efficient technique.
Our study confirmed that patients with advanced gastric cancer could benefit from aggressive en bloc surgical resection and should not render unresectable when pancreaticoduodenal region was found invaded or involved. With careful patient selection, gastrectomy with PD can be performed with acceptable morbidity and minimum mortality. Well histologic differentiation and negative resection margin are the most powerful determinants of survival following an extended resection. The survival rate in the palliative and explorative group was significantly (P = 0.0064) lower than in the combined PD group, partially because that their baseline conditions were different. And in this article, we did not analyze the survival rates of patients with TG or SG , because the number of the patients was too large to follow up. We have no complete data of these patients. So we can not compare the survival rates between patients with TG or SG and those with TG or SG combined with PD. It is the default of this study.
For advanced gastric carcinoma, gastrectomy with extensive lymph node dissection has been reported to acquire a substantial improvement in survival. But there are still some problems as for areas of resection for advanced gastric cancer involved local organs. For the anatomic reason, pancreaticoduodenal region involved in advanced gastric cancer was not scarce. But few articles about its surgical treatment have been reported. This study was to report the authors’ experience in undergoing pancreaticoduodenectomy (PD) and gastrectomy for advanced gastric cancer with pancreaticoduodenal region involved.
The focus of controversy for the advanced gastric cancer involved organ is either performing the extensive resection or giving up surgical resection. Arguments against enlarged resection are based on the observed increase in the morbidity and mortality rates, with little objective benefit in survival. And some surgeons still consider the invasion of adjacent organs by the carcinoma of stomach as a sign of incurable disease. But others believe patients with T4 gastric cancer will benefit from extended en bloc surgical resection.
This study confirmed that patients with advanced gastric cancer could benefit from aggressive en bloc surgical resection, which should not be rendered unresectable when pancreaticoduodenal region was found invaded or involved. Negative resection margin is an important factor for the patients with extended resection.
For the patients with advanced gastric cancer with pancreaticoduodenal region invaded, PD and extensive resection should be performed if the status of the patients permits.
This retrospective study assessed the performance of PD for advanced gastric cancer with pancreaticoduodenal region involvement, and observed that the 1-year and 3-year survival rates after total gastrectomy (TG) or distal subtotal gastrectomy (SG) combined with PD were 77% and 34%, which were significantly higher than in the patients with palliative treatment. It was also found that histological differentiation ad negative resection margin were most important predictors.
| 1. | Itoh H, Oohata Y, Nakamura K, Nagata T, Mibu R, Nakayama F. Complete ten-year postgastrectomy follow-up of early gastric cancer. Am J Surg. 1989;158:14-16. |
| 2. | Lee JS, Douglass HO Jr. D2 dissection for gastric cancer. Surg Oncol. 1997;6:215-225. |
| 3. | Ikeguchi M, Oka S, Gomyo Y, Tsujitani S, Maeta M, Kaibara N. Prognostic benefit of extended radical lymphadenectomy for patients with gastric cancer. Anticancer Res. 2000;20:1285-1289. |
| 4. | Pugliese R, Maggioni D, Berardi V, Scandroglio I, Pisani D, Mariani A, Di Lernia S, Valli C, Cocotta E. Extended (D2) lymphadenectomy in gastric cancer: a five year experience. Int Surg. 2000;85:209-215. |
| 5. | van de Velde CJ. Resection for gastric cancer in the community. Semin Oncol. 2005;32:S90-S93. |
| 6. | Shchepotin IB, Chorny VA, Nauta RJ, Shabahang M, Buras RR, Evans SR. Extended surgical resection in T4 gastric cancer. Am J Surg. 1998;175:123-126. |
| 7. | Martin RC 2nd, Jaques DP, Brennan MF, Karpeh M. Extended local resection for advanced gastric cancer: increased survival versus increased morbidity. Ann Surg. 2002;236:159-165. |
| 8. | Okano K, Maeba T, Ishimura K, Karasawa Y, Goda F, Wakabayashi H, Usuki H, Maeta H. Hepatic resection for metastatic tumors from gastric cancer. Ann Surg. 2002;235:86-91. |
| 9. | Peng S, Mou Y, Cai X, Peng C. Binding pancreaticoje-junostomy is a new technique to minimize leakage. Am J Surg. 2002;183:283-285. |
| 10. | Wilke H, Preusser P, Fink U, Achterrath W, Mayer HJ, Stahl M, Lenaz L, Meyer J, Siewert JR, Gerlings H. New developments in the treatment of gastric carcinoma. Cancer Treat Res. 1991;55:363-373. |
| 11. | Schulze-Bergkamen H, Zuna I, Teufel A, Stremmel W, Rudi J. Treatment of advanced gastric cancer with etoposide, folinic acid, and fluorouracil in the clinical setting: efficacy of therapy and value of serum tumor markers. Med Oncol. 2002;19:43-53. |
| 12. | Takeuchi K, Tsuzuki Y, Ando T, Sekihara M, Hara T, Yoshikawa M, Ohno Y, Kuwano H. Total gastrectomy with distal pancreatectomy and splenectomy for advanced gastric cancer. J Surg Res. 2001;101:196-201. |
| 13. | Fuchs CS, Mayer RJ. Gastric carcinoma. N Engl J Med. 1995;333:32-41. |
| 14. | Kodama I, Takamiya H, Mizutani K, Ohta J, Aoyagi K, Kofuji K, Takeda J, Shirouzu K. Gastrectomy with combined resection of other organs for carcinoma of the stomach with invasion to adjacent organs: clinical efficacy in a retrospective study. J Am Coll Surg. 1997;184:16-22. |
| 15. | Iriyama K, Ohsawa T, Tsuchibashi T, Noji M, Miki C, Ilunga K, Suzuki H. Results of combined resection of invaded organs in patients with potentially curable, advanced gastric cancer. Eur J Surg. 1994;160:27-30. |
| 16. | Korenaga D, Okamura T, Baba H, Saito A, Sugimachi K. Results of resection of gastric cancer extending to adjacent organs. Br J Surg. 1988;75:12-15. |
| 17. | Ozaki H, Kinoshita T, Kosuge T, Yamamoto J, Shimada K, Inoue K, Koyama Y, Mukai K. An aggressive therapeutic approach to carcinoma of the body and tail of the pancreas. Cancer. 1996;77:2240-2245. |
| 18. | Yonemura Y, Ooyama S, Matumoto H, Kamata T, Kimura H, Takegawa S, Kosaka T, Yamaguchi A, Miwa K, Miyazaki I. Pancreaticoduodenectomy in combination with right hemicolectomy for surgical treatment of advanced gastric carcinoma located in the lower half of the stomach. Int Surg. 1991;76:226-229. |
| 19. | Cho BC, Jeung HC, Choi HJ, Rha SY, Hyung WJ, Cheong JH, Noh SH, Chung HC. Prognostic impact of resection margin involvement after extended (D2/D3) gastrectomy for advanced gastric cancer: a 15-year experience at a single institute. J Surg Oncol. 2007;95:461-468. |
| 20. | Piso P, Bellin T, Aselmann H, Bektas H, Schlitt HJ, Klempnauer J. Results of combined gastrectomy and pancreatic resection in patients with advanced primary gastric carcinoma. Dig Surg. 2002;19:281-285. |
| 21. | Kaposztas Z, Kalmar K, Cseke L, Illenyi L, Kelemen D, Horvath OP. Prognostic factors in the surgical treatment of gastric cancer--10 years experience. Magy Seb. 2007;60:71-78. |