Published online May 21, 2008. doi: 10.3748/wjg.14.3006
Revised: March 16, 2008
Published online: May 21, 2008
AIM: To investigate transcriptional gene silencing induced by short hairpin RNAs (shRNAs) that target gene prompter regions of RUNX3 gene, and whether shRNAs homologous to DNA sequences may serve as initiators for methylation.
METHODS: According to the principle of RNAi design, pSilencer3.1-H1-shRNA/RUNX3 expression vector was constructed, The recombinant plasmid shRNA was transfected into human stomach carcinoma cell line SGC7901 with Lipofectamine 2000. Then, the positive cell clones were screened by G418. The mRNA and protein expression level of RUNX3 in the stable transfected cell line SGC7901 were determined by RT-PCR, Western blotting and immunocytochemistry. Characteristics of the cell lines including SGC7901, pSilencer3.1-H1/SGC7901 and pSilencer3.1-H1-shRNA/RUNX3/SGC7901 were analyzed with growth curves, clone formation rate and cell-cycle distribution. The activated level of RUNX3 was examined after treatment with the different density of 5’-aza-2’-deoxycytidine (5-Aza-CdR) by using semi-quantitative RT-PCR and Western blotting.
RESULTS: In the cell line SGC7901 transfected with pSilencer3.1-H1-shRNA/RUNX3, mRNA and protein expression of the RUNX3 gene was lost identified by RT-PCR, Western blotting and immunocytochemistry assay. The growth of pSilencer3.1-H1-shRNA/ RUNX3/SGC7901 cells without expression of RUNX3 was the fastest (P < 0.05), its rate of clone formation was the highest (P < 0.01), and the cell distribution in G0/G1 and S/M phases was lowest and highest, respectively (P < 0.05), compared with that of the transfected pSilencer3.1-H1 and non-transfected cells. Through RT-PCR and Western blot assay, inactivated RUNX3 could not be reactivated by 5-Aza-CdR.
CONCLUSION: We found that, although shRNAs targeted to gene prompter regions of RUNX3 could effectively induce transcriptional repression with chromatic changes characteristic of inaction promoters, this was independent of DNA methylation, and the presence of RNA-dependent transcriptional silencing showed that RNA-directed DNA methylation might be an existing gene regulatory mechanism relative to the methylated in humans.
-
Citation: Feng XZ, He XS, Zhuang YZ, Luo Q, Jiang JH, Yang S, Tang XF, Liu JL, Chen T. Investigation of transcriptional gene silencing and mechanism induced by shRNAs targeted to
RUNX3 in vitro . World J Gastroenterol 2008; 14(19): 3006-3014 - URL: https://www.wjgnet.com/1007-9327/full/v14/i19/3006.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.3006
The human runt-related transcription factor 3 gene (RUNX3), a novel tumor suppressor, was originally cloned as AML2 in human leukocythemia by Levanon in 1994 and is located on human chromosome 1p36.1[1]. RUNX3 contains two promoters, p1 and p2, one at the beginning of exon 1 and the other in front of exon 2. The mRNA expression of RUNX3 comes from transcription by p2, which has a high GC content due to a large conserved CpG island around it and contains Sp1 sites and a CCAAT box without a TATA box. Recently, RUNX3 has been considered to be a vital gene in the occurrence and development of gastric carcinoma[2].
Multiple genes participate in the occurrence and development of gastric carcinoma, which is at the top list of malignant tumors in China. Deactivation of anti-oncogenes is more frequent than activation of proto-oncogenes in the gastric carcinogenesis process. Anti-oncogenes are devitalized by chromosomal absence, gene mutation, and aberrant CpG island hypermethylation of gene promoters. Moreover, the data have suggested that, due to transcription termination of RUNX3 by aberrant CpG island hypermethylation at the promoter’s 5’-terminal CpG island area, the active level of RUNX3 decreased by 40% and 90%, respectively, in early and advanced gastric cancer. However, the inactivation mechanism of the aberrant CpG island hypermethylation is still unclear[3–6].
The phenomenon by which small interference RNA molecules that target gene prompter regions can induce transcriptional gene silencing in a DNA cytosine methylation-dependent manner (RNA-directed DNA methylation, RdDM) was initially discovered by Wassenegger et al in 1994 in plants infected with epiviruses[7]. This suggested that RdDM might have an important role in the loss of expression of tumor suppressor genes, and RUNX3. We constructed a recombinant plasmid, which expressed short hairpin RNAs (shRNAs) complementary to the CpG island, including the promoter of the human RUNX3 gene, to investigate transcriptional gene silencing and the mechanism mediated by shRNAs that target gene prompter regions of RUNX3 in human stomach carcinoma cell lines.
shRNAs that target the nucleotide sequence (GCCACTT-GATTCTGGAGGA) in the promter of human RUNX3 (NCBI, accession number AL023096) were designed by Ambion using restriction enzyme sites BamHI and HindIII, and synthesized by Shanghai Biological Engineering Limited Company (China). The oligonucleotide sequence was as follows: 5’-GATCCGCCACTTGATTCTGGAGGATTCAAGAGATCCTCCAGAATCAAGTGGCGGTTTTTTGGAAA-3’ and 5’-AGCTTTTCCAAAAAACCGCCACTTGATTCTGGAGGATCTCTTGAATCCTCCAGAATCAAGTGGCG-3’.
The eukaryotic expression vector pSilencer3.1-H1 (Ambion), which was kindly provided by Doctor Lei XiaoYong (Institute of Pharmacologic Research, Nanhua University), was digested by BamHIand HindIII restriction enzymes (MBI). The digested products, which were reclaimed using Gel-reclamation Kit (Omega), and the oligonucleotide sequence were ligated by T4 DNA ligase (MBI) at 4°C overnight to generate the recombinant pSilencer3.1-H1-shRNA/RUNX3. Then, the recombinant plasmid was validated by restriction enzymes digestion and sequencing.
Human gastric cancer SGC-7901, MKN-28, SGC7901, MGC803 and BGC823 cell lines, which were first cultured by Academia Sinica (Shanghai, China), were purchased from the Central South University (Hunan, China). All cells were cultured with RPMI 1640 (Gibco) medium and supplemented with 100 mL/L calf serum (Sijiqing, Hangzhou, China) at 37°C in a humidified atmosphere containing 50 mL/L CO2.
Fifty thousand SGC-7901 cells were seeded into each well in six-well plates and grown overnight. The medium was replaced with complete medium without fetal bovine serum (FBS). The recombinant pSilencer3.1-H1-shRNA/RUNX3 and the empty pSilencer3.1-H1 were transfected into SGC7901 cells using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. The medium was replaced with a fresh medium of calf serum (150 mL/L) after 5 h transfection. Two days later, the transfected cells were selected by G418 (200 &mgr;g/mL) (Huamei Biotechnology Company, Beijing, China) until positive clones were discovered after 10 d. The cells were cultured and finally selected by G418 (100 &mgr;g/mL) for a further 10 d. Single clones were selected to build a stable transfected cell line.
5'-Aza-2'-deoxycytidine (5-Aza-CdR), which is a DNA methyltransferase inhibitor, was purchased from Sigma (USA). It was diluted with PBS (pH 6.8), and finally prepared as 10 &mgr;mol/L mother liquor by filtrated sterilization. The mother liquor was stored at -70°C.
Total RNA was extracted from each sample using the Total RNA Extract Kit (Omega) following the manufacturer’s instructions. The concentration of RNA was measured by spectrophotometry. Total RNA was reverse-transcribed to cDNA with reverse transcriptase (RT) reagents (Promega) according to the manufacturer’s protocol. Briefly, the RT reaction was carried out in a final volume of 20 &mgr;L that contained 4 &mgr;L 25 mmol/L MgCl2, 2 &mgr;L 10 × RT buffer, 2 &mgr;L 10 mmol/L dNTP mixed liquor, 1 &mgr;L RNase inhibitor, 1 &mgr;L Oligo (dT) 15 primers, 15 U AMV retroviridase, 8.37 &mgr;L water free from enzyme, and 2 &mgr;L total RNA. The mixture was incubated at 70°C for 10 min and 42°C for 60 min, and RT was inactivated by heating at 95°C for 5 min, followed by incubation at 4°C for 5 min.
SGC-7901, pSilencer3.1-H1/SGC7901 and pSilencer3.1-H1-shRNA/RUNX3/SGC7901 cells were suspended in RPMI1640 medium and seeded in a 24-well plate at a density of 2.0 × 104 cells/well. Culture medium was changed every 2 d. The number of cells was counted consecutively for 8 d. Each experiment was done in triplicate.
Single cells were mixed with the semi-solid agar (3 g/L) with growth medium and plated on a basal agar layer (6 g/L) in three six-well plates. The cells were cultured at 37°C in a humidified atmosphere that contained 50 mL/L CO2 for 10 d. Then, colonies that contained > 100 cells in soft agar were counted under an inverted microscope and the rate of colony formation was calculated by the mean percentage of colonies.
The cells were passaged at a density of 5.0 × 105/mL. After 4 h, they were treated with serum-free medium for 12 h, followed by treatment with medium that contained calf serum (100 mL/L) for 24 h. Cells were collected by trypsinization and prepared as a single cell suspension by mechanical blowing with PBS, washed with cold PBS twice, and fixed with 700 mL/L alcohol at 4°C for 24 h. Fixed cells were washed with PBS and stained with PI (50 &mgr;g/mL in PBS) for 30 min at room temperature in the dark. DNA content in PI-stained cells was detected by FCM.
As shown in Table 1, specific primers for RUNX3 and β-actin genes were designed based on sequence data from the GenBank database. The primers were purchased from Shanghai Biological Engineering. Conditions for all PCRs were optimized on Cycler iQ (Bio-Rad, USA) and the optimum annealing temperature was 55°C. The following cycler running protocol was used: denaturation (95°C, 2 min), amplification and quantification repeated 35 times (94°C for 30 s, 55°C for 30 s, and 72°C for 1 min). In addition, a non-template control (ddH2O control) was analyzed for each template. All samples were amplified simultaneously in triplicate.
| Gene | Sequence | Amplified length (bp) |
| RUNX3 | 5’-GAGTTTCACCCTGACCA TCACTGTG-3’ | 870 |
| 5’-GCCCATCACTGGTCTTGAAGGTTGT-3’ | ||
| β-actin | 5’-CTACAATGAGCTGCGTGTGC-3’ | 500 |
| 5’-AGGAGGACTTCTTCTCGAT-3’ |
Cells were collected, washed three times with PBS, lysed in cell lysate that contained 0.1 mol/L NaCl, 0.01 mol/L Tris-HCl (pH 7.6), 0.001 mol/L EDTA (pH 8.0), 1 &mgr;g/mL aprotinin and 100 &mgr;g/mL PMSF, and then centrifuged at 13 000 ×g for 10 min at 4°C. The extracted protein sample (50 &mgr;g total protein/lane) was added to the same volume of sample buffer and subjected to denaturation at 100°C for 10 min. The samples were electrophoresed on 100 g/L SDS PAGE at 28 mA for 30 min until they reached the bottom of the spacer gel, separated on the separation gel at 120 V for 1.5 h, and finally transferred onto PVDF membrane at 105 mA for 3.5 h at 4°C. The PVDF membrane was treated with TBST that contained 50 g/L skimmed milk at room temperature for 2 h, followed by incubation with the primary antibody RUNX3 (1:100 dilution) (Boaosen Biotechnology Company, Beijing China; bs-0378R) at 37°C for 2 h or 4°C overnight. After being washed with TBST for 30 min, the corresponding secondary antibody (1:2000 dilution) (Zhongsan Jinqiao Biotechnology Company, Beijing, China) was added and incubated at room temperature for 1 h. The membrane was then washed three times for 15 min each with TBST. Fluorescence was produced from solution A and B that contained a chemiluminescence generator. Both RUNX3 and β-actin protein expression were quantitatively estimated by densitometric scanning performed with Imaginer 2200. RUNX3 protein concentration was normalized to β-actin level and expressed as a densitometric ratio.
Cells were seeded on the axenic cover glass in six-well plates at 1 × 105 cells/well, and were grown overnight. The cover glasses with adhered cells were washed three times with PBS, and fixed in 95% alcohol for 10 min. After being rinsed three times in PBS, the endogenous peroxidase activity was suppressed by 30 mg/L hydrogen peroxide for 15 min, followed by rinsing three times in PBS. Antigen repair was performed by immersing the sections in 10 mmol/L sodium citrate buffer (pH 6.0) and heating for 15 min in a microwave oven. Non-specific binding was blocked by incubation with 30 mL/L bovine serum albumin (BSA) for 40 min. The cells were treated for 16 h with goat anti-human polyclonal IgG antibodies of RUNX3 (Boaosen Biotechnology Company; bs-0378R) at 37°C for 2 h or 4°C overnight, according to the manufacturer’s recommended concentration (1:200 dilution). PBS was used as a negative control. After washing three times in PBS, the cover glasses were treated with biotinylated rabbit anti-goat immunoglobulin (Zhongsan Jinqiao Biotechnology Company) for 1 h at room temperature and then by horseradish peroxidase-streptavidin complex (Man Xin Biotechnology Company, Huzhou, China) for 30 min. The cover glasses were then washed three times in PBS and incubated in DAB for 2 min. Next, the cover glasses were rinsed gently with distilled water and counterstained with hematoxylin for 30 s, and dehydrated in alcohol prior to mounting. Images were collected by Olympus DD70 BX51 (Olympus, Japan) and analyzed by IMAGE-PRO plus 4.1 software (Media Cybernetics, USA). Eight visual fields in each section were randomly selected and the mean value of relative OD was measured and calculated by taking the OD of background as 1. The extent of immunohistochemical staining was categorized as positive (1-1.5) and strongly positive (> 1.5).
Experimental data in each group were expressed as mean ± SD. Analysis of variance (ANOVA) was performed with the Statistical Package for the Social Sciences (SPSS 13.0) for Windows by using one way ANOVA and pairwise comparison with Student’s t test. P < 0.05 was considered statistically significantly.
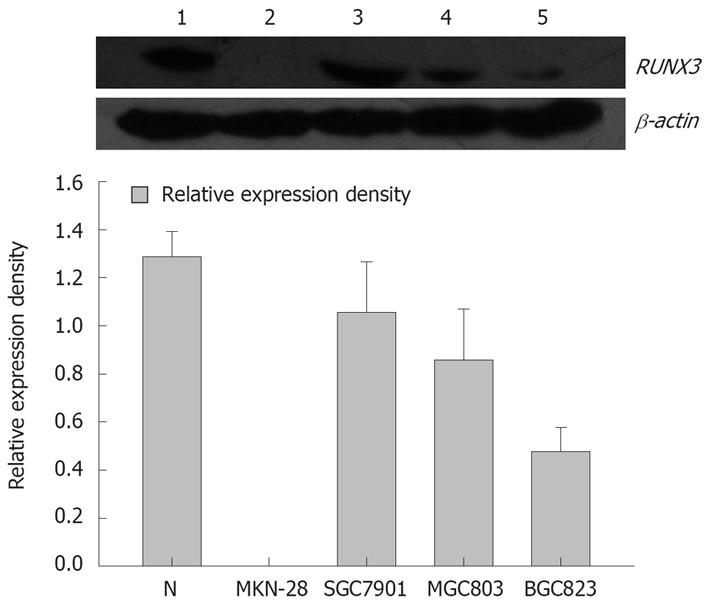
Western blot analysis showed that the relative densities of RUNX3 protein bands of normal gastric mucosa, well-differentiated gastric carcinoma cell line MKN-28, moderately differentiated gastric carcinoma cell line SGC7901, poorly differentiated gastric carcinoma cell lines MGC803 and BGC823 were: 1.279 ± 0.105, 0.003 ± 0.001, 1.057 ± 0.610, 0.857 ± 0.212, 0.477 ± 0.321 respectively (Figure 1). The moderately differentiated gastric carcinoma cell lines SGC7901 was selected for further experiments.
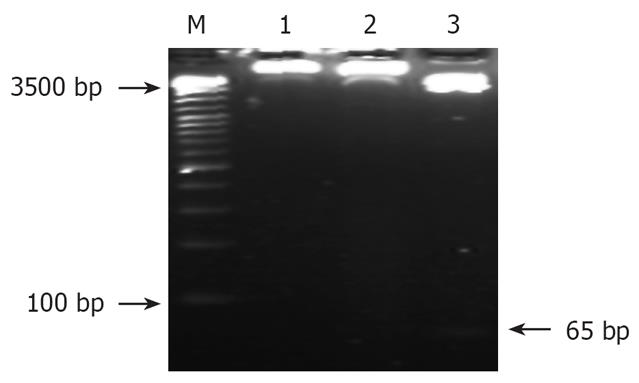
The recombinant eukaryotic expressing vector-pSilencer3.1-H1-shRNA/RUNX3 was cut by the double restriction enzyme BamHIand HindIII. Then the enzyme products were electrophoresis using the agar gel (27 g/L). And the objective fragment 65 bp was obtained (Figure 2).
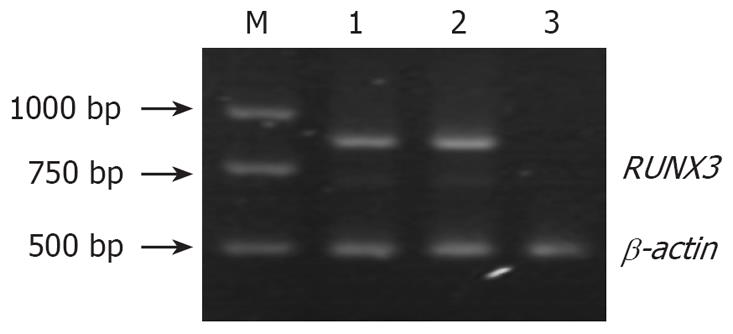
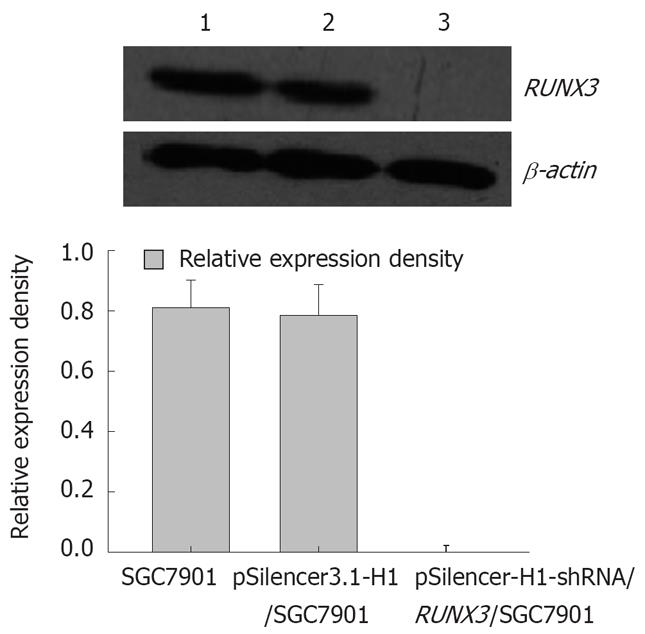
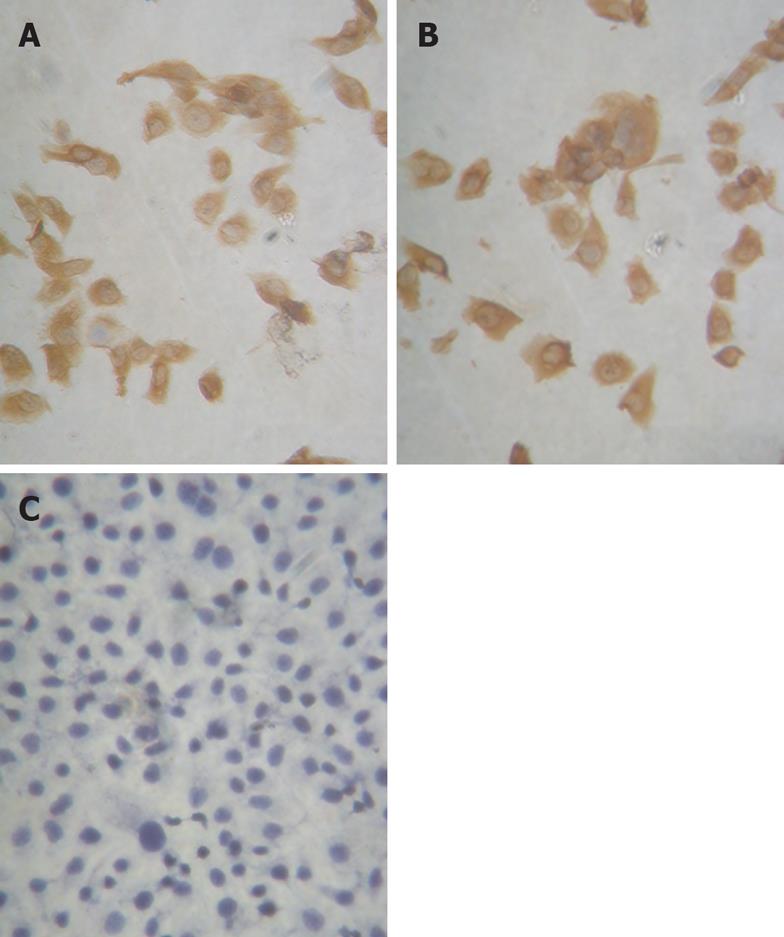
In SGC-7901, pSilencer3.1-H1/SGC7901 and pSilencer3.1-H1-shRNA/ RUNX3/SGC790 cells, RT-PCR showed that the relative densities of RUNX3 mRNA bands were 1.116 ± 0.217, 1.057 ± 0.187 and 0.002 ± 0.001, respectively (Figure 3), and Western blot analysis indicated that the relative densities of RUNX3 protein bands were 0.812 ± 0.091, 0.786 ± 0.103 and 0.021 ± 0.002, respectively (Figure 4). Immunocytochemistry disclosed that RUNX3 protein was located in the endochylema and the nucleus, and loss of expression of RUNX3 in SGC790 cells transfected with recombinant plasmid-pSilencer3.1-H1-shRNA/RUNX3 (Figure 5).
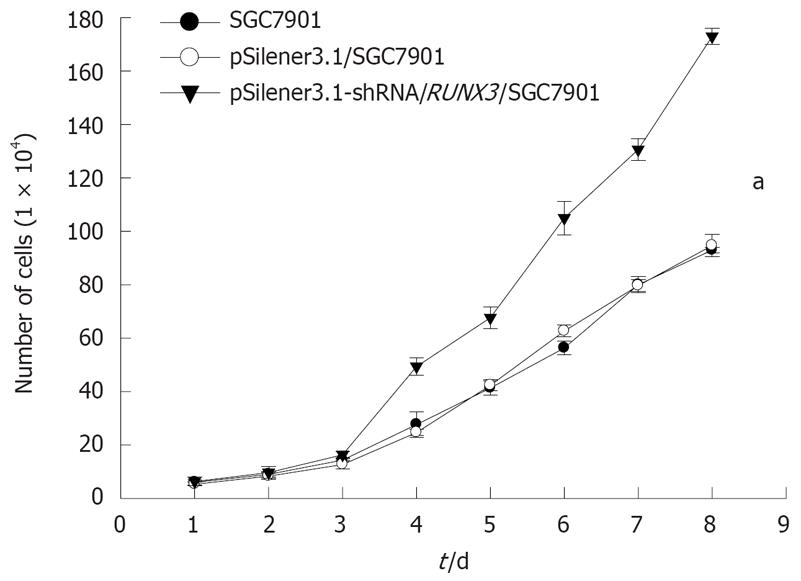
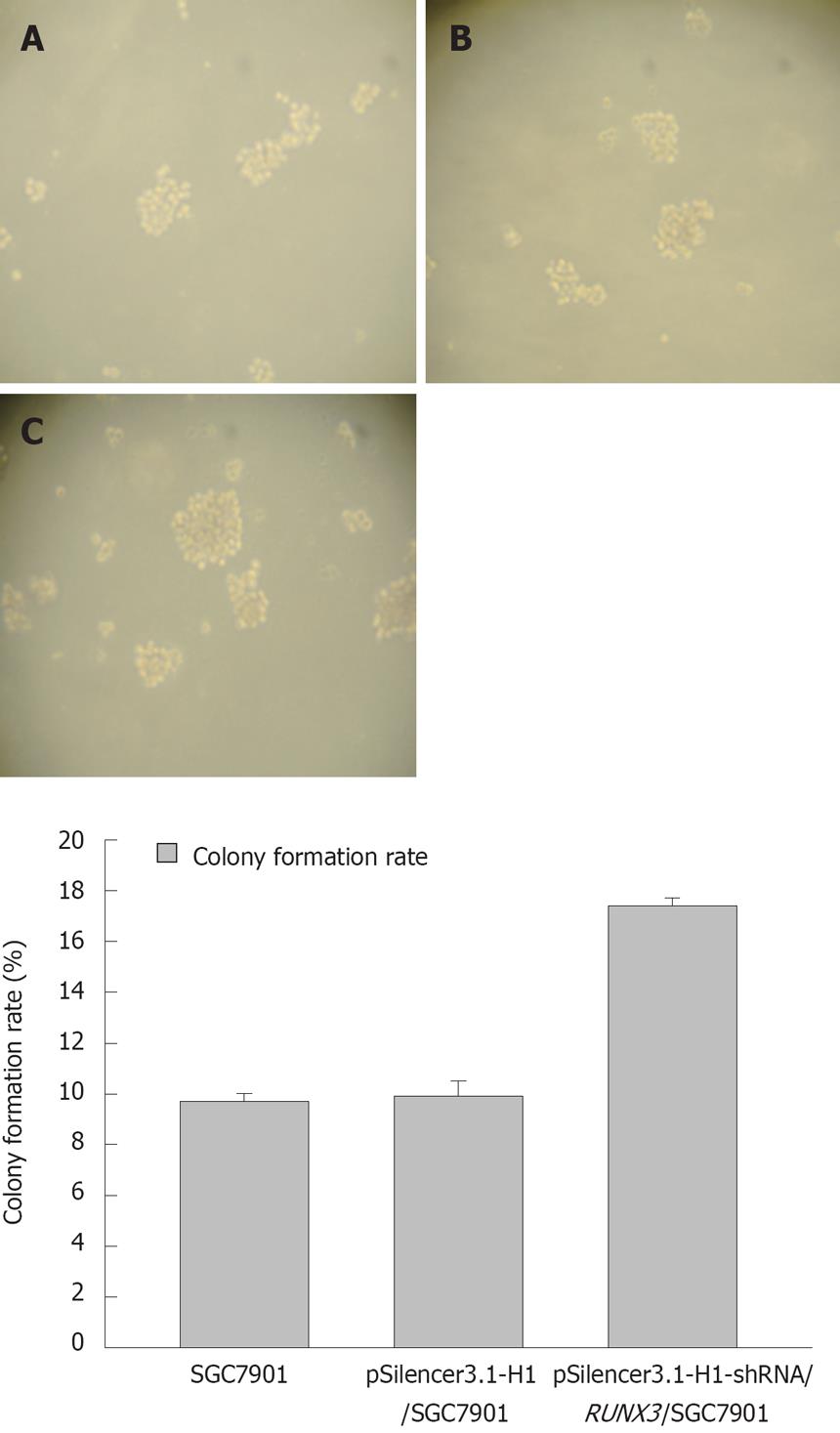
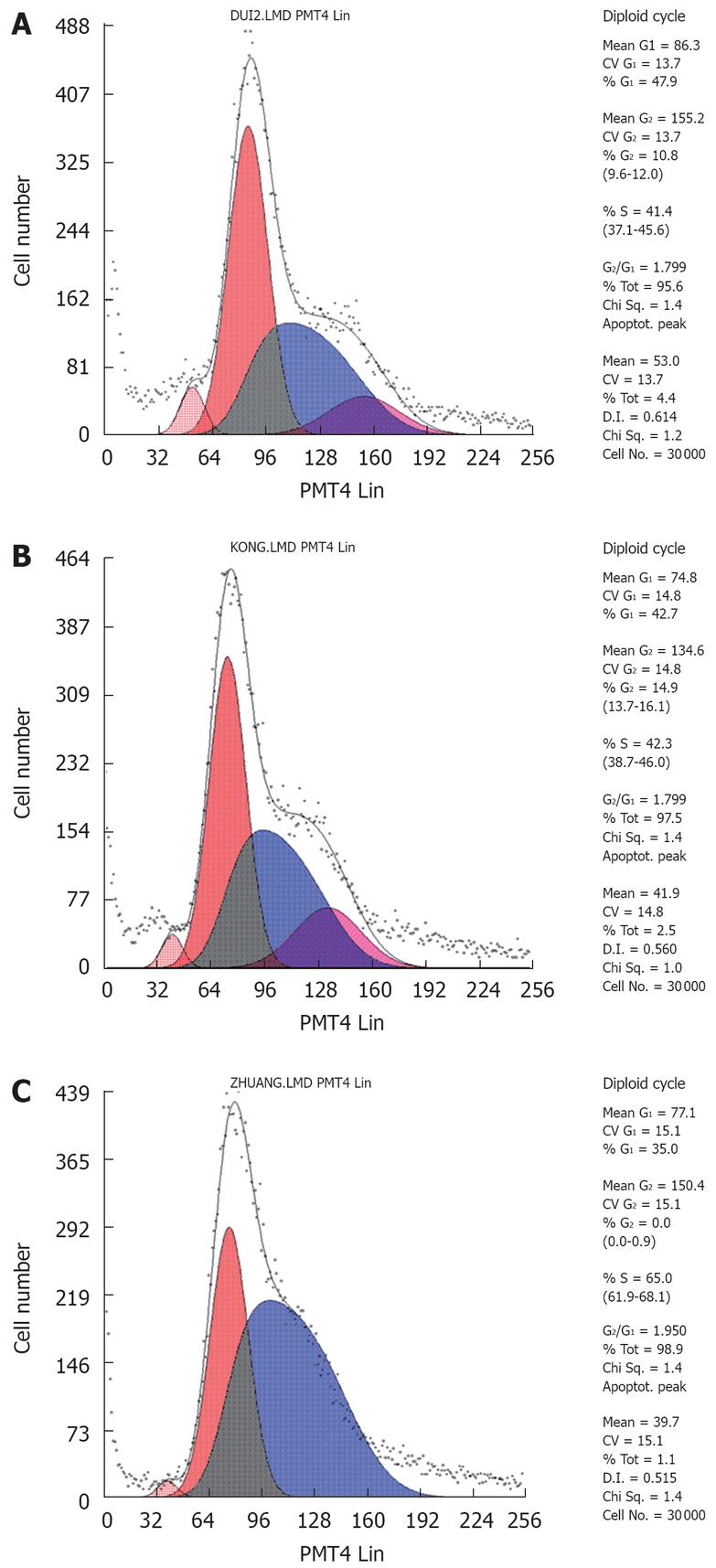
The growth curves showed that the pSilencer3.1-H1-shRNA/RUNX3/SGC7901 cells grew more quickly than the control cells, SGC7901 and pSilencer3.1-H1/SGC7901 cells (P < 0.05, Figure 6), but that the rate of cell growth was similar in the two control groups. The results of the soft-agar colony-formation experiment indicated that a significant increase in the colony-formation rate in the pSilencer3.1-H1-shRNA/RUNX3/SGC790 cells (17.4 ± 0.31)% was found compared with that in the control SGC-79011 (9.9 ± 0.3)% and pSilencer3.1-H1/SGC7901 (9.7 ± 0.6)% cells (P < 0.01, Figure 7). The colony-formation rates of the two control cells were similar. FCM with PI staining suggested that the proportion of cells in G0/G1 and S phases in pSilencer3.1-H1-shRNA/RUNX3/SGC7901 cells significantly decreased and increased, respectively (P < 0.05, Figure 8 and Table 2), compared with the control cells.
| Groups | G0/G1 | S | G2/M |
| SGC7901 | 43.2 ± 1.2 | 47.7 ± 1.1 | 9.0 ± 1.5 |
| pSilencer3.1-H1-shRNA/SGC7901 | 40.3 ± 2.0 | 49.3 ± 0.9 | 8.1 ± 0.3 |
| pSilencer3.1-H1-shRNA/RUNX3/SGC7901 | 37.2 ± 1.9 | 60.5 ± 0.8 | 7.3 ± 0.9 |
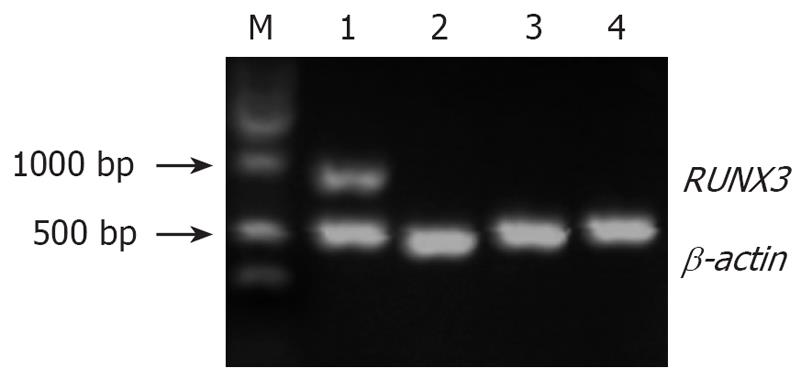
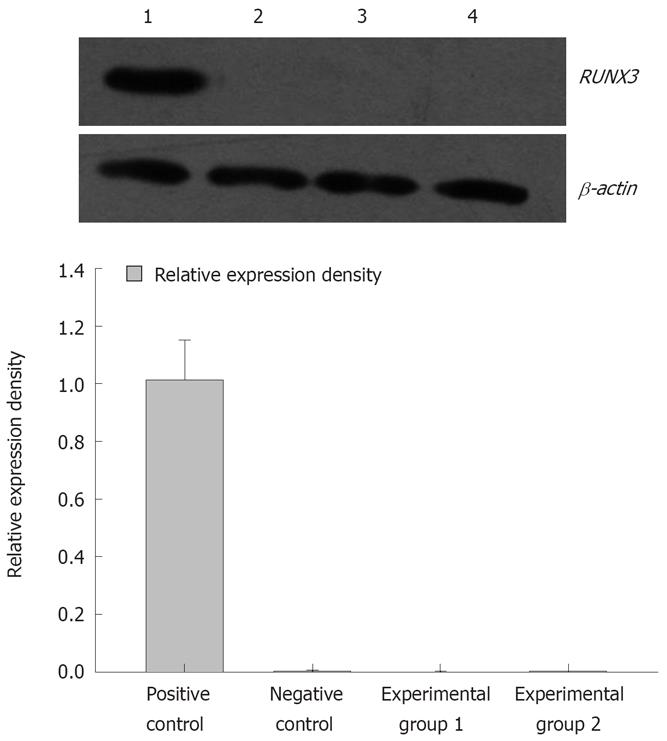
The pSilencer3.1-H1-shRNA/RUNX3/SGC7901 cells (1 × 106/100 mL) were treated with 5 × 10-6 mol/L and 1 × 10-5 mol/L 5-Aza-CdR. Cells were collected after 3 d treatment. The cells treated with 5 × 10-6 mol/L 5-Aza-CdR were named experimental group 1, and those treated with 1 × 10-5 mol/L 5-Aza-CdR were experimental group 2. RT-PCR showed that the relative densities of RUNX3 mRNA bands were 0.861 ± 0.167, 0.004 ± 0.001, 0.002 ± 0.001 and 0.002 ± 0.001, respectively (Figure 9), and Western blot analysis that the band densities of RUNX3 were 1.013 ± 0.138, 0.003 ± 0.001, 0.002 ± 0.001 and 0.005 ± 0.001, respectively (Figure 10).
In the occurrence and development of gastric carcinoma, the mechanism of abnormal silencing of anti-oncogenes mediated by epigenomics in the un-first-class structure levels of nucleotide sequence such as DNA methylation and histone modification, is still unclear. Many researchers have assumed that aberrant CpG island hypermethylation of the promoters of anti-oncogenes exists frequently in tumors. However, it is still not known how the aberrant CpG island hypermethylation takes place. Recently, the phenomenon by which siRNA molecules that target gene prompter regions can induce transcriptional gene silencing in a DNA cytosine methylation-dependent manner has attracted much attention.
siRNA can induce post-transcriptional gene silencing through RNA-RNA binding and transcriptional gene silencing through RNA-DNA binding. Transcriptional gene silencing refers to siRNA molecules that hinder production of mRNA from DNA before gene transcription, by modifying chromosomal DNA and histones. It include three patterns; RNA-directed DNA methylation, RNA-directed heterochromatinization and RNA-directed DNA ablation[8–10]. The idea of RNA-directed DNA methylation was obtained from a propagation study in Arabidopsis thaliana[11]. It is not known whether a similar mechanism exists in mammalian systems.
Some elements, such as the required constituents of RNA-directed DNA methylation in plants, have been discovered in mammals. They contain three DNA methyltransferases (DNMT) 1, which play a role in maintaining methyltransferases. DNMT3A and DNMT3B can be let in the new methylated sites. The two processes including the methylated maintenance and repeated methylation might exist to affect genome DNA in mammals as in plants[12]. DNMT3A and DNMT3B have been observed to participate in the orientation of siRNA that target gene promoters[13]. As to human HeLa cells, the percentage of the mature-type miR-21 located in the cytoplasm and the nucleus were 80% and 20%, respectively. After transfection with fluorescently-labeled siRNA/miR-21, the fluorescence which siRNA/miR-21 binds the complementary sequences was observed in the cytoplasm and the nucleus. A identical consequence has appeared in cells transfected with fluorescently-labeled siRNA/let-7a[14].
Some previous investigations have reported that siRNA molecules that target gene prompter regions can induce transcriptional gene silencing and DNA cytosine methylation around the promoter region. In the study by Morris et al, elongation factor 1 α (EF1α) was knocked down by the prompter-directed siRNA in HEK293 cells by transfection. After treatment with 5-Aza-CdR (4 &mgr;mol/L) and trichostatin A (TSA, 0.05 mmol/L), deactivation of EF1α was reversed and detected through nuclear run-on assays and RT-PCR. According to the above data, the transcriptional silencing of EF1a might be associated with DNA methylation of the targeted sequence[15]. According to research by Castanotto et al, shRNAs, molecules homologous to DNA sequences in the prompter and early transcribed regions of RASSF1, can direct the partial gene silencing and low levels of de novo DNA methylation. They used a methylation-specific polymerase chain reaction (MSP) and bisulfite sequencing in HeLa cells[16]. Moreover, Weinberg’s investigation showed that transcriptional silencing and DNA methylation of EF1a can be directed by antisense interference alone in human 293T cells transfected with EF1α siRNA. This targets the promoter of it using the peptide MPG, which transports siRNAs to the nucleus. This silencing is accompanied by increased methylation level in histone 3 lysine 9 (H3K9) and histone 3 lysine 27 (H3K27). Furthermore, siRNAs EF52 is associated with the transient expression of Flag-tagged DNMT3A, the targeted EF1α promoter, and trimethylated H3K27[17]. Previous studies have indicated that, in mammalian cells, the unique RNA endonuclease Dicer, which cuts long dsRNA to form siRNA, is situated in the cytoplasm[18–20]. RNA-induced silencing complex (RISC), which binds to siRNAs in the cell-substance, may gain access to the nucleus when caryotheca vanishes during cell division, or it may be transported into the nucleus, as in plants. Furthermore, siRNA can integrate the homologous DNA sequences (promoters) and induce transcriptional gene silencing in a DNA cytosine methylation-dependent manner[1121].
We confirmed that aberrant CpG island hyper-methylation of the promoter of RUNX3 is a critical pathway to cause down-regulation or loss of expression of the gene. How may the pathway be connected with the mechanism of RNA-directed DNA methylation? In our experiment, on the basis of the principle of RNAi design, pSilencer3.1-H1-shRNA/RUNX3 expression vector was constructed, and transfected in SGC7901 cells by liposomes. RT-PCR, western blot and immunocytochemistry showed that mRNA and protein expression of RUNX3 were absent in the stable cell line SGC7901 transfected with the recombinant plasmid. Moreover, compared with those transfected with pSilencer3.1-H1 and non-transfected cells, cells transfected with pSilencer3.1-H1-shRNA/RUNX3 grew most quickly. Both the clone number and size were largest following soft-agar colony-formation assay (P < 0.01) and the number of cells in G0/G1 and S/M phases was lowest and highest following FCM (P < 0.05). The above results indicated that, through RNA-dependent transcriptional silencing (RdTS), knockdown of the gene at the transcriptional stage was feasible and effective. After loss of expression of RUNX3 protein, the proliferation rate of SGC7901 cells increased. These results, like the previous description[3–6], suggested that RUNX3, a putative tumor suppressor, might have an important role in stomach tumorigenesis. However, after the deal with the different density of 5-Aza-CdR, which may reactivate anti-oncogenes silenced by de novo methylation, inactivated RUNX3 was not reactivated, as shown by RT-PCR and the silenced. The phenomenon was different from the RdDM described in plants. At the same time, similar results have been reported. In two human glioblastoma cell lines, U-87 and U-118, siRNA homologous to the promoter region of huntingtin gene can repress transcriptional expression of the target gene. However, no CpG methylation has been observed on the target sequences by bisulfite-mediated genomic sequencing[2122]. In the research of Ting et al, the human colorectal cancer cells, HCT116, were transfected with two 21-nucleotide-long dsRNAs (dsCDH1-1 and dsCDH1-2). The two sequences were homologous to the CpG island of the endogenous gene promoter of CDH1 but did not overlap any known transcribed sequences. The findings suggested that promoter-targeting dsRNAs could effectively silence CDH1 transcription, which results in a net decrease in mRNA and protein production, as demonstrated by RT-PCR and Western blot. Absence of DNA methylation at the targeted sequences was reviewed by MSP and bisulfite sequencing. Transfection of the human breast cancer cells, MCF-7, used a silencing strategy virtually identical to that above for CDH1. The transcriptional silencing without DNA methylation was reviewed and the results were identical[23].
What accounts for these opposing results? We believe the answer may lie in the types of DNA methylation assays performed. In the work by Morris et al, the DNA methylation data remain to be verified because DNA methylation was not as extensive, and there was indirect evidence for 5-Aza-CdR and TSA, a histone-deacetylase inhibitor. Co-administration alleviating the silencing was difficult to interpret as the reason of the EF1α silencing. In other investigations, MSP distinguishes between non-methylated and methylated alleles by using two sets of primers to amplify either non-methylated or methylated sequences after bisulfite treatment, which specifically converts non-methylated cytosines to uracils. The shortcoming of MSP analysis is that it examines a few CpG sites in the sequences that are recognized by the primers, and the design of primers is very difficult. Mismatch sequencing was used to verify the overall target region by unbiased primers that did not contain CpG dinucleotides to amplify the bisulfite-converted promoter region. Due to the existence of incomplete mismatch conversions, unconverted cytosine residues in both CpG and CpG contexts remained as cytosine and created a false negative result. Furthermore, the PCR primers that were used previously to obtain the initial mismatch sequencing template that contains CpG sites may bias the amplification step and produce problematic mismatch sequencing results[5]. Such non-conversions may partly explain the different results in the mismatch sequencing. In the current study, the level of DNA methylation was testified preliminary and indirect. The finding that inactivation of RUNX3 was not reactivated with 5-Aza-CdR could not confirm sufficiently that the transcriptional silencing of RUNX3 was independent of DNA methylation. Therefore, further study is needed.
Taken together, the presence of RdTS indicated that RdDM might be an existing gene regulatory mechanism relevant to methylation in humans, even though we are currently unable to verify this. A recent study has shown that, in A. thaliana, RdDM is studied in the progeny of plants being silenced, and DNA methylation may still be involved in a prolonged silencing event in human cells[24]. Therefore, further experiments, which include a thorough examination of the long-term and full-scale outcomes of RdTS, are needed to research RNA-directed DNA methylation in mammalian systems.
Aberrant CpG island hypermethylation of the promoter of RUNX3, a tumor suppressor, is associated with its transcriptional silencing and the loss of gene functions in stomach cancer. However, the mechanism of the aberrant CpG island hypermethylation is still unclear.
In plants, siRNA molecules that target gene prompter regions can induce transcriptional gene silencing in a DNA cytosine methylation-dependent manner (RdDM). Whether a similar mechanism exists in mammalian systems is a vital and controversial issue. Recently, the RdDM theory on gene regulation has attracted close attention from researchers and has become a highlight in tumor studies.
This article focuses on the relationship between siRNA molecules and aberrant CpG island hypermethylation in the promoter domain of RUNX3, through the RNA interference principle and gene transfection technology in human gastric cancer cell lines.
RUNX3 is an important anti-oncogene. Its mechanism of methylation may contribute to the study of the etiology of gastric cancer, and offer a theoretical basis for gene therapy of gastric cancer
RNA interference is an evolutionarily conserved mechanism of gene silencing. It can affect transcriptional gene silencing induced by siRNA that target gene prompter regions in a DNA cytosine methylation-dependent manner.
This is a good study in which the authors demonstrated that, shRNAs that targeted gene prompter regions of RUNX3 could effectively induce transcriptional repression with chromatic changes characteristic of inaction promoters, but it was independent of DNA methylation. The study was well designed and the results are convincing.
| 1. | Levanon D, Negreanu V, Bernstein Y, Bar-Am I, Avivi L, Groner Y. AML1, AML2, and AML3, the human members of the runt domain gene-family: cDNA structure, expression, and chromosomal localization. Genomics. 1994;23:425-432. |
| 2. | Li QL, Ito K, Sakakura C, Fukamachi H, Inoue K, Chi XZ, Lee KY, Nomura S, Lee CW, Han SB. Causal relationship between the loss of RUNX3 expression and gastric cancer. Cell. 2002;109:113-124. |
| 3. | Wei D, Gong W, Oh SC, Li Q, Kim WD, Wang L, Le X, Yao J, Wu TT, Huang S. Loss of RUNX3 expression significantly affects the clinical outcome of gastric cancer patients and its restoration causes drastic suppression of tumor growth and metastasis. Cancer Res. 2005;65:4809-4816. |
| 4. | Homma N, Tamura G, Honda T, Matsumoto Y, Nishizuka S, Kawata S, Motoyama T. Spreading of methylation within RUNX3 CpG island in gastric cancer. Cancer Sci. 2006;97:51-56. |
| 5. | So K, Tamura G, Honda T, Homma N, Endoh M, Togawa N, Nishizuka S, Motoyama T. Quantitative assessment of RUNX3 methylation in neoplastic and non-neoplastic gastric epithelia using a DNA microarray. Pathol Int. 2006;56:571-575. |
| 6. | Zeng C, He XS, Luo Q, Zhao S, Deng M, Li YN. The expression and the mechanism of the down regulation of RUNX3 in the gastric carcinoma. Shijie Huaren Xiaohua Zazhi. 2006;14:250-255. |
| 7. | Wassenegger M, Heimes S, Riedel L, Sunger HL. RNA-directed de novo methylation of genomic sequences in plants. Cell. 1994;76:567-576. |
| 8. | Kawasaki H, Taira K, Morris KV. siRNA induced transcrip-tional gene silencing in mammalian cells. Cell Cycle. 2005;4:442-448. |
| 9. | Kawasaki H, Taira K. Transcriptional gene silencing by short interfering RNAs. Curr Opin Mol Ther. 2005;7:125-131. |
| 10. | Gaur RK, Rossi JJ. The diversity of RNAi and its applications. Biotechniques. 2006;Suppl:4-5. |
| 11. | Matzke MA, Birchler JA. RNAi-mediated pathways in the nucleus. Nat Rev Genet. 2005;6:24-35. |
| 12. | Szyf M. DNA methylation and demethylation as targets for anticancer therapy. Biochemistry (Mosc). 2005;70:533-549. |
| 13. | Morris KV. siRNA-mediated transcriptional gene silencing: the potential mechanism and a possible role in the histone code. Cell Mol Life Sci. 2005;62:3057-3066. |
| 14. | Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, Tuschl T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell. 2004;15:185-197. |
| 15. | Morris KV, Chan SW, Jacobsen SE, Looney DJ. Small interfering RNA-induced transcriptional gene silencing in human cells. Science. 2004;305:1289-1292. |
| 16. | Castanotto D, Tommasi S, Li M, Li H, Yanow S, Pfeifer GP, Rossi JJ. Short hairpin RNA-directed cytosine (CpG) methylation of the RASSF1A gene promoter in HeLa cells. Mol Ther. 2005;12:179-183. |
| 17. | Weinberg MS, Villeneuve LM, Ehsani A, Amarzguioui M, Aagaard L, Chen ZX, Riggs AD, Rossi JJ, Morris KV. The antisense strand of small interfering RNAs directs histone methylation and transcriptional gene silencing in human cells. RNA. 2006;12:256-262. |
| 18. | Hutvagner G, McLachlan J, Pasquinelli AE, Balint E, Tuschl T, Zamore PD. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 2001;293:834-838. |
| 20. | Schmitter D, Filkowski J, Sewer A, Pillai RS, Oakeley EJ, Zavolan M, Svoboda P, Filipowicz W. Effects of Dicer and Argonaute down-regulation on mRNA levels in human HEK293 cells. Nucleic Acids Res. 2006;34:4801-4815. |
| 21. | Svoboda P, Stein P, Filipowicz W, Schultz RM. Lack of homologous sequence-specific DNA methylation in response to stable dsRNA expression in mouse oocytes. Nucleic Acids Res. 2004;32:3601-3606. |
| 22. | Park CW, Chen Z, Kren BT, Steer CJ. Double-stranded siRNA targeted to the huntingtin gene does not induce DNA methylation. Biochem Biophys Res Commun. 2004;323:275-280. |
| 23. | Ting AH, Schuebel KE, Herman JG, Baylin SB. Short double-stranded RNA induces transcriptional gene silencing in human cancer cells in the absence of DNA methylation. Nat Genet. 2005;37:906-910. |
| 24. | Aufsatz W, Mette MF, Matzke AJ, Matzke M. The role of MET1 in RNA-directed de novo and maintenance methylation of CG dinucleotides. Plant Mol Biol. 2004;54:793-804. |