Published online May 14, 2008. doi: 10.3748/wjg.14.2832
Revised: March 27, 2008
Published online: May 14, 2008
AIM: To determine the effects of allopurinol, an inhibitor of xanthine oxidase, and apocynin, an inhibitor of NADPH oxidase, on oxidant stress and liver injury caused by hepatic ischemia/reperfusion (I/R) procedure in mice.
METHODS: Mice were pretreated with a xanthine oxidase inhibitor, allopurinol, or NADPH oxidase (NOX) inhibitor, apocynin before the hepatic I/R procedure. Then treated or untreated mice underwent the hepatic I/R procedure. The effects on hepatic injury and superoxide anions were determined after starting reperfusion.
RESULTS: A standard warm hepatic I/R procedure led to a marked increase in superoxide anion production as indicated by a superoxide anion tracer, MCLA. At the same time, the procedure caused profound acute liver injury, as indicated by elevated serum alanine aminotransferase and tumor necrosis factor-α levels, reduced liver glutathione levels and elevated malondialdehyde contents, as well as a high apoptotic cell count. All these changes were reversed by the use of apocynin or allopurinol prior to the hepatic I/R procedure.
CONCLUSION: Allopurinol and apocynin exerted protective effects on hepatic ischemia/reperfusion injury. The protection is associated with blocking the generation of superoxide anions during the hepatic I/R procedure by inhibiting xanthine oxidase and NADPH oxidase activity.
- Citation: Liu PG, He SQ, Zhang YH, Wu J. Protective effects of apocynin and allopurinol on ischemia/reperfusion-induced liver injury in mice. World J Gastroenterol 2008; 14(18): 2832-2837
- URL: https://www.wjgnet.com/1007-9327/full/v14/i18/2832.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2832
Excessive reaction oxygen species (ROS) cause tissue damage and cell death by binding and altering cellular macromolecules, including DNA, proteins and lipids, and affect their function. One main chemical source which has been shown to contribute significantly to overall pronounced oxidant stress during hepatic ischemia/reperfusion (I/R) procedure is xanthine oxidase (XO), which generates superoxide anions (O2-) during the conversion of hypoxanthine to xanthine. It is known from many studies that much of the sustained injury during organ transplantation, including the liver, is triggered by ROS via activated XO, because allopurinol, an XO inhibitor, provided some protection against the hepatic I/R-induced injury[1].
NADPH oxidase (NOX), using NADPH as the source of electrons, catalyzes one electron reduction of molecular oxygen to generate O2-[2], which is a central and initial ROS molecule and may convert to more active and toxic ROS, such as hydrogen peroxide (H2O2), hydroxyl radical (HO-), or peroxide nitrite (ONOO-) in the presence of H+, H2O2, and nitric oxide (NO)[3]. These O2--derived ROS participating in the inflammatory process, are thought to be key mediators for the activation of Kupffer cells[4] and thus, are crucial in the apoptotic and/or necrotic cell death of the parenchymal cells and sinusoidal endothelial cells (SEC) in the liver[56]. The generation of superoxide anions by NADPH oxidase serves as a host defense mechanism against invading microorganism infection and the enzyme is present in phagocytic cells, such as monocytes and neutrophils[7]. Although the evidence exists that ROS generated by NOX participate in many cellular responses, and may be involved in many injuring processes, few studies are available that investigate the role of NOX in the contribution to pronounced oxidant stress during the ischemia/reperfusion-induced hepatic injury[78]. Apocynin is a naturally occurring methoxy-substituted catechol that effectively inhibits NADPH oxidase through preventing the assembly of its multi-subunits[9]. Thus, we used this inhibitor to explore the role of NADPH oxidase in the generation of superoxide anions during the hepatic I/R procedure and investigate whether apocynin confers any protection against the injury in a mouse model of warm ischemia/reperfusion-induced acute liver injury.
ICR mice, from Charles River Laboratory, Wilmington, MA, were fed a pellet diet and water ad libitum and maintained on a 12 h-light/dark cycle. The animal experiment was performed according to a protocol approved by the UC Davis Institutional Animal Care and Use Committee (IACUC). The protocol was prepared in accordance with the National Institutes of Health animal use guidelines. Mice were pretreated with either an XO inhibitor, allopurinol (50 mg/kg, i.p. from Sigma Chemical Co. St. Louis, MO) or a NOX inhibitor, apocynin (3 mg/kg, i.p. from Acros Organics, Geel, Belgium) one day and one hour before the hepatic I/R procedure. A warm hepatic I/R procedure was performed as reported previously by us[10]. In brief, mice were anesthetized with pentobarbital sodium (60 mg/kg, i.p.). Laparotomy was made with a middle incision to expose the lobes of the liver. Following surgical exposure of the portal vein, mice were injected with heparin (100 unit/kg) via tail vein to prevent the formation of blood clot during the ischemia duration. The portal vein and hepatic artery were occluded for 30 min with a microaneurysm clamp to induce hepatic ischemia. Then, the clamp was removed to allow blood to flow through the liver again (reperfusion).
A total of six groups (6 mice in each group) were included in the experiment. Group A: sham-operated group, in which mice received normal saline (N.S.), and underwent a sham operation without I/R procedure; Group B (N.S. control group) in which mice underwent hepatic I/R procedure plus N.S. injection; Group C (allopurinol treatment group), in which animals received prior allopurinol injections and subsequent hepatic I/R procedure; Group D (apocynin treatment group), in which animals received prior apocynin injections and subsequent hepatic I/R procedure. Six and twenty four hours after starting the reperfusion, blood samples were collected from the vena cava before sacrifice. The liver was rapidly excised after drawing blood from the vena cava. Portions of liver tissue were fixed in 10% neutralized formalin for histological evaluation or snap frozen in liquid nitrogen, and maintained at -80°C until homogenization for the various biochemical assays.
Serum levels of alanine aminotransferase (ALT) served as an indicator of liver injury and were analyzed using a commercially available diagnostic kit (Catachem Inc. Bridgeport, Connecticut), and expressed as unit/L.
2-Methyl-6-(P-methoxyphenyl)-3,7-dihydroimidazo(1,2-α)pyrazine-3-one (MCLA) enhanced chemiluminescence was used to determine O2- generation, as reported by us previously[10]. Approximately, 10 mg of frozen liver tissue was homogenized on ice in 1 mL of homogenization buffer containing 20 mmol/L of N-2-Hydroxyethylpiperazine-N’-2-ethanesulfonic acid (HEPES) and 10 mmol/L ethylene diamine tetraacetic acid (EDTA). The homogenate was subjected to low speed centrifugation (1000 g) for 10 min to remove debris. Luminometer vials containing 2 mL of prewarmed Krebs-HEPES buffer (99 mmol/L NaCl, 4.7 mmol/L KCl, 1.2 mmol/L MgSO4, 1 mmol/L KH2PO4, 1.9 mmol/L CaCl2, 25 mmol/L NaHCO3, 11.1 mmol/L glucose, and 20 mmol/L HEPES, pH 7.4) with 1.0 &mgr;mol/L of MCLA were placed in the dark for at least 20 min. After the dark adaptation, background readings were recorded in a luminometer (Lumat LB; Berthold Technologies, BmbH &Co. KG, Germany), and then 20 &mgr;L of homogenate supernatant was added to each vial containing MCLA. Chemiluminescent emission in relative light units (RLU) was recorded during a plateau phase of each recording period, and corrected by subtracting the background reading. The calculated value was used to express the integrated values of chemiluminescence (RLU/second/&mgr;g protein).
Levels of the reduced form of glutathione (GSH) and the lipid peroxidation product, malondialdehyde (MDA) in liver tissue were measured spectrophotometrically 6 h and 24 h after starting reperfusion by commercially available kits (OXIS Research, Portland, OR). The levels of GSH and MDA are expressed as nanomoles per milligram of protein.
Fixed liver specimens were embedded in paraffin, sectioned in 4 &mgr;m thickness, and stained with hematoxylin and eosin (HE) for the evaluation of liver injury. Photomicrographs were taken with a digital camera under a microscope. In addition, frozen sections of the livers were stained with the terminal deoxynucleotidyl transferase (TdT) for detecting of apoptotic cells by an in situ Apoptosis Detection Kit according to the manufacturer’s instructions (Chemicon Inc., Temecula, CA), and reported previously by us[11].
Serum tumor necrosis factor-α (TNF-α) levels after the hepatic I/R procedure were determined with an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, Minneapolis, MN) and expressed as nanograms per milliliter.
Most data were expressed as mean ± SD of the mean (SEM), and evaluated with an ANOVA analysis and Newman-Keuls tests for multiple comparisons among groups. A P value less than 0.05 was considered statistically significant.
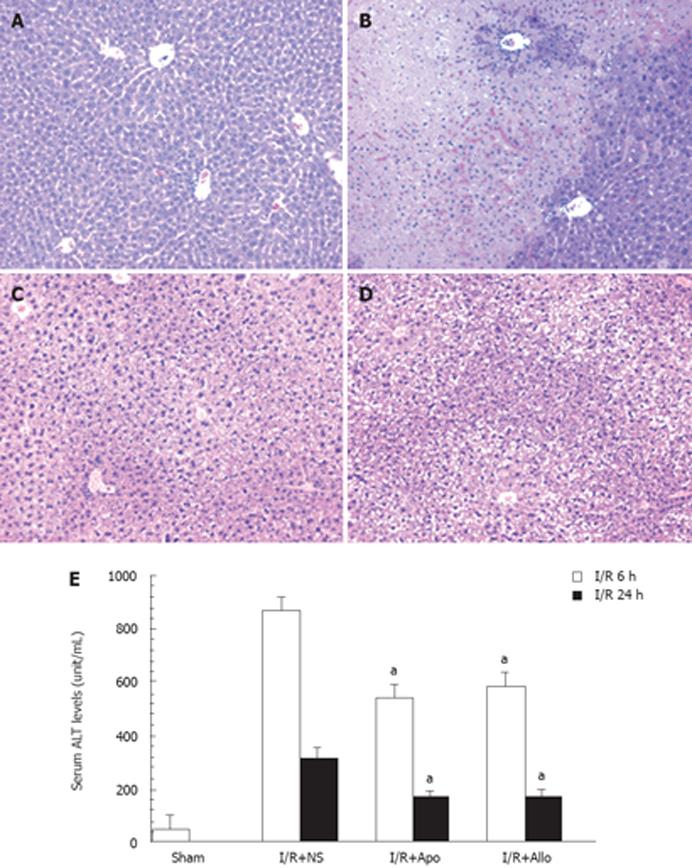
As shown in Figure 1E, the hepatic I/R procedure caused a marked increase in serum ALT levels 6 h to 24 h after starting the reperfusion. Prior intraperitoneal injection of either allopurinol or apocynin led to a marked decrease in serum ALT levels compared to animals with I/R plus N.S. injection at both 6-h and 24-h points (P < 0.01). It appeared that either allopurinol or apocynin tended to exert an improved protection to the same extent, and that the differences were not statistically significant at both time points between these two groups. These findings were consistent with the histological findings as shown in Figure 1A-D. The hepatic I/R procedure led to marked necrosis in the central lobule (Zone III) with significant inflammatory infiltration (Figure 1B). A significant reduction in necrosis was found in the livers of animals receiving injections of allopurinol or apocynin (Figure 1C and D) compared to the control group (Figure 1A). For controls, additional animals were injected with allopurinol or apocynin without the hepatic I/R procedure. Serum ALT levels in animals receiving apocynin (65 ± 17 unit/mL, n = 3) or allopurinol (57 ± 14 unit/mL, n = 3) were similar to levels in N.S. controls (46 ± 27 unit/mL, n = 3). These data indicate that neither apocynin nor allopurinol is toxic to the liver.
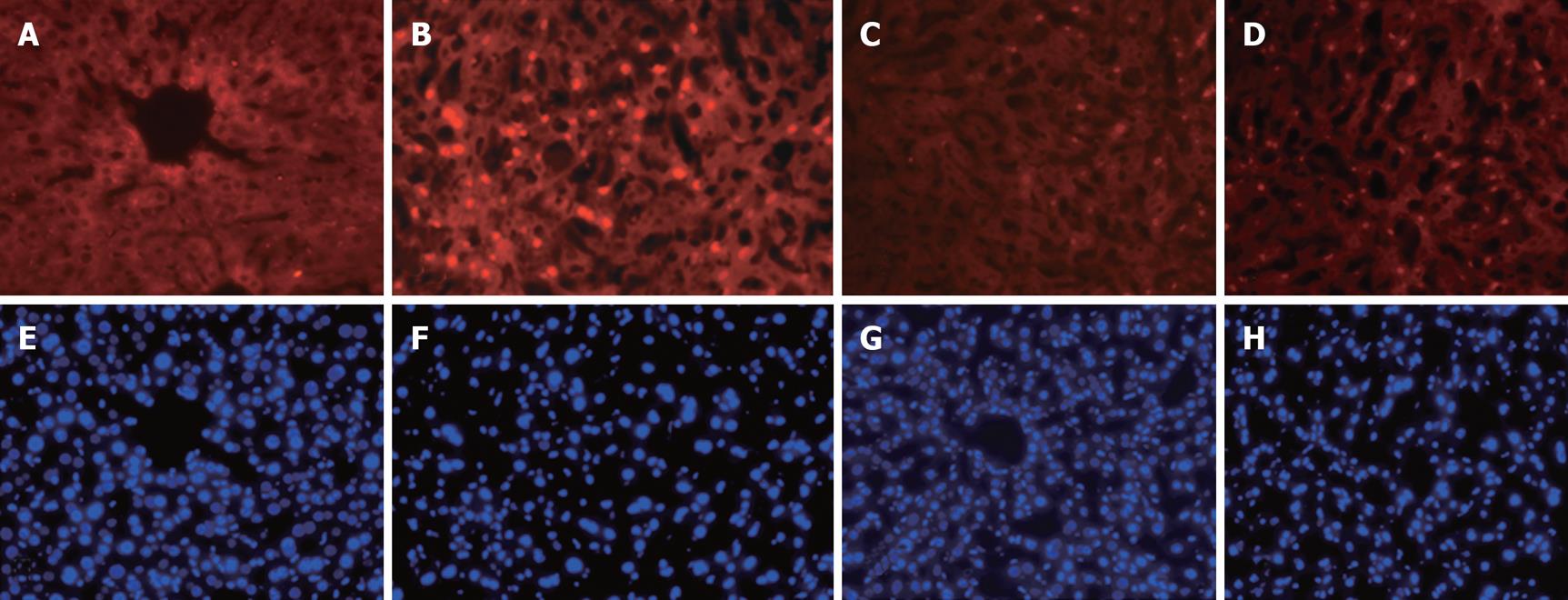
Apoptotic cells in liver sections were detected by in situ staining using TUNEL assay. As shown in Figure 2, 6 h after starting reperfusion, profound apoptotic cells with positive TUNEL red fluorescent staining in nuclei were visualized in animals with the hepatic I/R procedure (Figure 2B) compared to sham-operated controls (Figure 2A). Prior treatment of allopurinol or apocynin prevented apoptotic cell death caused by the hepatic I/R procedure (Figure 2C and D).
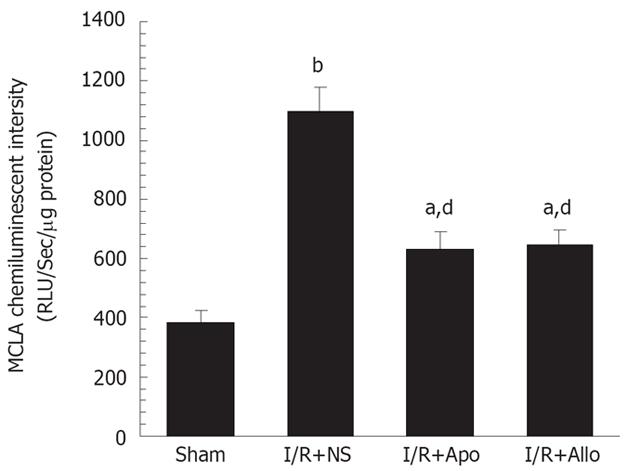
We employed MCLA, which is proportional to levels of O2- and singlet oxygen, to investigate whether the hepatic I/R procedure enhanced the chemiluminescent emission from the liver homogenates. We found that light emission from the liver homogenates was elevated 3-fold 6 h after starting the perfusion, compared to animals with sham operation. The enhanced chemiluminescent emission, caused by the I/R procedure, was markedly inhibited by prior administration of allopurinol or apocynin (Figure 3). This finding provides convincing evidence that NADPH oxidase or xanthine oxidase is the equally important source of superoxide anions, which contributes to pronounced oxidant stress during the hepatic I/R procedure.
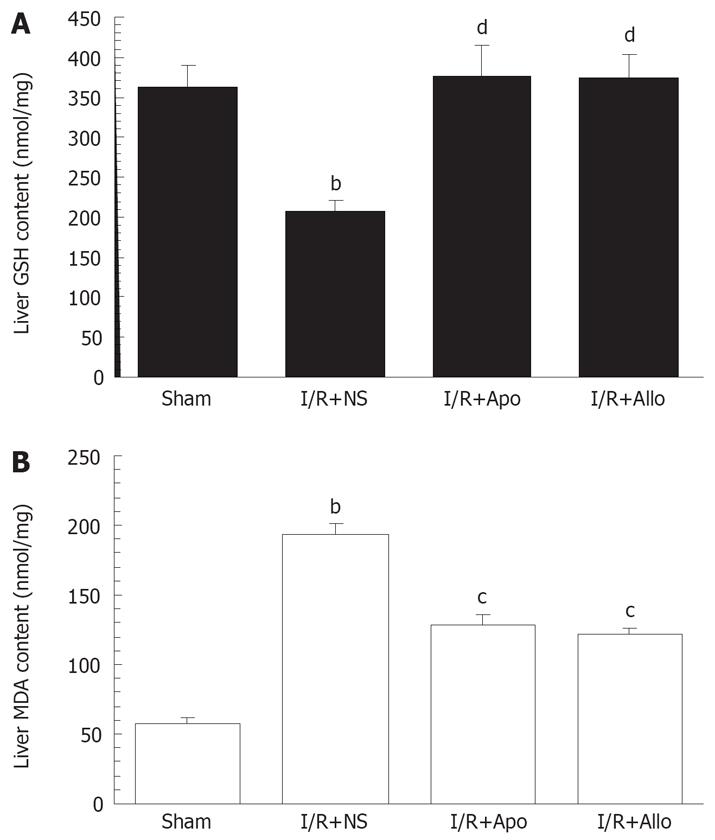
Liver GSH and MDA levels were determined as indicators of oxidant stress and lipid peroxidation during the hepatic I/R procedure. As shown in Figure 4, both allopurinol or apocynin led to a restoration of decreased GSH levels and a reversion of elevated MDA levels in the liver (P < 0.05-0.01). The GSH levels in the treated groups were close to controls 6 h after starting the reperfusion.
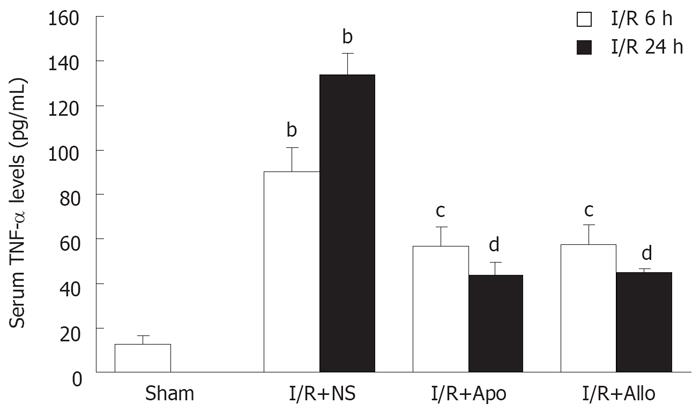
Serum TNF-α was determined using an ELISA kit 6 and 24 h after starting the reperfusion. It is clear that the hepatic I/R procedure caused an elevation of serum TNF-α levels, and that the prior administration of either allopurinol or apocynin reduced the TNF-α levels when compared to the I/R+N.S. controls (P < 0.05-0.01, Figure 5).
In the present study, we demonstrated that prior administration of either allopurinol or apocynin prevented the hepatic I/R-induced acute liver injury in mice; the protection was due to a blockage of xanthine oxidase (XO) and NADPH oxidase (NOX) by allopurinol and apocynin, respectively. The findings indicate that both XO and NOX play a critical role in the generation of superoxide anion and superoxide anion-derived ROS during the hepatic I/R procedure. Thus, our findings further confirm the involvement of NOX activation in the hepatic I/R-induced acute injury.
Our previous study showed that pronounced oxidant stress is the major cause of the hepatic I/R injury, and the delivery of either extracellular superoxide dismutase or catalase gene markedly attenuated the hepatic I/R-induced injury in mice[10]. It is known that XO plays a critical role in generating superoxide anions and administering allopurinol, a blocker of XO, reduced the injury. It is not clear whether other pathways of oxidative reactions also contribute to the enhanced oxidant stress based on the fact that allopurinol failed to improve the progression of remote hepatic parenchymal injury caused by hind limb I/R in mice[7]. This drove us to identify other possible pathologic pathways which lead to enhanced oxidant stress in the hepatic I/R process.
Apocynin is a potent inhibitor of NADPH oxidase by reacting with thiol groups required for enzyme assembly. Apocynin is thought to acquire activation by reacting with hydrogen peroxide and extracellular peroxidase. Active NADPH oxidase is comprised of five subunits (p22phox, p40phox, p47phox, p67phox and gp91phox)[12]. In resting cells, three of these subunits (p22phox, p47phox, and p67phox) form a cytosolic complex. Activation of NOX initiates with the phosphorylation of p47phox, which in turn results in the migration of the cytosolic complex to the membrane and subsequent association with membrane-bound p22phox and gp91 to form the active NADPH oxidase[13]. Several homologues of gp91phox-Nox1, Nox3, Nox4 and Nox5 have been identified in non-phagocytic cells[14]. NADPH oxidase has been shown to have specific subcellular localizations. The mechanisms by which receptors activate NADPH oxidase and regulate ROS production are poorly understood. One may speculate that ROS may be generated during the early and late phases of the hepatic I/R procedure by xanthine oxidase (XO), mitochondrial respiration, or by Kupffer cell-associated and polymorphonuclear neutrophil (PMN)-associated NADPH oxidase[515]. Thus, blockage of either XO or NOX with their specific inhibitors decreased the production of superoxide anions, as indicated by less chemiluminescent emission with MCLA, and in turn reduced oxidant stress as reflected by restoration of GSH levels and reversed MDA content in the liver. Consequently, lower levels of serum ALT and TNF-α, improved liver histology, as well as fewer apoptotic cell counts, demonstrating the protective effects of these two inhibitors in the hepatic I/R model system. Our results are consistent with a recent report showing that inhibition of NADPH oxidase prevented alcohol-induced liver injury[16], hemorrhagic shock-induced organ injury[17] and hepatic I/R injury[18], likely by inhibiting ROS formation via NADPH oxidase. However, in the study by Hasegawa et al[18], a less specific NADPH oxidase inhibitor, diphenyleneiodonium chloride (DPI), was used, and superoxide anion levels in the liver were not determined. The cell types which often have high levels of NADPH oxidase include neutrophils, monocytes and Kupffer cells. The former two cell types are recruited during the I/R process and contribute to the damaging process by releasing superoxide anions and proteases[19]. Activation of Kupffer cells also participates in the enhanced oxidant stress, and increases the production of TNF-α and other cytotoxic factors because the depletion of Kupffer cells resulted in reduced hepatic I/R-associated injury in rodent models[2021]. Nevertheless, further studies are needed to elucidate the role of each cell type that significantly produces superoxide anions via the activation of NADPH oxidase during the hepatic I/R process.
In conclusion, the findings in the present study demonstrate that the pretreatment of both allopurinol and apocynin prevented ischemia/reperfusion-induced acute liver injury and that the protection is associated with the blockage of either xanthine oxidase or NADPH oxidase activity. The activation of both enzymes contributes to the generation of superoxide anions and related reactive oxygen species during the hepatic ischemia/reperfusion-induced liver injury. This study implies a new pharmacological intervention approach by blocking either xanthine oxidase or NADPH oxidase, or in combination to improve the donor organ quality and survival after the transplantation in recipients.
Ischemia/reperfusion-associated donor liver damage is inevitable during the harvest, preservation, transportation and implantation to a recipient. Enhanced oxidant stress has been linked to the donor organ damage; however, the source of reactive oxygen species (ROS) for enhanced oxidant stress has not been well defined. Superoxide anion generated from the reaction of hypoxathine to xanthine catalyzed by xanthine oxidase (OX) has been thought to be one source of ROS, but an intrinsic source of ROS, via the activation of NADPH oxidase (NOX), has not been well studied in a hepatic ischemia/reperfusion model system.
We employed a sensitive chemiluminescent tracer, MCLA, which reflects tissue levels of superoxide anion, one initial and very active oxygen species, to investigate the importance of superoxide anion via the NADPH oxidase reaction in a model of standard hepatic ischemia/reperfusion procedure in mice.
We found that there was a marked increase in superoxide anion release during the ischemia/reperfusion procedure, and that the use of apocynin, a specific inhibitor of NADPH oxidase, effectively minimized the superoxide anion release, oxidant stress and liver damage caused by a hepatic ischemia/reperfusion procedure in mice.
The study implies a new pharmacological intervention approach by blocking either xanthine oxidase or NADPH oxidase, or in combination to improve the donor organ quality, function and survival after the transplantation in recipients.
This study of the beneficial effects of allopurinol and apocynin on the healing of ischemia/reperfusion-induced liver injury is original. It is a well-designed and well-written paper. Allopurinol and apocynin exerted protective effects on hepatic ischemia/reperfusion injury. The protection is associated with blocking the generation of superoxide anions during the hepatic I/R procedure by inhibiting xanthine oxidase and NADPH oxidase activity.
| 1. | Rhoden E, Pereira-Lima L, Lucas M, Mauri M, Rhoden C, Pereira-Lima JC, Zettler C, Petteffi L, Bello-Klein A. The effects of allopurinol in hepatic ischemia and reperfusion: experimental study in rats. Eur Surg Res. 2000;32:215-222. |
| 2. | Yamamori T, Inanami O, Nagahata H, Kuwabara M. Phosphoinositide 3-kinase regulates the phosphorylation of NADPH oxidase component p47(phox) by controlling cPKC/PKCdelta but not Akt. Biochem Biophys Res Commun. 2004;316:720-730. |
| 3. | Arteel GE. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology. 2003;124:778-790. |
| 4. | Czaja MJ. Induction and regulation of hepatocyte apoptosis by oxidative stress. Antioxid Redox Signal. 2002;4:759-767. |
| 5. | Mochida S, Arai M, Ohno A, Masaki N, Ogata I, Fujiwara K. Oxidative stress in hepatocytes and stimulatory state of Kupffer cells after reperfusion differ between warm and cold ischemia in rats. Liver. 1994;14:234-240. |
| 6. | Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 2003;125:1246-1257. |
| 7. | Dorman RB, Wunder C, Saba H, Shoemaker JL, MacMillan-Crow LA, Brock RW. NAD(P)H oxidase contributes to the progression of remote hepatic parenchymal injury and endothelial dysfunction, but not microvascular perfusion deficits. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1025-G1032. |
| 8. | Harada H, Hines IN, Flores S, Gao B, McCord J, Scheerens H, Grisham MB. Role of NADPH oxidase-derived superoxide in reduced size liver ischemia and reperfusion injury. Arch Biochem Biophys. 2004;423:103-108. |
| 9. | Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol. 1994;11:95-102. |
| 10. | He SQ, Zhang YH, Venugopal SK, Dicus CW, Perez RV, Ramsamooj R, Nantz MH, Zern MA, Wu J. Delivery of antioxidative enzyme genes protects against ischemia/reperfusion-induced liver injury in mice. Liver Transpl. 2006;12:1869-1879. |
| 11. | Wu J, Liu L, Yen RD, Catana A, Nantz MH, Zern MA. Liposome-mediated extracellular superoxide dismutase gene delivery protects against acute liver injury in mice. Hepatology. 2004;40:195-204. |
| 13. | Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131-140. |
| 14. | Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology. 2000;32:169-173. |
| 15. | Kono H, Rusyn I, Uesugi T, Yamashina S, Connor HD, Dikalova A, Mason RP, Thurman RG. Diphenyleneiodonium sulfate, an NADPH oxidase inhibitor, prevents early alcohol-induced liver injury in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1005-G1012. |
| 16. | Abdelrahman M, Mazzon E, Bauer M, Bauer I, Delbosc S, Cristol JP, Patel NS, Cuzzocrea S, Thiemermann C. Inhibitors of NADPH oxidase reduce the organ injury in hemorrhagic shock. Shock. 2005;23:107-114. |
| 17. | Yabe Y, Kobayashi N, Nishihashi T, Takahashi R, Nishikawa M, Takakura Y, Hashida M. Prevention of neutrophil-mediated hepatic ischemia/reperfusion injury by superoxide dismutase and catalase derivatives. J Pharmacol Exp Ther. 2001;298:894-899. |
| 18. | Hasegawa T, Malle E, Farhood A, Jaeschke H. Generation of hypochlorite-modified proteins by neutrophils during ischemia-reperfusion injury in rat liver: attenuation by ischemic preconditioning. Am J Physiol Gastrointest Liver Physiol. 2005;289:G760-G767. |
| 19. | Gujral JS, Hinson JA, Farhood A, Jaeschke H. NADPH oxidase-derived oxidant stress is critical for neutrophil cytotoxicity during endotoxemia. Am J Physiol Gastrointest Liver Physiol. 2004;287:G243-G252. |
| 20. | Vega VL, Mardones L, Maldonado M, Nicovani S, Manriquez V, Roa J, Ward PH. Xanthine oxidase released from reperfused hind limbs mediate kupffer cell activation, neutrophil sequestration, and hepatic oxidative stress in rats subjected to tourniquet shock. Shock. 2000;14:565-571. |
| 21. | von Frankenberg M, Golling M, Mehrabi A, Nentwich H, Thies J, Schaeffer F, Jahnke C, Bud O, Gebhard MM, Otto G. Destruction of Kupffer's cells increases total liver blood flow and decreases ischemia reperfusion injury in pigs. Transplant Proc. 1999;31:3253-3254. |