INTRODUCTION
Although organ transplantation has become an established treatment, the shortage of donor organs remains a serious problem, for which tissue engineering is a possible solution. A bioartificial liver (BAL) system consists of three components: cells, a scaffold for the cells, and growth-regulating factors. Ideally, the cells for a BAL will exhibit similar functions to normal human mature hepatocytes while maintaining long-term viability and proliferative activity. However, a method of culturing human hepatocytes for a prolonged period has not been established so far.
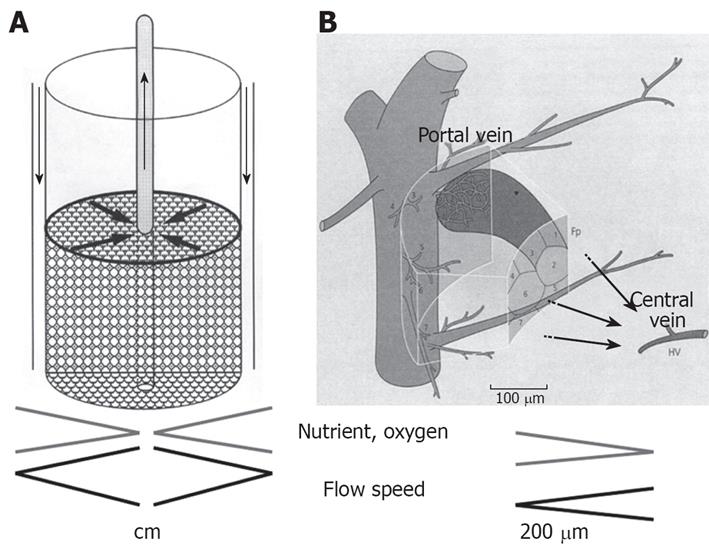
We have developed a radial flow bioreactor (RFB), which is a 3-dimensional culture system that can be used for high-density culture[1–4], achieving a cell density 10 times greater than that obtained with hollow-fiber culture[56]. A cylindrical bioreactor is filled with porous cellulose beads that act as microcarriers, and culture medium flows from the periphery toward the center of the reactor (Figure 1A)[27]. To achieve a high-density cell culture, it is essential to minimize any variations in the distribution of oxygen and nutrients between the culture medium at the inlet and outlet of the reactor. If medium flows from the periphery towards the center, a high perfusion rate can provide an adequate supply of oxygen and nutrients to cells at the center of the bioreactor even though oxygen and nutrients are consumed by cells at the periphery. Thus, there are similarities between the RFB and the anatomy of hepatic lobules (Figure 1B)[27].
Figure 1 The principle of RFB system.
A: A cylindrical bioreactor is filled with porous cellulose beads that act as microcarriers and culture medium flows from the periphery toward the center of the reactor. Biases in distribution of oxygen and nutrients between the culture medium at the inlet and outlet of the reactor are minimized; B: The RFB system is similar to the organization of the hepatic primary lobe Figure 1B is reproduced from reference 7.
Because of the easy availability, recent reports point out a clinical use of BAL systems that utilize animal cells[89]. Fetal porcine hepatocytes have a high proliferative potential in vitro. Previous studies have shown that minimal immunogenicity is exhibited by tissues harvested at the earliest available gestational age[10–12]. Thus, the earliest time after organogenesis is established appears to be preferable for human transplantation. In fact, Friedman et al[13] have shown that maximal liver growth and function were achieved at the earliest teratoma-free gestational age of embryonic day (E) 28. In this study, the possibility of teratoma development was also taken into consideration, and construction of a BAL system was achieved with a RFB and fetal porcine livers obtained on E35 as the cell source, while E56 cells were also examined for comparison. Furthermore, this study also focused on the effect of hepatocyte growth factor (HGF), which was originally reported as a hepatocyte-specific mitogen with an important role in liver regeneration[14].
MATERIALS AND METHODS
Isolation of fetal hepatocytes and nonparenchymal epithelial cells
Pregnant mini swines (CSK-MS) weighing 30 to 35 kg were purchased from Chugai Institute of Medical Science (Nagano, Japan). Fetal hepatic cells were isolated from the porcine livers of embryos at 5 and 8 wk of gestation (NIBS strain) by the two-step liver perfusion method of Seglen with some modifications[15–17]. Five weeks of gestation was designated as E35 and 8 wk of gestation was designated as E56. For controls, mature porcine liver cells were used. After intraportal infusion of collagenase (0.05%) and dispase (1000 U/mL), the hepatic tissue was minced into pieces and shaken in collagenase solution. Then the collagenase-digested liver cell suspension was centrifuged at 50 g for 1 min. The E35 cells were divided into 3 fractions and the E56 cells were divided into 2 fractions. The hepatocyte fraction (lowest fraction) was maintained on ice in serum-free ASF 104 medium (Ajinomoto, Tokyo). The one and two upper fractions, respectively, were centrifuged once at 350 g for 5 min, and the cell pellet (containing non-parenchymal cells) was also maintained on ice in serum-free ASF 104 medium.
Oxygen consumption in the RFB
An RFB system with a 15-mL capacity was placed in an aseptic room maintained at 37°C. The system was composed of an RFB and a conditioning vessel connected to a tank containing fresh medium and a recovery tank. The system was automatically controlled by a computer that monitored pH, glucose, and oxygen consumption. The oxygen tension in the culture medium was measured both within the reservoir and at the outlet of the bioreactor, and the oxygen consumption was monitored on the basis of the oxygen tension gradient (Do in-Do out: ΔO2 ppm). Oxygen consumption was used as an index of the activity of the cells in the RFB system.
Culture of cells in the RFB
The RFB (Biott, Tokyo, Japan) is a cylindrical bioreactor with a capacity of 15 mL that contains cellulose beads (Asahi Kasei, Tokyo, Japan). The RFB is attached to a reservoir containing culture medium and an automatic controller that maintains the oxygen content and pH of the medium. Both hepatocytes (5 × 107) and non-parenchymal cells (5 × 107) were inoculated into the reservoir, which was filled with ASF104 medium containing 2% fetal bovine serum (FBS). The bioreactor was perfused in a closed circuit fashion for 2 h to maximize the efficiency of cell attachment to the cellulose beads, after which the reactor was switched to the open-circuit mode and the medium was changed to ASF104 without FBS. For this study, incubation in the RFB system was performed for one week before morphological and functional examinations.
Factor mix and HGF
The factor mix (FM) that we formulated consists of 10-7 mol/L insulin, 10-7 mol/L dexamethasone, 100 ng/mL oncostatin M (OSM), 25 ng/mL epidermal growth factor (EGF), 1 &mgr;g/mL L-ascorbic acid phosphate magnesium salt, 0.1 mmol/L nicotinamide, and antibiotics, which was added to the culture medium. HGF was also added at 20 ng/mL (low-dose group) or 100 ng/mL (high-dose group). HGF was administered three times (d 1, 3, and 5) by closed-circuit perfusion for 2 h.
Experimental groups
The following groups were studied: FME35 group (ASF104 + FM), LFE35 group (ASF104 + FM + HGF 20 ng/mL: low-dose), and HFE35 group (ASF104 + FM + HGF 100 ng/mL: high-dose) for E35 cells, with corresponding groups for E56 cells. Two other groups, i.e., the FMH group (Adult: group H, ASF104 + FM) and the HFH group (Adult: group H, ASF104 + FM + HGF 100 ng/mL), were studied as control groups. Each group was set at n = 3.
Ammonia loading test
In order to assess the performance of hepatocyte functions, the ammonia (NH3) loading test was performed. In consideration of the results of oxygen consumption and morphological features, the NH3 loading test compared the HFE35 group, FME35 group, and HFE56 group. After incubation for one week (d 7) in the RFB, ammonium chloride (NH4Cl) was added at three concentrations (1 mmol/L + 2 mmol/L + 3 mmol/L) every 8 h for 24 h, followed by 3 mmol/L for a further 24 h[4]. Then NH3 and urea levels were measured on d 8 and d 9 using an automatic high-speed amino acid analyzer JLC-300 (JEOL Ltd., Tokyo).
Immunohistochemistry
For immunochemical study of cytokeratin 19 (CK19) (DakoCytomation), the streptoavidin-biotin (SAB) technique was used[1819]. Specimens of RFB cultures were fixed with Methacarn solution (methanol:chloroform:glacial acetic acid = 6:3:1), embedded in paraffin, cut into 3 &mgr;m sections, and deparaffinized with graded xylene series. Endogenous peroxidase was inhibited by adding 0.3% H2O2 methanol.
For immunofluorescence study of connexin 32 (Cx32), specimens of RFB cultures were fixed in cold absolute acetone for 10 min. Immunohistochemistry with a rabbit polyclonal anti-Cx32 (Zymed, South San Francisco, CA) was performed. Cx32 was visualized using Alexa Fluor 488-conjugated goat anti rabbit immunoglobulin G (Molecular Probes, Eugene, OR). For comparison, immunohistochemical staining as also performed on parenchymal cells and non-parenchymal cells in monolayer cell culture. Samples were examined with an epifluorescence microscope (Nikon, Tokyo, Japan) and a laser-scanning confocal microscope (MRC 1024; Bio-Rad, Hercules, CA,).
Transmission electron microscopy (TEM)
For TEM, cultured cells were fixed with 2.0% glutaraldehyde in 0.1 mol/L phosphate buffer (PB) for 1 h and postfixed with 1% OsO4 in 0.1 mmol/L PB for 1 h at 4°C. Specimens were dehydrated in ethanol and embedded in a mixture of Epon-Araldite. Thin sections (60 nm) were cut with a diamond knife mounted on an LKB ultratome, and stained with aqueous uranyl acetate. Then the sections were examined under a JEOL 1200EX electron microscope (JEOL Ltd., Tokyo, Japan).
Statistical analysis
The oxygen consumption, the NH3 and urea levels were reported as a mean ± SD. For individual parameters, differences between groups were assessed by repeated measures ANOVA[2021] and Bonferron`s multiple comparison test. All calculations were performed using Stat View-J statistical soft ware (SAS Institute, Cary, NC) with P < 0.05 considered significant.
RESULTS
Morphological features of fetal porcine liver cells
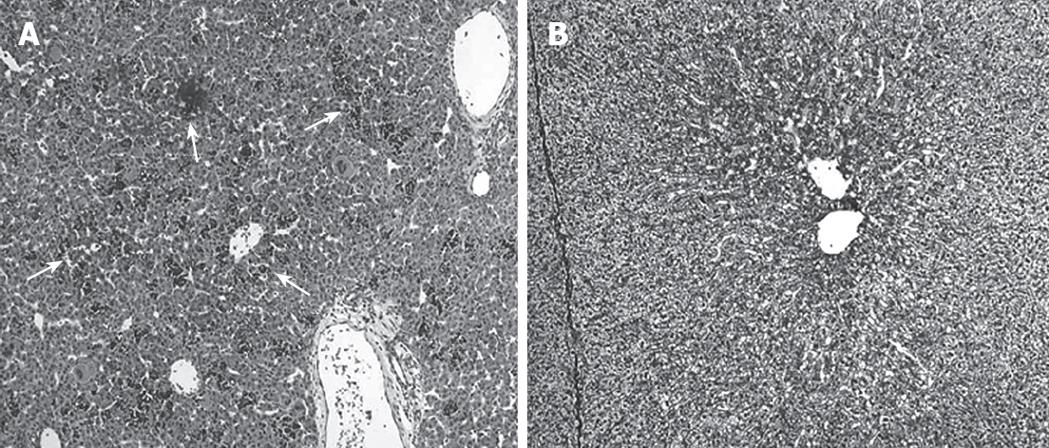
Figure 2A demonstrates the histological features of E35 porcine liver. Although the hepatic lobular structure is present, extramedullary hematopoiesis was also observed and the cells are largely immature. On the other hand, the E56 liver shows a definite lobular structure and a decrease of extramedullary hematopoiesis, so it more closely resembles the adult porcine liver (Figure 2B). Liver cells isolated on E56 were divided into two layers (parenchymatous cells and non-parenchymatous cells) after centrifugation of the cell suspensions in accordance with these histological findings; but, E35 liver cell suspensions were divided into three layers. The upper layer, the middle layer, and the lower layer consisted of parenchymal cells, extramedullary-hematopoiesis cells including immature hepatic cells, and non-parenchymal cells, respectively.
Figure 2 Histological findings of fetal porcine liver (HE staining).
A: Extramedullary hematopoiesis was observed (arrow) and the cells are largely immature at embryonic d 35 (× 100); B: A definite lobular structures and a decrease of extramedullary hematopoiesis were observed at embryonic d 56 (× 100).
Oxygen consumption
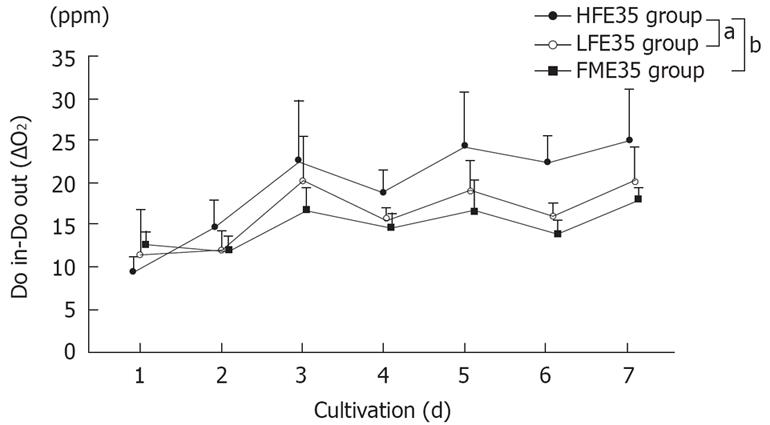
Changes in the oxygen consumption in each group, which is an index of cellular activity in RFB culture, are shown in Figure 3. Although viability of the FME35 group was maintained for one week, viability of the FMH group was difficult to maintain (data not shown). Although the LFE56 group did not show a significant change of oxygen consumption over time compared with the FME35 group (P = 0.2792), the HFE35 group showed a significant change of oxygen consumption with time (P < 0.01). Also compared to the LFE35 group, the HFE35 group showed a significant change over time (P < 0.05). E56 cells gave the same results as E35 cells when cultured under the same conditions (data not shown). The HFH group, which was treated with a high concentration of HGF, also maintained viability for one week (data not shown).
Figure 3 Changes of the oxygen consumption in the HFE35, LFE35, and FME35 groups.
HFE35 group vs FME35 group (P < 0.01), HFE35 group vs LFE35 group (P < 0.05), LFE35 group vs FME35 group (no significant). The data show the mean values, while error bars represent corresponding standard deviations. aP < 0.05, bP < 0.01.
Architecture of cells cultured in the RFB
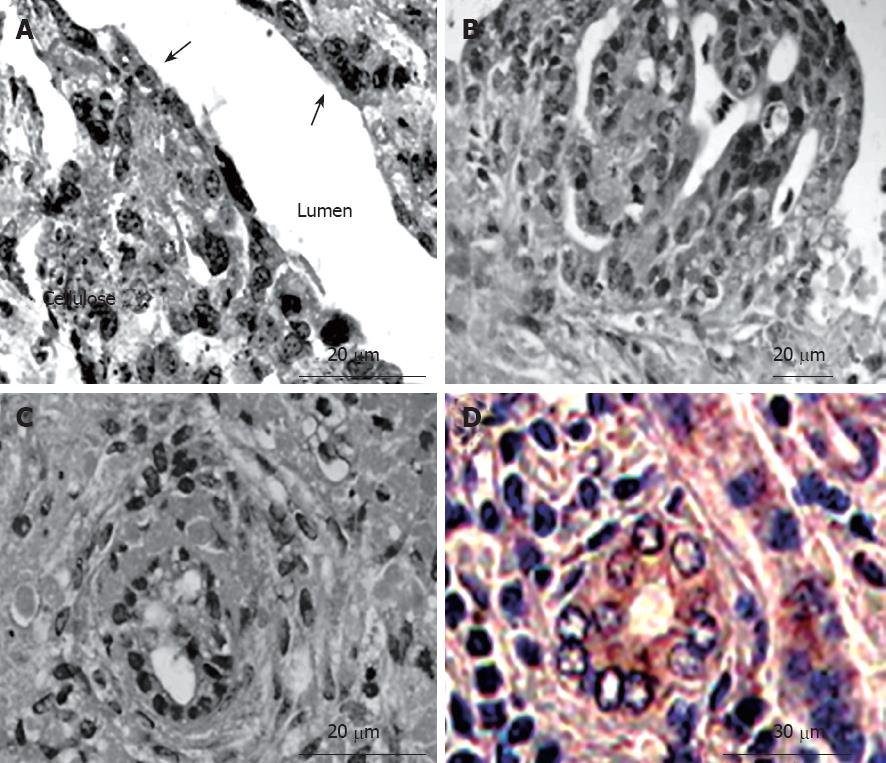
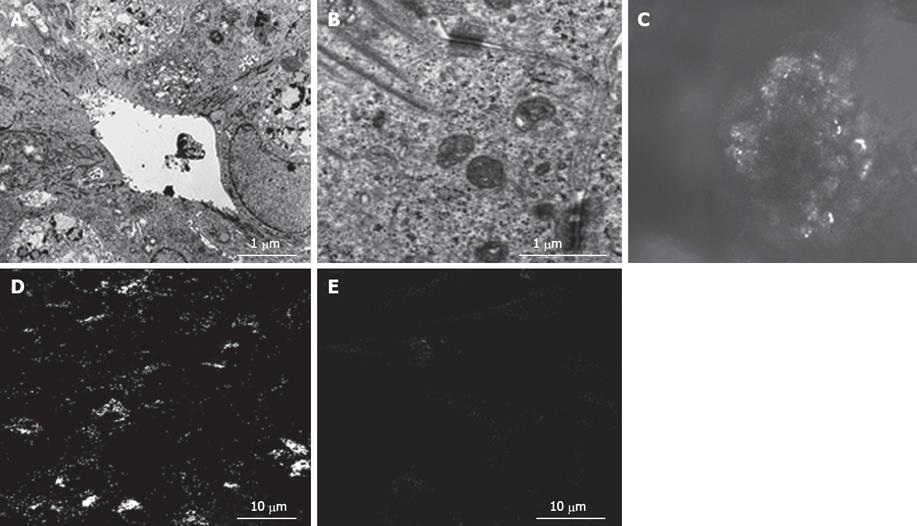
In the bioreactor, cultured cells formed layers on the cellulose beads. Non-parenchymal cells had a flat shape and existed on the surface of the perfused side (Figure 4A). Moreover, cell clumps of 0.4 to 0.8 cm3 separated from the cellulose beads to float in the RFB. In particular, these clumps were conspicuous in the HFE35 group. On histological examination of these clumps, a palisading structure (Figure 4B) and bile duct-like structures were also observed (Figure 4C), so reconstruction of a “mini-liver organoid” was achieved. Furthermore, CK19 which is the marker of a bile duct was also observed in bile duct-like structures (Figure 4D). TEM images revealed microvilli inside bile duct-like structures (Figure 5A). Moreover, junctional complexes were well-developed and tight junctions were also observed (Figure 5B). Immunohistochemical staining for Cx32, which is the main protein of gap junctions in hepatocytes of the organoid, showed expression in a whirl-shaped pattern (Figure 5C). These morphological characteristics were more notable in the HFE35 group. The expression of Cx32 in the parencymal cells in monolayer culture was reduced compared to that of the clump in RFB (Figure 5D). Furthermore, the expression of Cx32 in non-parenchymal cells in monolayer culture was not observed (Figure 5E).
Figure 4 Histological findings of cultured cells in the RFB (hematoxylin-eosin staining and immunohistochemistry).
A: Cultured cells formed layers on the cellulose beads. Non-parenchymal cells had a flat shape and exited on the surface of the perfused side (arrow); B: A palisading structure was observed in cell clumps; C: Bile duct-like structures were also observed in cell clumps; D: The expression of cytokeratin 19 (CK19) was observed in bile duct-like structures.
Figure 5 A: Transmission electron microscopic images revealed microvilli inside bile duct-like structures; B: Junctional complexes were well developed and tight junctions were also observed; C: Connexin 32 (Cx32) in liver organoid, showed expression in a whirl-shaped pattern; D: Cx32 in parenchymal cells in monolayer culture was fewer than that of the organoid; E: Cx32 in npon-parenchymal cells in monolayer culture was not observed.
Ammonia loading test
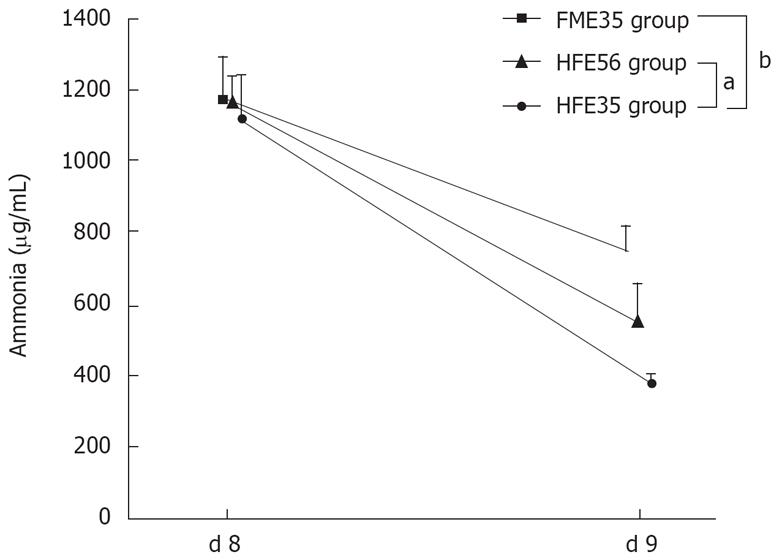
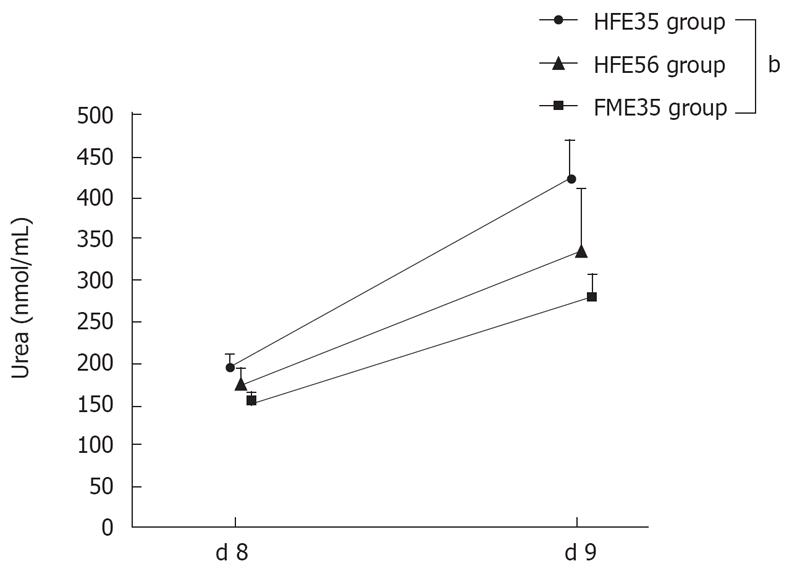
The ammonia loading test was performed in the HFE35 group, which showed good oxygen consumption and morphological reconstruction of a mini liver, the FME35 group, and the HFE56 group. Compared with the FME35 group, the NH3 concentration of the HFE35 group was decreased significantly (P < 0.01) (Figure 6), and the urea concentration increased significantly along with a reduction of NH3 (P < 0.01) (Figure 7). Moreover, a significant reduction of the NH3 concentration was seen in the HFE35 group compared with the HFE56 group (P < 0.05) (Figure 6). On the other hand, the change of the urea concentration in the HFE35 group was marked, but there was no significant difference with the HFE56 group (Figure 7).
Figure 6 The NH3 loading test compared the HFE35 group, FME35 group, and HME56 group.
HFE35 group vs FME35 group (P < 0.01), HFE35 group vs HFE56 group (P < 0.05). FME35 vs HFH56 (no significant). aP < 0.05, bP < 0.01.
Figure 7 Changes of urea concentration in the HFE35, HFE56, and FME35 groups.
HFE35 group vs FME35 group (P < 0.01), HFE35 vs HFE56 and HFE56 vs FME35 (no significant). bP < 0.01.
DISCUSSION
In this study, fetal porcine hepatic cells with proliferative capacity were cultured in a RFB system that allowed high-density, three-dimension culture[1–4], and the usefulness of the RFB system as a BAL and the possibility of liver reconstruction with implantable organoids were examined. Although the high proliferative capacity of embryonic cells is well known, it has been unclear at which gestational age the fetal liver cells show optimal proliferation and functional capacity when used as a source of cells for a BAL or for organoid reconstruction. In this study, fetal porcine livers (E35 and E56) were selected based on the findings of Friedman et al, who examined the timing of liver organogenesis in embryonic swines[13]. They determined distinct gestational time windows for the embryonic porcine liver and precursor cells, with maximal liver growth and function being achieved at the earliest teratoma-free gestational age of E28. In their tissue transplantation experiment, no development of teratomas was seen on E28 (0/23 transplants)[13]. Examination of hepatocyte function based on the serum albumin level also showed that E28 is the optimal gestational age for transplantation, and albumin secretion was decreased at E56 and E80[13]. Although this study was performed in vitro using the RFB system, in consideration of the possibility of the teratoma development because the environment was similar to that in vivo, E35 was chosen as a safe gestational age and comparison with E56 was also performed.
At present, using cells from the fetal human liver is ethically problematic and great difficulties in obtaining such cells are expected. For this reason, fetal porcine liver cells were used in the present study. The following three points are also worth considering: (1) The anatomical and physiological characteristics of the porcine liver are similar to those of the human liver; (2) The gestation period of pigs is comparatively short (about 112-116 d), and breeding is easy; (3) Breeding of specific-pathogen-free animals is also possible, so zoonoses can be avoided[2223]. However, the swine is immunologically different from humans and hyperacute rejection of porcine organs by human recipients is mediated via antibodies directed against Galα-1, 3-Gal on porcine cells, posing an immediate barrier to successful clinical xenotransplantation[24–26]. In addition, infection of the human host by porcine endogenous retroviruses poses a major problem[27]. It was reported that infection can be avoided by breeding GalT KO swines that show no infectivity for human cells in vitro[2829]. Thus, porcine organs may be used for xenotransplantation in donors with no alternative.
With respect to growth factors, the factors that were considered to be necessary for organogenesis by inducing proliferation, differentiation, and maturation of co-cultured fetal hepatic parenchymal cells and non-parenchymal cells were added to the culture medium[30–35]. Hamazaki et al[35] demonstrated that the important growth factors for hepatogenesis are fibroblast growth factor (FGF) at an early stage, HGF in the middle stage, and OSM, insulin, and dexamethasone at the late stage. OSM and dexamethasone are especially important for the maturation of hepatoblasts to hepatocytes[3136]. On the other hand, it is generally accepted that HGF/scatter factor (SF) is an important paracrine mediator of epithelial-mesenchymal cell interactions, with potential involvement in organogenesis and angiogenesisis[3738]. In particular, a morphogenic effects of HGF/SF on the organization of focal contacts and cellular junctions has been documented[39].
Morphological examination of each group showed that fetal hepatic parenchymal cells were adherent to the cellulose beads, while fetal hepatic non-parenchymal cells existed on side of perfusion (Figure 4A)[2]. Also, small organoids that had separated from the beads were observed floating in the RFB. In the HFE35 group, larger organoids were observed. Microscopic examination of the organoids from a high-HGF groups revealed a palisading architecture and bile duct-like structures with the expression of CK19, so the organoids could be called mini livers. Furthermore, TEM showed microvilli on the luminal surfaces of bile duct-like structures and junctional complexes, the basis of the cytoskeleton of epithelial tissue. Finally, strong expression of Cx32, which is the main protein of hepatocyte gap junctions, was observed[4041]. These findings were considered to be the features of organoids which had adhered to the beads firmly until reaching a certain size.
Oxygen consumption was used as an index of cellular activity and it showed a significant change over time in the HFE35 group compared to the FME35 group, and a high level of oxygen consumption was maintained in the HFE35 group. Although the LFE35 group also showed a high consumption at the time of HGF addition compared to the FME35 group, there was no significant change over time. The E56 group also showed similar changes of oxygen consumption under the same conditions. The ammonia loading test was performed for evaluation of functionality of mature hepatocytes. Among 3 groups tested, the reduction of NH3 and the increase of urea production were highest in the HFE35 group, suggesting that the urea cycle was activated by elevation of the ammonia concentration. Although similar changes were observed in the HFE56 group, the extent of change was smaller than in the HFE35 group. These results showed that the organoids possessed some of the functions of mature hepatocytes.
Both proliferative activity and differentiation of cells are needed as an ideal source for a BAL. In this study, the combination of embryonic porcine liver (E35) cells and pulsing with a high concentration of HGF in our RFB provided a system for which clinical application may eventually be possible in the fields of BAL and transplantation medicine.
COMMENTS
Background
As an alternative to liver transplantation, numerous researchers have been working towards the goal of development of a fully functional artificial liver. Researchers are, therefore, concentrating their efforts on hybrid systems incorporating human-or animal-derived cells. While research on bioartificial liver (BAL) system is still in its infancy, the urgent goal is to develop a sophisticated BAL suitable for clinical applications.
Research frontiers
The flow bioreactor (RFB) system is a 3-dimensional culture system that can be used for high-density culture, achieving a cell density 10 times greater than that obtained with hollow-fiber culture. The culture medium flows from the periphery toward the center of the reactor. When medium flows from the periphery towards the center, a high perfusion rate can provide an adequate supply of oxygen and nutriments to cells at the center of the bioreactor even though oxygen and nutrients are consumed by cells at the periphery. This system simulates the anatomy of hepatic lobules. On the other hand, both proliferative activity and differentiation of cells are needed as an ideal source for a BAL. Fetal cells have a high proliferative potential in vitro and we selected the fetal porcine hepatocytes as a cell source. Furthermore, we examined the earliest teratoma-free gestational age of embryonic day. Furthermore, the efficacy of pulsed administration of a high concentration hepatocytes growth factor (HGF) was examined.
Innovations and breakthroughs
In this study, the combination of embryonic porcine liver (embryonic d 35: E35) cells and pulsing a high concentration of HGF in our RFB provided a system for which clinical application may eventually be possible in the fields of BAL and transplantation medicine.
Applications
Our RFB system may be able to perform clinical application as extracorporeal BAL for acute hepatic failure. Furthermore, the liver organoids in RFB have the potential future in vivo application (implantable bioartificial liver etc.).
Peer review
The manuscript reports the hepatic reconstruction from fetal porcine liver cells using a radial flow bioreactor. The authors evidenced that cells organized in organoids with the presence of bile duct-like structure. They also showed that HGF favored differentiation and survival of cells in the bioreactor. Despite the fact that this bioreactor was described useful from maintenance of expression of CYP3A4 by human hepatocytes and that the use of pig cells is ethically problematic for tissue engineering and development of extracorporeal bioartificial liver, the results are interesting and well documented.