Published online Jan 14, 2008. doi: 10.3748/wjg.14.231
Revised: September 25, 2007
Published online: January 14, 2008
AIM: To describe a new clinical and pathological subtype of microscopic colitis in children.
METHODS: A selected group of children with abdominal pain, constipation and/or diarrhoea showing discrete or no macroscopic abnormalities on endoscopy was described.
RESULTS: Multiple biopsies of colon showed large mononuclear clear cells in lamina propria of mucous membrane provided that good quality histological sections were performed and observed under a higher magnification. Otherwise, they could be misinterpreted as artefacts. Their presence in routine histology might suggest a systemic storage disease (Whipple’s disease), and neuronal intestine dysplasia. Using immunohistochemical staining and electron microscopy we confirmed their origin from CD68 positive mononuclear macrophages.
CONCLUSION: The presence of large clear cells is a constant microscopic feature. Failure of transient large bowel stationary macrophages plays a role in the pathogenesis of this benign microscopic clear cell colitis, sometimes coexisting with allergy.
- Citation: Józefczuk J, Wozniewicz BM. Clear cell colitis: A form of microscopic colitis in children. World J Gastroenterol 2008; 14(2): 231-235
- URL: https://www.wjgnet.com/1007-9327/full/v14/i2/231.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.231
Microscopic colitis constitutes a group of diseases with unknown aetiology and causes neither destruction nor rebuilding of large bowel mucosa. Microscopic colitis is rarely recognized in children[12] and may be misinterpreted as inflammatory bowel disease[3]. Various forms of microscopic colitis have been identified in adults[4–11], including lymphocytic, collagenous, multinucleated giant cells. No description of microscopic colitis with the presence of giant mononuclear clear cells in lamina propria in children is available. Several staining methods can be used to identify the phenotype of these cells. This is probably not a new disease but it is frequently overlooked due to routine use of low magnifications and poor quality of histological staining for paraffin-embedded materials. The presence of large amounts of these cells in our series of biopsies is characteristic in children with chronic abdominal pain, diarrhoea or constipation. In three cases, the preliminary diagnosis was Whipple’s disease, neuronal dysplasia and even Gaucher disease. Definitive diagnosis of clear cell colitis (CCC) was made by immunohistochemistry methods and electron microscopy. A higher diagnostic profile is possible for identification of various forms of microscopic colitis.
Clinical, endoscopic and morphological analyses of CCC were described in series of large bowel biopsies. Characteristic giant mononuclear clear cells were found in sections by routine hematoxyllin and eosin staining. Magnifications larger than 200 × must be used and high quality paraffin histology sections must be obtained to detect the cells. Otherwise, CCC may be overlooked. Using immunocytochemical and electron microscopic procedure, these clear cells were identified as activated macrophages which were CD68 positive and contained multiple vacuoles with characteristic amorphic homogenous lipid like material. The cells were found only in various parts of large intestine but not in small intestine or gastric or duodenal mucosa. Ten-year observation of patients in whom repeated biopsies were performed, allowed us to conclude that CCC is a form of microscopic colitis with chronic persistent benign course and persistent constant histological pattern. During the last 3 years, 20 patients underwent a prolonged (6-mo to a 1-year) therapy with probiotics. Clinical symptoms improved and the number of clear cells decreased in microscopic examinations. In 6 cases the cells disappeared completely from mucous membranes. The remaining patients received a continuous long-term follow-up.
Clinical and morphological study on CCC involved 81 children aged between 17 mo and 17 years selected out of 5000 large bowel biopsies taken from children who underwent treatment in paediatric centres between 1994 and 2005. The group included 63% boys and 18% girls. The majority of patients (68%) were inhabitants of urban areas, whilst the rest (32%) lived in the countryside. Colon dysfunction was the reason for diagnostic investigation. The majority of patients reported recurring abdominal pain, diarrhoea, constipation, and poor appetite. Their stools were of various consistency and contained mucus. Each child had at least two endoscopic examinations of the final part of the alimentary tract (23 had rectoscopy and 58 had colonoscopy).
Clinical, laboratory and endoscopic evaluations were performed carefully. Clinical diagnosis was established based on the number of stools per week, the presence of mucus, general comfort of a child, intensity of stomach aches, the presence of fever, ESR, haemoglobin concentration, state of nutrition and the presence of parenteral manifestations. Evaluating the rate for changes in endoscopic image, we took into consideration vascular drawing, granulation of mucous membrane.
The presence of intestinal pathogens (Salmonella, Shigella, Campylobacter, Escherichia coli, Yersinia, Clostridium difficile) and Giardia infection was verified. Diagnostic research for the presence of enteroviruses was performed in the Center of Virology of the National Institute of Hygiene on the Hep-2 and RD cell lines supplied by the WHO. The presence of retrovirus was checked in order to obtain a full virological evaluation. Tests of the latex reaction with Slidex Rota-kit 2 set and ELISA serological examination (using the DAKO rabbit anti-rotavirus (Human) reactants recommended by the WHO) were performed.
During colonoscopy 3 specimens were collected from each of the 58 patients. Colonoscopy was performed 2 times and a total number of 248 biopsies were examined. A comparison of long-term changes occurring in the course of CCC was possible. Rectoscopy was performed 3 times in 23 cases. A total number of 317 biopsies were collected. Paraffin-embedded specimens were cut into serial sections which were stained with hematoxyllin and eosin. In 81 cases, 162 immunological staining procedures were performed with 16 monoclonal antibodies (to CD3, CD4, CD22, CD34, CD31, CD68, PCNA, Ki67, chromogranin, synaptophysin, neurofilament, actin, p53, GFAP, IL-beta, TNF alpha) and 4 histological staining procedures were performed for mucicarmin, PAS, PAS diastase. A total of 2916 staining procedures were reviewed (Table 1). The phenotypes of clear cells were identified.
| Marker | Clear cells |
| CD3 (T cell) | Negative |
| CD4 (T cell) | Negative |
| CD22 (B cell) | Negative |
| CD34 (precursor cell) | Negative |
| CD31 (Endothelial) | Negative |
| CD68 (Macrophages) | +++ Strongly positive |
| PCNA-proliferation antigen | Negative2 |
| Ki67 mitotic activity | Negative1 |
| Chromogranin | Negative |
| Synaptophysin | Negative |
| Naurofilament | Negative |
| Actin | Negative |
| P53 mutations | Negative |
| GFAP glial marker | Negative |
| TNF alpha | Negative |
| IL2 | Negative |
| Mucicarmin | Negative |
| PAS | Strong positive |
| PAS diastase | Digested |
| Osmium tetraoxide | Positive |
In 10 cases, examination under an electron microscope (Jeol 100CX) was also performed. Preparations of intestinal mucosal biopsies were fixed in 4% glutaraldehyde in cacodylate buffer and then in OsO4, followed by a typical Epon procedure. Ultra-thin sections were counterstained with lead citrate and uranyl acetate.
The incidence of CCC as a form of microscopic colitis (MC) was 2% in children with abdominal pain, diarrhoea and constipation. ESR < 100 mm and MHC/hemoglobin concentration > 8.5 g/dL were found in all children. During the 10 years of observation, no clinical or endoscopic changes in IBD were noticed. Before analysis, children did not take any medicines or drugs that could caused pathological changes in mucous membrane of the large intestine. No treatment was administered. In some cases, a pre-treatment with salazopyrine for a few months did not result in regression of endoscopic or histological changes. Clinical symptoms were improved when probiotics was administered in 20 patients. Clear cells disappeared on mucosal membranes in the second biopsy from 6 patients. The remaining patients remained on treatment with Saccharomyces boulardi.
The dominant symptoms in both girls and boys included recurrent abdominal pain, stiff and (or) loose stools, even diarrhoeal stools with mucus additive. Some patients were admitted to gastroenterology hospital departments mainly because of suspected IBD. Some showed atopic skin inflammation and (or) recurring upper respiratory system inflammation. Five children with bronchial asthma were treated. Laboratory peripheral blood and urine tests and microbiologically parasitological stool tests did not reveal any pathological changes. Abdominal pain occurred in all cases. The pain was mild or moderate. Less than 18 stools per week were counted in 7 children, 18-35 stools in 59 cases and 36-60 stools per week in the remaining 15 cases, constipation in 19 cases. A subjective evaluation of general feeling of children was good. Parenteral symptoms were noted in 19 out of 81 children, mainly upper respiratory tract infection. Forty-three children had ESR less than 40 mm, 20 had 50-100 mm ESR after 1 h. Haemoglobin concentration was above 10 g/dL in all cases. The state of nutrition (Cole and Stanfield) was over 85% in 27 cases, 80%-85% in 46 cases and under 80% in 8 cases. Bacteriological and virological examination of stools gave negative results.
Endoscopic examinations were done with a stiff rectoscope. Eighty-two percent of the patients had also a supplementary colonoscopy. Macroscopically, all patients appeared to have normal mucosa or granulated mucosa, with sharply outlined vascularization and a thin layer of mucus in the rectum and sigmoid colon. In children with CCC undergoing colonoscopy, characteristic fine granulation and increased sharpness of vascular outline in the descending and transverse colon were found and changes in the ascending colon were also observed in 6 children. Susceptibility to bleeding was increased in 9 children. Three to six biopsies were taken during each endoscopic examination of the large intestine, observation was repeated 3 times during the 5-10 years. One specimen was collected at each rectoscopy during the 5-10 years of observation. In the consecutive rectoscopic and endoscopic examinations, the mucosal pattern of CCC was not changed. Persisting granulation and increased sharpness of the vascular pattern were revealed. Endoscopic examination of the upper part of alimentary tract gave normal results.
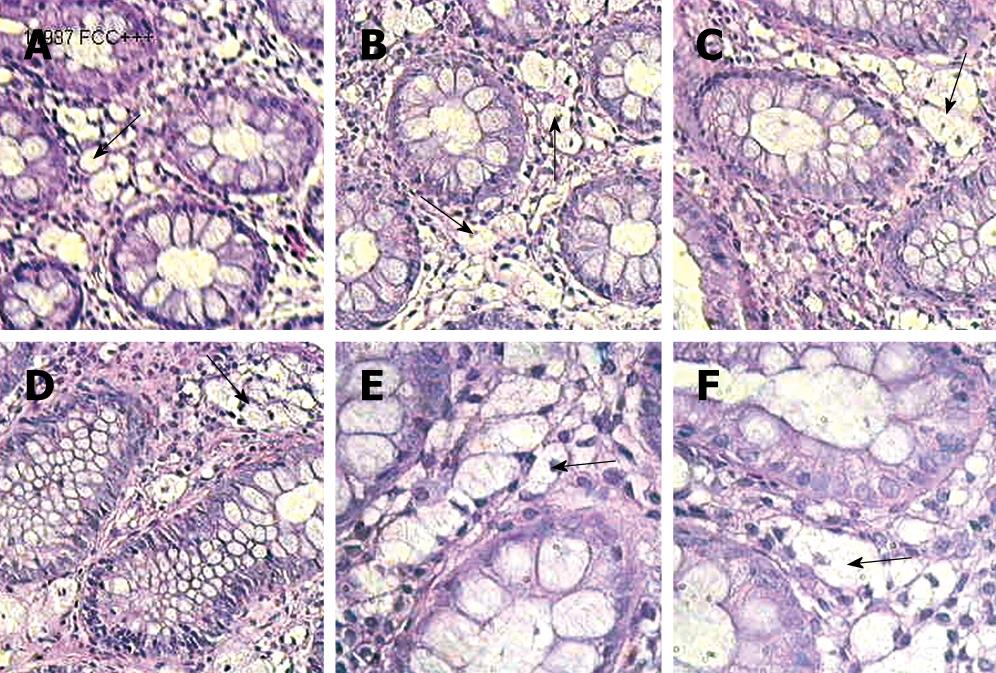
Microscopic images of routinely HE-stained sections were analysed. There were large clear cells in the lamina propria of mucosa, appearing either separately or in groups (Figure 1). The cells occupied the entire lamina propria from the covering epithelium to the basis of cryptal glands and the level of muscularis mucosa. Distribution of the clear cells allowed identification of 3 degrees of clear cell density: grade 1-single cells at various levels of lamina propria, grade 2-the presence of aggregates of 3-10 cells, grade 3-clear cells uniformly distributed in lamina propria. The presence of clear cells in patients was permanently observed during the 5-10 years. Clear cells showed a positive reaction with neutral mucopolisacharides in the PAS method with periodic acid and the Shiff’s reagent (PAS- positive reaction), and negative reaction after digestion with diastase. Staining with mucicarmine was negative. No intraepithelial lymphocytosis was noted and the base membrane was normal. About 243 serial sections stained with HE were reviewed microscopically.
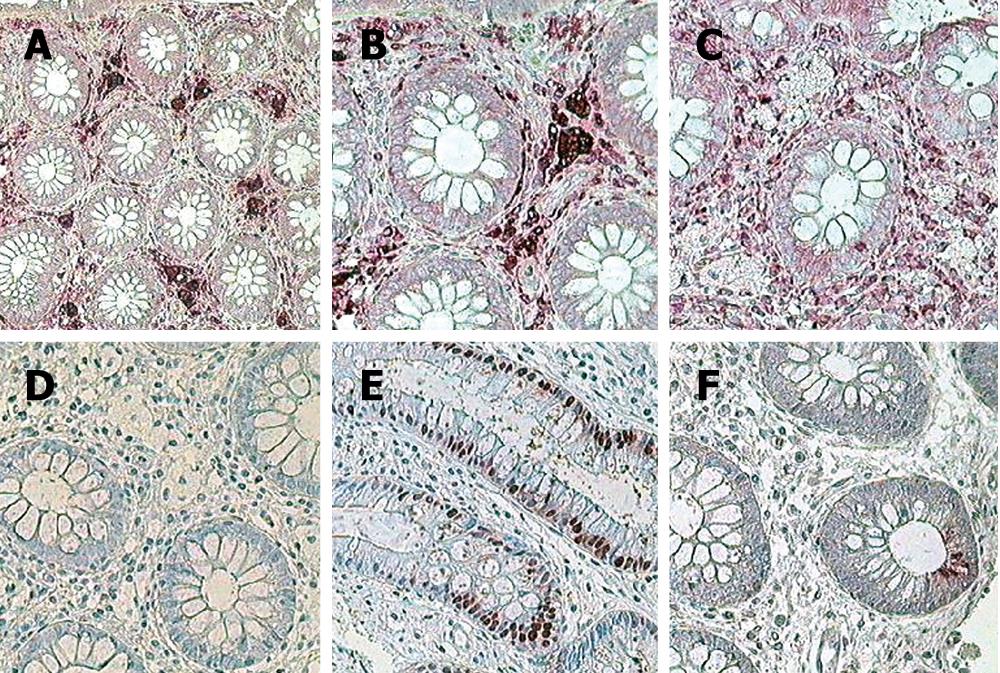
Clear cells presented a strong positive expression in the reaction with the CD68 antibody (Figure 2A-F, Table 1). Sixteen immunocytochemical reactions were performed 3 times in 81 patients during the period of observation in order to identify the phenotype of large clear cells.
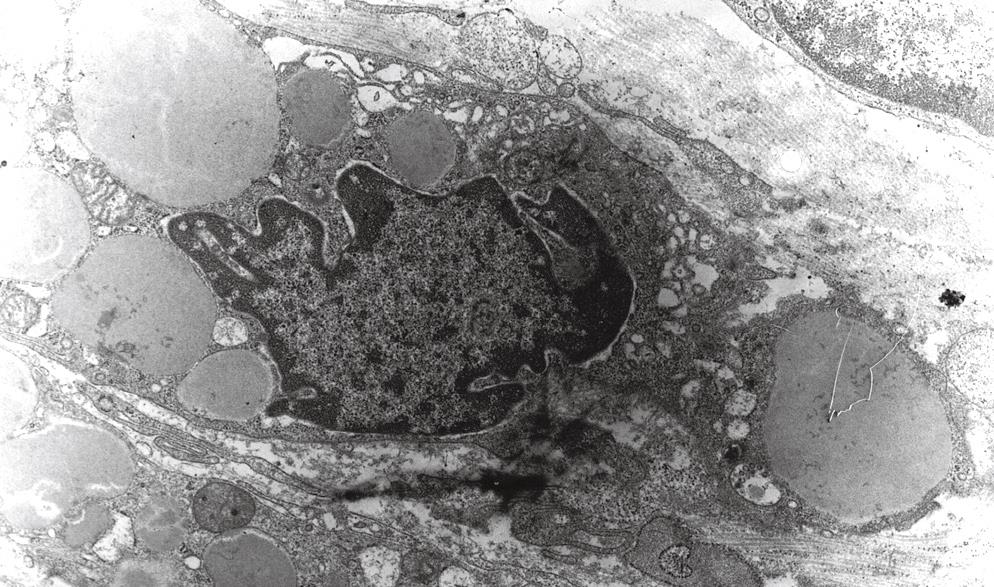
In contrast to primary metabolic diseases, electron microscopic examination of clear cells revealed the presence of single nuclei with nucleoli and multiple secondary phagosomes with accumulation of homogenous osmophilic materials (Figure 3). Other structures of the large intestine mucosa were normal. The material for electron microscopic analysis was taken from 10 patients with CCC confirmed previously by histological examination.
Microscopic colitis is the term suggested by some authors for abdominal pain, alternating chronic diarrhoea and constipation and endoscopic image close to normal[4–8]. A characteristic microscopic picture of adults is in the form of collagenous and lymphocytic colitis[9–11]. The cause for microscopic colitis is unknown. Several concepts are published, including abuse of medications[12], increased concentration of antigens and cytokine expression[13], auto-antibodies in blood[14], prostaglandin level[15]. MC may overlap with indeterminate inflammatory bowel disease[16], but in contrast to CCC, TNF alpha expression may be positive in silent inflammatory bowel disease[17].
CCC in children as a form of microscopic colitis is characterized by the presence of various large mononuclear clear cells in lamina propria. The cells are easily recognized when high magnification microscopy is used and section staining of microscopic slides is good. CCC does not manifest as destruction or reconstruction of glands. In the present study, basic membrane was not damaged, no lymphocytic or calagenous colitis or other forms of microscopic colitis described elsewhere were observed. Clear cells are mononuclear in contrast to other microscopic colitis, where there are giant multinucleated cells[18]. Clear cells described here are different from other muciphages[19]. On the other hand, Hamrock[20] has reported clear cells in patients with AIDS. The cells look like Whipple cells but they can be overloaded with inclusion material (Mycobacterium). Some authors suggested that rectal biopsy should be performed in cases of irritable bowel disease since they may be classified as MC[21].
Differential diagnosis should also consider neuronal dysplasia[22] of intestine, in which large, bright ganglion cells can be very similar to the giant clear cells in CCC. The reaction with neurofilaments, synaptophisine or (and) chromogranine is helpful. Some authors suggest that Salmonella typhimurium type can mobilize phagocytosis in macrophages of the large intestine but we have not found bacterial or viral particles in the phagosomes of clear cells. Different macrophages described in Erdheim-Chester disease exhibit neoplastic characteristics[23]. Possible persistent changes (e.g. after the rotaviral diarrhoea) and the presence of enteroviruses in clear foamy cells can be ruled out on the ground of electron microscopy and negative results of blood and stool test.
Xanthelasma of rectum containing sialomucin, is a different disease rather than CCC. However, patients with similar symptoms suffer from diarrhoea, constipation or haemorrhages. The presence of these symptoms is a result of mechanical injuries of mucosa in the course of chronic constipation. In contrast to muciphages, clear cells were negative for mucicarmine staining in our study.
The mononuclear phagocytic system consists of macrophages widely distributed in the body as circulating or stationary cells. Macrophages of large bowel mucosa belong to stationary cells and have various functions. In some special unknown conditions, they can transform into large clear cells and exhibit transient malfunction with accumulation of lipid material. It should be emphasized that one third of our patients had allergic extra intestinal manifestations. Finally, application of probiotics (Saccharomyces bulardi) has a beneficial effect in children with CCC. Confirmation of this finding requires further long-term observation.
Clear cell colitis (CCC) is a description of a new form of microscopic colitis in children. This is a benign disease recognized in some children showing symptoms of recurrent alternate diarrhoea and constipation. Patents are directed by physicians to gastroenterology units of district hospitals. Endoscopic examination shows normal or minor changes which do not constitute a qualification for further therapy. Pathologists using usually low magnifications can overlook the presence of large clear cells in lamina propria, and describe mucosa as normal. The lesions are seldom found in academic gastroenterological centers, where selected patients with more serious symptoms are ordered.
Detection of giant clear cells is very important if no other lesions in large intestine musosa or other organs are found. Otherwise, false diagnosis of primary metabolic disease or neuronal displasia can be made. Before application of immunocytochemical tests, electron microscopy is useful in differential diagnosis, even microscopic examinations of conjunctiva and liver are recommended.
The reason why giant clear cells are aggregated is not clear. The presence of so called muciphages, described previously as a phenomenon in adults can be excluded on the basis of negative mucicarmine staining and different electron microscopic pictures.
As the children included in the study showed symptoms of allergy, and even asthma, selective activation of lipid and protein storing macrophages is considered to be associated with mucosa auto-immunological response, probably under influence of antigenemia occurring in children suffering from constipation. It should be emphasized that the presence of giant macrophages is chronic and the clinical course is stable. In the present paper, no therapy was needed at the end of a long term clinical and morphological observation period. During the last two years, some improvement was achieved after application of probiotics. Usage of probiotics (among others of Lactobacilus casei) in all children is the aim in future studies.
Clear cell colitis is a form of microscopic colitis in children with accumulation of giant clear cells.
This manuscript appears to be reasonably well written. The authors described a new form of CCC in children and presented evidence to support their contention.
| 1. | Sanderson IR, Boyle S, Williams CB, Walker-Smith JA. Histological abnormalities in biopsies from macroscopically normal colonoscopies. Arch Dis Child. 1986;61:274-277. |
| 2. | Mashako MN, Sonsino E, Navarro J, Mougenot JF, Gargouri A, Boige N, Cezard JP. Microscopic colitis: a new cause of chronic diarrhea in children? J Pediatr Gastroenterol Nutr. 1990;10:21-26. |
| 3. | Domizio P. Pathology of chronic inflammatory bowel disease in children. Baillieres Clin Gastroenterol. 1994;8:35-63. |
| 4. | da Silva JG, De Brito T, Cintra Damiao AO, Laudanna AA, Sipahi AM. Histologic study of colonic mucosa in patients with chronic diarrhea and normal colonoscopic findings. J Clin Gastroenterol. 2006;40:44-48. |
| 5. | Pardi DS, Smyrk TC, Tremaine WJ, Sandborn WJ. Microscopic colitis: a review. Am J Gastroenterol. 2002;97:794-802. |
| 6. | Warren BF, Edwards CM, Travis SP. ‘Microscopic colitis’: classification and terminology. Histopathology. 2002;40:374-376. |
| 7. | Nyhlin N, Bohr J, Eriksson S, Tysk C. Systematic review: microscopic colitis. Aliment Pharmacol Ther. 2006;23:1525-1534. |
| 8. | Nyhlin N, Bohr J, Eriksson S, Tysk C. A higher diagnostic profile is possible for microscopic colitis. Aliment Pharmacol Ther. 2006;24:562. |
| 9. | Barta Z, Mekkel G, Csipo I, Toth L, Szakall S, Szabo GG, Bako G, Szegedi G, Zeher M. Microscopic colitis: a retrospective study of clinical presentation in 53 patients. World J Gastroenterol. 2005;11:1351-1355. |
| 10. | Liszka L, Woszczyk D, Pajak J. Histopathological diagnosis of microscopic colitis. J Gastroenterol Hepatol. 2006;21:792-797. |
| 11. | Jaskiewicz K, Rzepko R, Adrych K, Smoczynski M. Micro-scopic colitis in routine colonoscopies. Dig Dis Sci. 2006;51:241-244. |
| 12. | Leong RW, Chan FK. Drug-induced side effects affecting the gastrointestinal tract. Expert Opin Drug Saf. 2006;5:585-592. |
| 13. | Tagkalidis PP, Gibson PR, Bhathal PS. Microscopic colitis demonstrates a T helper cell type 1 mucosal cytokine profile. J Clin Pathol. 2007;60:382-387. |
| 14. | Holstein A, Burmeister J, Plaschke A, Rosemeier D, Widjaja A, Egberts EH. Autoantibody profiles in microscopic colitis. J Gastroenterol Hepatol. 2006;21:1016-1020. |
| 15. | Ribardo DA, Crowe SE, Kuhl KR, Peterson JW, Chopra AK. Prostaglandin levels in stimulated macrophages are controlled by phospholipase A2-activating protein and by activation of phospholipase C and D. J Biol Chem. 2001;276:5467-5475. |
| 16. | Yantiss RK, Odze RD. Diagnostic difficulties in inflammatory bowel disease pathology. Histopathology. 2006;48:116-132. |
| 17. | Akazawa A, Sakaida I, Higaki S, Kubo Y, Uchida K, Okita K. Increased expression of tumor necrosis factor-alpha messenger RNA in the intestinal mucosa of inflammatory bowel disease, particularly in patients with disease in the inactive phase. J Gastroenterol. 2002;37:345-353. |
| 18. | Libbrecht L, Croes R, Ectors N, Staels F, Geboes K. Microscopic colitis with giant cells. Histopathology. 2002;40:335-338. |
| 19. | Bejarano PA, Aranda-Michel J, Fenoglio-Preiser C. Histochemical and immunohistochemical characterization of foamy histiocytes (muciphages and xanthelasma) of the rectum. Am J Surg Pathol. 2000;24:1009-1015. |
| 20. | Hamrock D, Azmi FH, O'Donnell E, Gunning WT, Philips ER, Zaher A. Infection by Rhodococcus equi in a patient with AIDS: histological appearance mimicking Whipple's disease and Mycobacterium avium-intracellulare infection. J Clin Pathol. 1999;52:68-71. |
| 21. | MacIntosh DG, Thompson WG, Patel DG, Barr R, Guindi M. Is rectal biopsy necessary in irritable bowel syndrome? Am J Gastroenterol. 1992;87:1407-1409. |
| 22. | Nogueira A, Campos M, Soares-Oliveira M, Estevao-Costa J, Silva P, Carneiro F, Carvalho JL. Histochemical and immunohistochemical study of the intrinsic innervation in colonic dysganglionosis. Pediatr Surg Int. 2001;17:144-151. |
| 23. | Egan AJ, Boardman LA, Tazelaar HD, Swensen SJ, Jett JR, Yousem SA, Myers JL. Erdheim-Chester disease: clinical, radiologic, and histopathologic findings in five patients with interstitial lung disease. Am J Surg Pathol. 1999;23:17-26. |