Published online May 7, 2008. doi: 10.3748/wjg.14.2731
Revised: February 16, 2008
Published online: May 7, 2008
AIM: To characterize the mitochondrial dysfunction in experimental cirrhosis and to study whether insulin-like growth factor-I(IGF-I) therapy (4 wk) is able to induce beneficial effects on damaged mitochondria leading to cellular protection.
METHODS: Wistar rats were divided into three groups: Control group, untreated cirrhotic rats and cirrhotic rats treated with IGF-Itreatment (2 &mgr;g/100 g bw/d). Mitochondrial function was analyzed by flow cytometry in isolated hepatic mitochondria, caspase 3 activation was assessed by Western blot and apoptosis by TUNEL in the three experimental groups.
RESULTS: Untreated cirrhotic rats showed a mitochondrial dysfunction characterized by a significant reduction of mitochondrial membrane potential (in status 4 and 3); an increase of intramitochondrial reactive oxigen species (ROS) generation and a significant reduction of ATPase activity. IGF-Itherapy normalized mitochondrial function by increasing the membrane potential and ATPase activity and reducing the intramitochondrial free radical production. Activity of the electron transport complexes Iand III was increased in both cirrhotic groups. In addition, untreated cirrhotic rats showed an increase of caspase 3 activation and apoptosis. IGF-Itherapy reduced the expression of the active peptide of caspase 3 and resulted in reduced apoptosis.
CONCLUSION: These results show that IGF-Iexerts a mitochondrial protection in experimental cirrhosis leading to reduced apoptosis and increased ATP production.
- Citation: Pérez R, García-Fernández M, Díaz-Sánchez M, Puche JE, Delgado G, Conchillo M, Muntané J, Castilla-Cortázar I. Mitochondrial protection by low doses of insulin-like growth factor-Iin experimental cirrhosis. World J Gastroenterol 2008; 14(17): 2731-2739
- URL: https://www.wjgnet.com/1007-9327/full/v14/i17/2731.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2731
Insulin-like growth factor-I(IGF-I) is an anabolic hormone produced mainly in the liver in response to growth hormone (GH) stimulation[1]. In cirrhosis, the reduction of receptors for GH in hepatocytes and the reduced ability of synthesis of the hepatic parenchyma cause a progressive decrease in serum IGF-Ilevels[23]. We have shown previously that short courses of treatment with IGF-Iin rats with carbon tetrachloride-induced cirrhosis induced many systemic beneficial effects[4–11] and showed hepatoprotective, antioxidant and antitibrogenic properties[12–15]. However, these mechanisms of IGF-Iactivities regarding the improvement of liver function and fibrosis are not fully understood. We have suggested previously that the described hepatoprotection by IGF-Itherapy in rats with carbon tetrachloride-induced cirrhosis could be related to mechanisms of mitochondrial protection[12].
Mitochondria are a major source of reactive oxygen species (ROS) under physiologic conditions, because 2% to 3% of the O2 consumed is converted to O2-. Intramitochondrial ROS production increases after peroxidation of mitochondrial membrane lipids[16–20]. Mitochondria are particularly sensitive to ROS-induced injury in the pathogenesis of disease. Oxidative stress exerts deleterious effects on mitochondrial function by directly impairing oxidative phosphorylation through direct attack of proteins or membrane lipids. ROS can also induce mitochondrial DNA deletions and mitochondrial membrane permeability transition (MMPT)[21]. MMP pore opening activates caspases which is an endpoint to initiate cell death. Furthermore, it has been implicated in the pathogenesis of many fibroproliferative diseases including liver fibrogenesis[22]. Recently, a large number of studies have associated mitochondrial dysfunction caused by ROS to both accidental cell death (necrosis) and programmed cell death (apoptosis)[192324]. In order to give a better insight into the mechanisms by which IGF-Iexerts an hepatoprotective action on damaged liver and supposing that mitochondria could be one of the main cellular target of IGF-I, the aims of the present study were: (1) to characterize the mitochondrial dysfunction in experimental liver cirrhosis and (2) to study whether IGF-I(4 wk) induces beneficial effects on damaged mitochondria leading to cellular protection. Thus, we have extended our study to analyze the effect of IGF-I on mitochondrial function (Mitochondrial Potencial Membrane, intramitochondrial free radical production and “in vitro” complex activities), caspase 3 activation and apoptosis. In this series we included three experimental groups of Wistar rats: healthy control group (CO); untreated cirrhotic rats (CI) and cirrhotic rats treated with IGF-Iat low doses (2 &mgr;g/100 g bw/d, CI + IGF-I).
All experimental procedures were performed in conformity with The Guiding Principles for Research Involving Animals[25]. Cirrhosis was induced as previously described[512]. Briefly, male Wistar rats (3 wk old, 130-150 g) were subjected to CCl4 inhalation (Merck, Darmstadt, Germany) twice a week for 11 wk with a progressively increasing exposure time from 1 to 5 min. From that time since 12 wk until the 16 wk rats were exposed to CCl4 once a week for 2 min (5 additional doses of CCl4).
During the whole period of cirrhosis induction animals received phenobarbital (Luminal, Bayer, Leverkusen, Germany) in the drinking water (400 mg/L). Both food (standard semipurified diet for rodents; B.K. Universal, Sant Vicent del Horts, Spain) and water were given ad libitum. Healthy, age and sex-matched control rats were maintained under the same conditions, but received neither CCl4 nor phenobarbital.
IGF-Itherapy or saline was administered the last 4 wk (13th-16th wk, 28 d), cirrhotic rats were randomly assigned to receive either vehicle (saline) (group CI, n = 6) or recombinant human IGF-I(Chiron Company, San Francisco, CA) (20 &mgr;g/kg per day in two divided doses, subcutaneously) (Group CI + IGF-I, n = 6) for 4 wk. Healthy control rats (Group CO, n = 6) received saline during the same period.
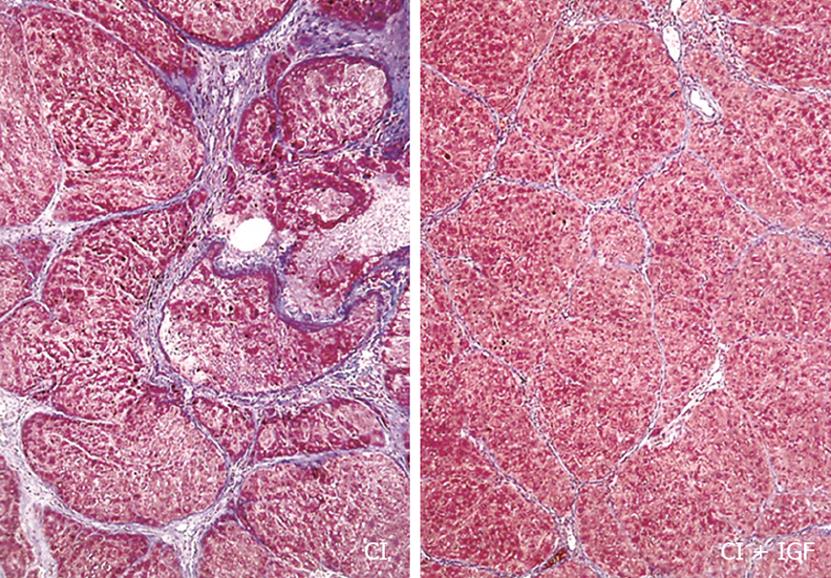
In the morning of the 29th day (after 4 wk of treatment), rats were weighed, blood was obtained from the retroocular plexus and animals were killed by decapitation. After the abdominal cavity was opened, the livers were dissected and weighed. No animals developed ascites in this series that was considered as compensated cirrhosis. A sample from the left major liver lobe and the testes was processed for histological examination (fixed in Bouin's solution) and the righ lobe was placed in ice-cold isolation buffer. Bouin-fixed tissues were processed and sections (4 &mgr;m) were stained with haematoxylin and eosin and Masson’s trichrome. Liver cirrhosis was confirmed in all animals treated with CCl4 following the criteria previously reported[1215].
Liver function tests were determined by routine laboratory methods using a Hitachi 747 autoanalyzer (Boehringer Mannheim, Mannheim, Germany).
Lipid peroxidation is a well-established mechanism of cellular injury in animals and is used as an indicator of oxidative stress in cells and tissues. Lipid peroxides are unstable and decompose to form a complex series of compounds including reactive carbonyl compounds.
Polyunsaturated fatty acid peroxides generate malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE) upon decomposition. Measurement of malondialdehyde and 4-hydroxyalkenals have been used as an indicator of lipid peroxidation[26]. The LPO-586 method (Biotech, Oxis International Inc, USA) is designed to assay MDA in combination with 4- hydroxynonenal.
The LPO-586™ assay is based on the reaction of a chromogenic reagent, N-methyl-2-phenylindole, with MDA and 4-HNE at 45°C. One molecule of either MDA or 4-hydroxyalkenal reacts with 2 molecules of reagent N-methyl-2-phenylindole to yield a stable chromophore with maximal absorbance at 586 nm. For simultaneous determination of MDA and 4-HNE, one must use the procedure utilizing methanesulfonic acid (MSA) as the acid solvent.
PCC was determined by the method of Levine et al[27]. The sample was divided into two portions containing 1-2 mg protein each. To one portion, an equal volume of 2 mol/L HCl was added and incubated at room temperature for one hour, and shaken intermittently. The other portion was treated with an equal volume of 10 mmol/L DNPH in 2 mol/L HCl and incubated for one hour at room temperature. After incubation, the mixture was precipitated with 10% TCA and centrifuged. The precipitate was washed with ethanol:ethyl acetate (1:1), twice dissolved in 1 mL of 6 mol/L guanidine HCl, centrifuged at low speed and the supernatant was taken. The difference in absorbance between the DNPH-treated and HCl-treated samples was determined at 366 nm.
Liver mitochondrial fraction was prepared according to the method described by Schneider and Hogeboom with modifications. Liver samples were homogenized (1:10 w/v) in an ice-cold isolation buffer pH 7.4, containing sucrose 0.25 mmol/L, KH2PO4 5 mmol/L, MOPS 5 mmol/L, and 0.1% BSA. The homogenate was centrifuged at 800 g for 10 min. The resulting supernatant was centrifuged at 8500 g for 10 min. The supernatant was discarded and the pellet was diluted in cold isolation buffer and centrifuged at 8500 g for 10 min three times. The final mitochondria pellet was resuspended in a minimal volume and aliquots were stored at -80°C until use in enzyme assays. All procedures were conducted at 4°C.
Mitochondrial membrane potential was evaluated using rhodamine 123 dye (RH123) obtained from Molecular Probes, Inc. (Eugene, OR). It has an absorbance maximum of 500 nm and an emission maximum of 523. Mitochondrial suspensions (40-50 &mgr;g mitochondrial prot/mL) were incubated in isolation buffer for 5 min in the dark with RH123 (0.5 &mgr;g/mL), after adding various agents. For state 4 conditions, mitochondrial samples were incubated with rotenone 25 &mgr;mol/L and energized with sodium succinate 5 mmol/L, and with rotenone 25 &mgr;mol/L, sodium succinate 5 mmol/L + ADP 200 &mgr;mol/L for state 3 conditions. The uncoupler CCCP 10 &mgr;mol/L was added to confirm that the uptake of RH123 was related to mitochondrial membrane potential.
The mitochondrial free radical production was measured by RH123 production, a fluorescent molecule derivated from DihydroRh123 without charge (DHR123) (Molecular Probes Inc.) employing the cytometry method performed by O'Connor[28] with a small modification.
The mitochondrial homogenates (100 &mgr;g/mL) were incubated with DHR123 8.2 nmol/L and radish peroxidase 7 U/mL for 5 min, at room temperature. Fluorescence values from the substrates were normalized with that obtained with peroxidase + uncoupler CCPC. H2O2 1 mmol/L was employed as positive control.
After incubation, the suspensions were analyzed immediately. Gated mitochondrial population was chosen by flow cytometry, based on forward scatter (FS) and side scatter (SS) within mitochondria samples.
Cytofluorometric analysis was performed by using a flow cytometer EPICS XL (Beckman-Coulter, CA). Green fluorescence was detected with a wide band filter for RH123 centred in 525 ± 20 nm (FL1).
The fluorescence intensity of dyes reflecting a minimum of 15 000 individual mitochondria per sample were analyzed in an Epics XL flow cytometer (Beckman-Coulter, CA) using System II version 3.0. Software.
Mitochondrial suspensions were thawed and diluted with potassium phosphate. Activities of the respiratory chain enzymes were measured at 37°C in Cobas Mira (ABXMicro, Alemania). In vitro mitochondrial activities were assessed as follow.
Complex I NADH-ubiquinone oxidoreductase (E.C. 1.6.99.3.): The activity was measured by following the decrease in absorbance due to oxidation of NADH to NAD at 340 nm[29]. The reaction mixture contained potassium phosphate 1.0 mol/L (pH 8), NaN3 0.1 mol/L, EDTA 1 mmol/L, NADH 10 mmol/L, Coenzyme Q1 1 mmol/L. The reaction was initiated by the addition of the mitochondrial suspensions and was monitored for 10 min.
Complex II Succinate-ubiquinone oxidoreductase (E.C. 1.3.5.1.): The activity was measured by following the decrease in absorbance due to coupled reduction of 2,6-dichlorophenolindophenol (DCPIP) at 600 nm. The reaction mixture contained sodium succinate 1.0 mol/L, potassium phosphate 1.0 mol/L (pH 7), 10% Triton X-100, EDTA 1 mmol/L, NADH 10 mmol/L, 0.1% DCPIP, Coenzyme Q2 0.01 mg/mL. The reaction was initiated by the addition of the mitochondrial suspensions and was monitored for 10 min.
Complex III Ubiquinol-ferricytochrome c oxidoreductase (E.C.1.10.2.2.): The activity was measured by following the increase in absorbance due to the reduction ferricytochrome c at 550 nm. The reaction mixture contained sodium succinate 1.0 mol/L, potassium phosphate 0.04 mol/L (pH 7.4), NaN3 0.02 mol/L, EDTA 0.1 mmol/L, 0.1% bovine serum albumin, (CoQ2) H2 0.05 mg/mL. Mitochondrial sample was added and reaction was initiated by the addition of ferricytochrome c 3 mg/mL and was monitored for 2 min. Ferricytochrome c was prepared according to Trounce et al[30].
Complex IV Ferrocytochrome c-oxygen oxidoreductase (E.C.1.9.3.1.): The activity was measured by following the decrease in absorbance due to the oxidation of ferrocytochrome c at 550 nm. The assay mixture consisted of potassium phosphate 0.1 mol/L (pH 7.4) and 1% ferrocytochrome c. The reaction was initiated by the addition of mitochondria and the reaction was monitored for 2 min. Ferrocytochrome c was prepared according to Trounce et al[30].
Complex V ATPase (E.C: 3.6.1.34.): The activity was assayed by coupling the reaction to the pyruvate kinase and lactate dehydrogenase systems and measuring NADH oxidation at 340 nm. The assay system contained Tris-HCL buffer 65 mmol/L (pH 7.5), sucrose 300 mmol/L, MgCl2 4.75 mmol/L, ATP 4 mmol/L, NADH 0.4 mmol/L, phosphoenolpyruvate 0.6 mmol/L, KCN 5 mmol/L, PK 700 U/mL and LDH 1000 U/mL.
Frozen livers (1 g) were homogenized (Ultra-turrax T25; Janke & Kunkel IKA-laboratory) in lysis solution (1% SDS, Tris-HCl 10 mmol/L, EDTA 50 mmol/L, PMSF 1 mmol/L, aprotinin 1 &mgr;g/mL and leupeptin 1 &mgr;g/mL) pH 7.4 at 4°C for 10 min, transferred to Eppendorf tubes and centrifuged at 20 800 g at 4°C for 5 min. Proteins (100 &mgr;g) were separated by 12% SDS-PAGE and transferred to nitrocellulose. The membranes for measuring caspase-3 activation were incubated with anti-caspase-3 (dilution 1:150) rabbit polyclonal antibodies (Santa Cruz Biotechnology, Inc., California, USA) as primary antibodies and anti-rabbit-IgG-alkaline phosphatase (Sigma Chemical Co.) as secondary antibodies using BCIP-NBT as alkaline phosphatase substrate.
TUNEL was performed in deparaffinized 4-&mgr;m-thick sections of liver from the three experimental groups with an ApopTag kit (Oncor Gaithersburg, Maryland) according to the supplier’s instructions. Sections were counterstained with hematoxylin. The number of TUNEL-positive hepatocytes was recorded in the whole preparation (at × 200 magnification). The results were expressed as stained cells per area (arithmetic mean of 4 areas).
Data are expressed as mean ± SEM. Statistical significance was estimated with paired or unpaired t-test as appropriate. A P-value of < 0.05 was considered significant. All analyses were performed by using the SPSS version 10.0. (SPSS Inc, Chicago, USA) statistical package.
At baseline, before the onset of IGF-Itreatment, groups CI and CI + IGF-Ishowed similar serum levels of alanine aminotransferase, aspartate aminotransferase, glucose, cholesterol, alkaline phosphatase, bilirubin, total protein and albumin which were all of them significantly abnormal when compared to those in control rats (Table 1). According to previous data, the biochemical results in this series showed an improvement of liver function tests as it is summarized in Table 1.
| CO, healthy control group | CI, untreated cirrhotic group | CI + IGF-I, cirrhotic rats treated with IGF-I | ||
| Aspartate transaminase (IU/L) | d 0 | 75.0 ± 5.0 | 142.0 ± 35.0 | 150.0 ± 24.0 |
| d 30 | 86.0 ± 4.0 | 168.0 ± 29.0b | 128.0 ± 15.0c | |
| Alanine transaminase (IU/L) | d 0 | 34.0 ± 1.0 | 69.0 ± 10.0b | 73.0 ± 10.0b |
| d 30 | 33.0 ± 2.0 | 58.0 ± 7.0b | 58.0 ± 9.0bc | |
| Alkaline phosphatase (IU/L) | d 0 | 63.0 ± 4.0 | 110.0 ± 14.0a | 124.0 ± 21.0a |
| d 30 | 60.0 ± 2.0 | 100.0 ± 12.0b | 77.0 ± 8.0c | |
| Billirubin (mg/dL) | d 0 | 0.1 ± 0.1 | 0.4 ± 0.2a | 0.4 ± 0.1a |
| d 30 | 0.1 ± 0.0 | 0.3 ± 0.1 | 0.2 ± 0.1c | |
| Cholesterol (mg/dL) | d 0 | 49.0 ± 4.0 | 76.0 ± 5.0b | 80.0 ± 7.0b |
| d 30 | 46.0 ± 2.0 | 61.0 ± 9.0 | 57.0 ± 5.0c | |
| Triglycerides (mg/dL) | d 0 | 151.0 ± 22.0 | 119.0 ± 6.0 | 101.0 ± 11.0 |
| d 30 | 102.0 ± 12.0 | 82.0 ± 11.0 | 70.0 ± 7.0 | |
| Glucose (mmol/L) | d 0 | 11.32 ± 0.63 | 6.97 ± 0.43b | 6.45 ± 0.51b |
| d 30 | 9.27 ± 0.34 | 6.4 ± 0.45b | 7.45 ± 0.32bc | |
| Albumin (g/dL) | d 0 | 3.6 ± 0.1 | 3.4 ± 0.1 | 3.4 ± 0.1 |
| d 30 | 3.8 ± 0.1 | 3.5 ± 0.2 | 3.7 ± 0.1 | |
| Total proteins (mmol/L) | d 0 | 6.9 ± 0.1 | 6.6 ± 0.2 | 6.5 ± 0.1 |
| d 30 | 6.8 ± 0.8 | 6.2 ± 0.3 | 6.6 ± 0.1 |
In agreement with previous results, a reduction of fibrosis (Figure 1) and liver oxidative products were also observed in CI-IGF group: 4-hydroxynonenal, CO = 0.64 ± 0.05; CI = 0.78 ± 0.03; CI + IGF-I= 0.73 + 0.04 (nmol/mg prot.); Protein carboxyl content (nmol/mg prot.): CO = 6.48 ± 0.71; CI = 8.15 ± 0.60; CI + IGF-I= 6.80 ± 0.64 (P < 0.05 CI vs CO), although the reduction was less notable than in previous studies because in this series (see Methods) cirrhotic animals received CCl4 doses also during the 4 wk of treatment (with IGF-Ior saline).
Gated mitochondrial population was chosen by flow cytometry (FCM), based on forward scatter (FS) and side scatter (SS) within mitochondria samples.
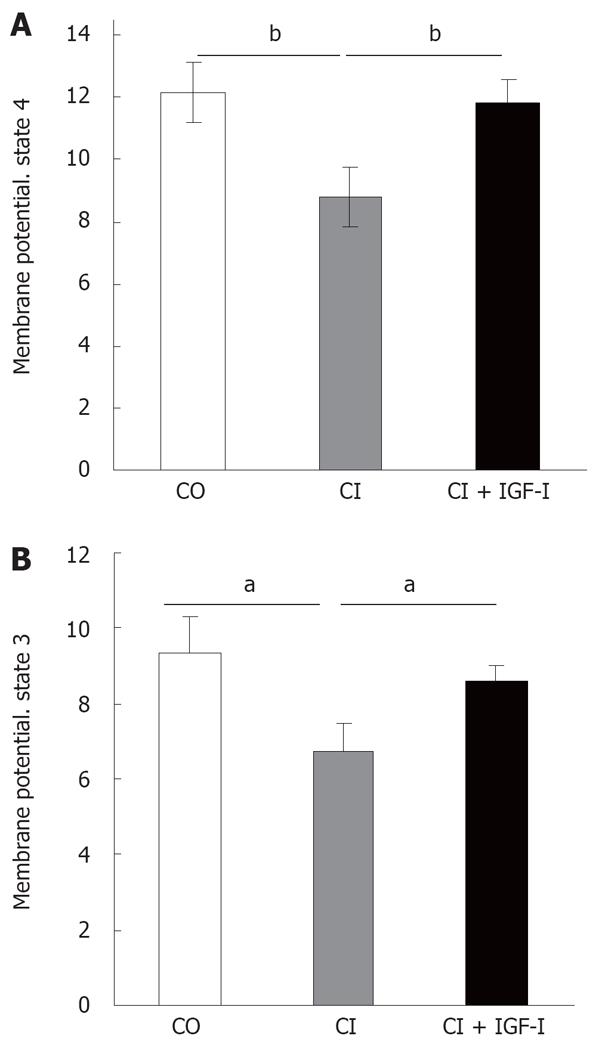
The membrane potential was monitored by fluorescence quenching of RH123 in mitochondria from liver rats under respiratory state 4. Under these conditions, the mitochondrial membrane potential is an index of mitochondrial energy status. Figure 2A shows that the membrane potential in state 4 was significantly decreased in the CI animals compared to control, and was restored upon IGF-Itreatment (CO, healthy controls = 12.18 ± 0.96, CI, untreated cirrhotic rats = 8.83 ± 0.88, CI + IGF-I, cirrhotic rats treated with IGF-I= 11.80 ± 0.85 Fluorescence Arbitrary Units -FAU-).
In a similar way, in the state 3 (addition of ADP as substrate), expressed as fluorescence arbitrary units, both the control rats and the CI + IGF-Irats showed significantly higher values than untreated cirrhotics rats treatment (CO = 9.33 ± 0.97, CI = 6.71 ± 0.58, CI + IGF-I= 8.62 ± 0.45 FAU) (Figure 2B).
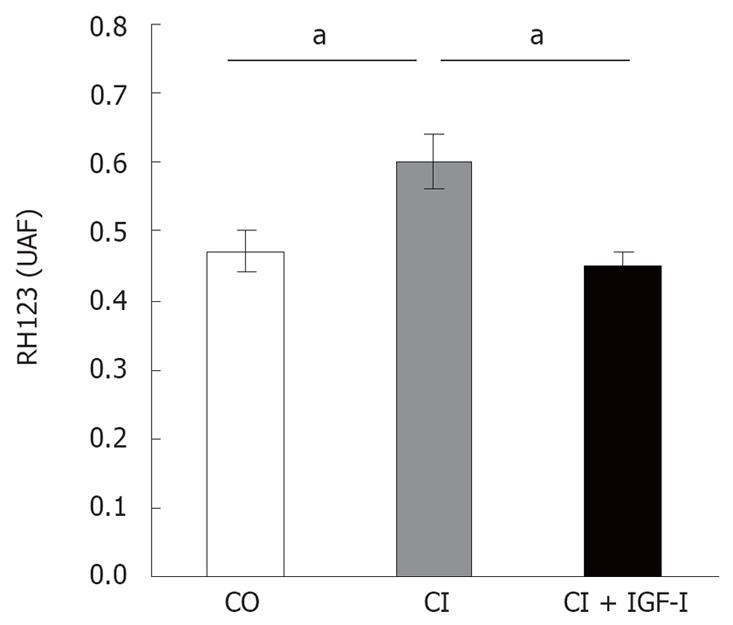
Mitochondria from untreated cirrhotic rats showed a significant increase in ROS generation (CI = 0.58 ± 0.03 FAU, P < 0.05 vs CO) when compared to controls (CO = 0.47 ± 0.02 FAU) and cirrhotic rats treated with IGF-I(CI + IGF-I= 0.45 ± 0.01 FAU, P < 0.05 vs CI): see Figure 3.
Table 2 summarizes the activities of the electron transport complexes in mitochondria from the three experimental groups. As shown in Table 2, activities of complexIand III increased significantly in cirrhotic animals. This increase was significantly higher in the CI + IGF-Igroup. Therefore, activity of complex IV was slightly increased in CI and CI + IGF-Igroups.
| CO, healthy control group | CI, untreated cirrhotic group | CI + IGF, cirrhotic rats treated with IGF-I | |
| I-NADH-ubiquinone oxidoreductase (nmol/min per mg prot) | 7.68 ± 2.19 | 11.01 ± 1.60c | 16.10 ± 2.03 |
| II-Succinate-ubiquinone oxidoreductase (&mgr;mol/min per mg prot) | 0.38 ± 0.02 | 0.33 ± 0.04 | 0.35 ± 0.03 |
| III-Ubiquinol-ferricytochrome oxidoreductase (nmol/min per mg prot) | 89.57 ± 6.54 | 148.75 ± 23.26a | 163.15 ± 21.38b |
| IV-Ferrocytochrome c-oxygen oxidoreductase (pmol/min per mg prot) | 3.91 ± 0.85 | 5.22 ± 1.01 | 5.66 ± 0.97 |
By contrast, complex II activity was slightly decreased in cirrhotic rats when compared to control group.
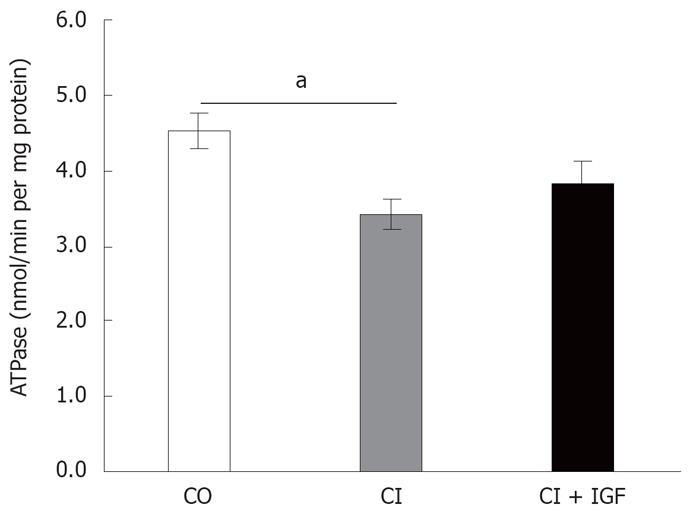
As shown in Figure 4, ATPase activity declined in untreated cirrhotics rats. However, there was no significant difference between the CO and CI + IGF-Igroups in complex V activity treatment (CO = 4.52 ± 0.23, CI = 3.43 ± 0.21, CI + IGF-I= 3.83 ± 0.31 nmol/min per mg prot).
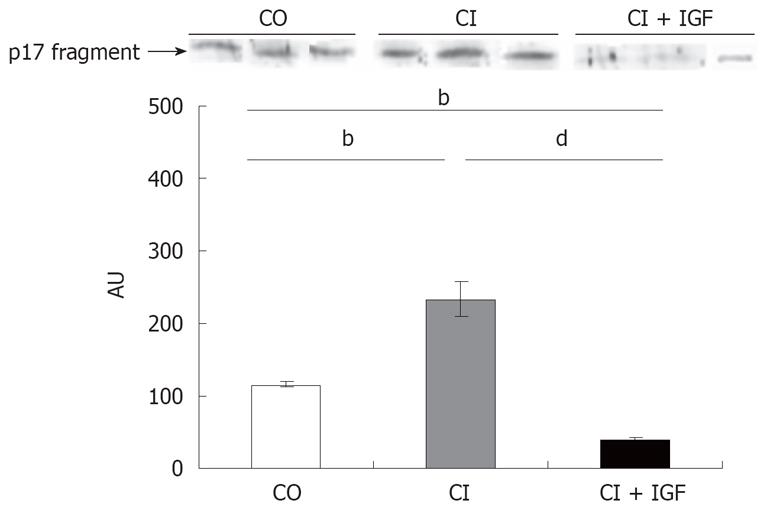
Western blotting for fragment-17 of caspase 3 showed a significant increase of caspase 3 activation in untreated cirrhotic rats when compared to healthy controls (Figure 5). However a notable reduction in the expression of this fragment was observed in cirrhotic animals treated with IGF-I.
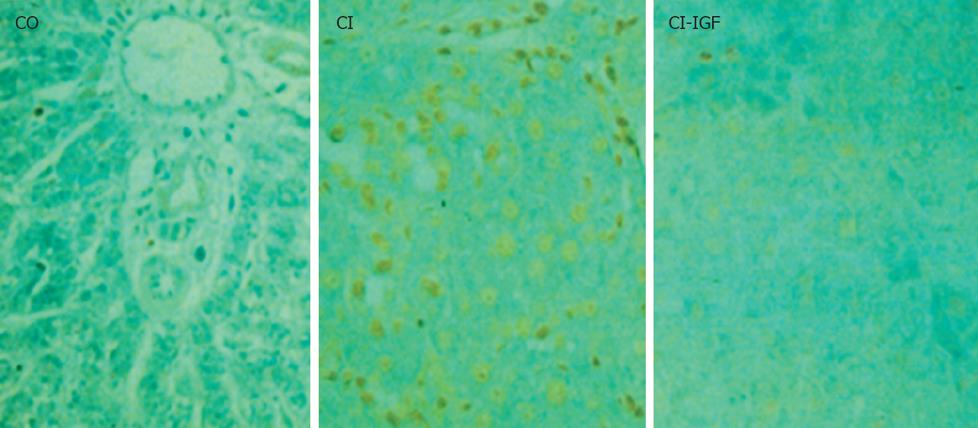
The number of TUNEL-positive hepatocytes was significant increased in untreated cirrhotic group as compared to controls. IGF-Itreatment reduced apoptosis in hepatocytes as shown in Figure 6 and Table 3.
This study analyzes the effect of IGF-Ion mitochondrial damage, caspase 3 activation and hepatocyte apoptosis in rats with CCl4-induced cirrhosis. In previous studies, we have shown that low doses of IGF-Iimproved liver function and reduced oxidative liver injury and fibrosis, suggesting a mitochondrial protection[121415]. The mechanisms of this hepatoprotection are understood only partially; but, data in this paper give some insight.
When compared to healthy rats, untreated cirrhotic rats showed altered liver function tests and increased hepatic lipid peroxidation and protein carbonyl content (PCC) which was -in agreement with previous data[121415] and improved with IGF-Isubstitution.
The present work shows that mitochondrial dysfunction in rats with CCl4-induced cirrhosis is characterized by a significant depletion of the mitochondrial membrane potential (MMP), a reduction of ATPase activity, and a significant increase of intramitochondrial ROS generation.
In addition, these results indicate that the activities in vitro (in optimal conditions) of complexIand III were highest in cirrhotic animals when compared to control animals. It would be an adaptive mechanism as in fact it is not possible to produce a normal proton gradient (Figure 2) in untreated cirrhotic rats. The increased mitochondrial production of O2· at complexesIand III and consequently of H2O2 is a common finding in oxidative mitochondrial damage[3132]. Uncontrolled mitochondrial formation of ROS promotes the activation of the mitochondrial membrane permeability transition[31].
Several research groups have tried to characterize the effect of chronic ethanol consumption and CCl4-induced liver injury showing morphological alterations in mitochondria and deficient substrate oxidation, ADP phosphorylation, cytochrome c and enzyme content[163133–37]. On the other hand, other authors have concluded that in chronic CCl4-treated rats, liver mitochondria are morphologically and functionally intact[38]. In the present work, the described alteration in mitochondrial function occurred at least after 16 wk of CCl4 exposure.
The major finding of this work is that mitochondrial dysfunction leading to hepatocyte apoptosis is improved by IGF-Itherapy. These results give new evidence of the hepatoprotective properties of IGF-Iin experimental cirrhosis[1215].
In the present work, oxygen consumption by isolated mitochondria was not assessed and consequently proton leak data (oxygen consumption/MMP) are not available. However, the observed reduction of MMP with an increased generation of ROS suggests that oxygen is wasted by damaged mitochondria producing H2O2 instead of a normal proton gradient, driving force of ATP synthesis through the proton pumping F1F0 adenosine triphosphate synthase (ATP synthase)[31].
The described mitochondrial dysfunction in this paper is corrected by IGF-Itherapy since mitochondria from IGF-Itreated cirrhotic rats showed a normal MMP and a recovered ATPase activity resulting in increased activity of complexIand III, but reduced ROS generation. Taken together, all of these data suggest an extramitochondrial protection of mitochondria by IGF-I.
Previously, we have reported that IGF-Itreatment restored the expression of several protease inhibitors such a alpha-1-antichymotrypsin, the serine protease inhibitor 2[13], that could contribute to the described mitochondrial protection. Further studies are necessary to elucidate all mechanisms involved in the hepatoprotection induced by IGF-I. In the previously mentioned work, we provided evidence that IGF-Itreatment in cirrhotic rats modulates gene expression, reverting the global hypomethylation of the genomic DNA observed in cirrhosis[13].
On the other hand, the mitochondrial dysfunction in the present study was associated with an overexpression of the active fragment of caspase 3 and an increased number of TUNEL positive hepatocytes in untreated cirrhotic rats. In accordance with the improvement of the mitochondrial function described in CI + IGF-Igroup, IGF-Itreated animals showed a significant reduction of caspase 3 activation and apoptosis of hepatocytes.
In recent years, mitochondria have been recognized as regulators of cell death[3940]. Cellular dysfunction induced by intra- or extracellular insults converge on mitochondria and induce a sudden increase in permeability on the inner mitochondrial membrane, the so-called mitochondrial membrane permeability transition (MMPT). MMPT is caused by the opening of pores in the inner mitochondrial membrane, matrix swelling and outer membrane rupture. The MMPT is an endpoint to initiate cell death and a putative target for cellular protection since MMPT and the release of mitochondrial cytochrome c activate the apoptotic pathway by which initiator caspases (i.e. caspase 8 and 9) are converted to their active forms, which in turn activate downstream effector caspases (i.e. caspases 3, 6 and 7). Finally, cellular targets of the effector caspases include endonucleases and cytoskeletal proteins[41].
These results show that IGF-Iinduces cell resistance to apoptosis by oxidative stress through mitochondrial protection. Mitochondria seem to be the main cellular targets of IGF-Iaction. Likewise, our data are in agreement with the observation that the effect of serum withdrawal on the autophagy of dysfunctional mitochondria is prevented by the addition of IGF-I[42].
Accordingly, it has been reported that IGF-Iinhibited the reduction of MMP, cytochrome c release, caspase 3 activity and apoptosis in several cell lines and experimental procedures[43–45].
The described improvement of mitochondrial function in cirrhotic rats by IGF-Itherapy resulted in an increment of the ATP synthesis. Interestingly, a recent trial with cirrhotic patients showed that the IGF-Itherapy induced an improvement in albumin and metabolism increasing resting energy expenditure (REE) (kcal/d)[46]. Although the mechanism by which IGF-Iinfluences REE in cirrhotic patients remains to be clarified, it could be related with an increment of ATP availability after IGF-Itherapy.
In conclusion, these results show that IGF-Iexerts a mitochondrial protection in experimental cirrhosis leading to reduced caspase activation and apoptosis and increasing ATP production. This work provides new evidence of the beneficial effect of IGF-Isupplementation in experimental liver cirrhosis and experimental basis for further studies at exploring the potential of IGF-Iin the treatment of human cirrhosis.
Liver cirrhosis is a condition of “IGF-Ideficiency”. The endogenous administration of low doses of insulin-like growth factorI(IGF-I) exerts a mitochondrial protection in experimental cirrhosis leading to reduced apoptosis and increased ATP production.
Liver cirrhosis. Antioxidant therapies. New strategies in chronic liver diseases. Axis GH/IGF-Iin liver cirrhosis.
Mitochondrial dysfunction in cirrhosis. Mitochondrial protection induced by IGF-Iat low doses.
This work provides new evidence of the beneficial effect of IGF-Isupplementation in experimental liver cirrhosis and experimental basis for further studies at exploring the potential of IGF-Iin the treatment of human cirrhosis.
This paper provides evidence in order to give a better insight into the mechanisms by which IGF-Iexerts an hepatoprotective action on damaged liver and demonstrates that mitochondria are one of the main cellular targets of IGF-I. This study contributes to the characterization of mitochondrial dysfunction in experimental liver cirrhosis and shows the beneficial effects on damaged mitochondria leading to cellular protection induced by IGF-Itherapy.
| 1. | Sara VR, Hall K. Insulin-like growth factors and their binding proteins. Physiol Rev. 1990;70:591-614. |
| 2. | Caufriez A, Reding P, Urbain D, Golstein J, Copinschi G. Insulin-like growth factor I: a good indicator of functional hepatocellular capacity in alcoholic liver cirrhosis. J Endocrinol Invest. 1991;14:317-321. |
| 3. | Hattori N, Kurahachi H, Ikekubo K, Ishihara T, Moridera K, Hino M, Saiki Y, Imura H. Serum growth hormone-binding protein, insulin-like growth factor-I, and growth hormone in patients with liver cirrhosis. Metabolism. 1992;41:377-381. |
| 4. | Picardi A, de Oliveira AC, Muguerza B, Tosar A, Quiroga J, Castilla-Cortazar I, Santidrian S, Prieto J. Low doses of insulin-like growth factor-I improve nitrogen retention and food efficiency in rats with early cirrhosis. J Hepatol. 1997;26:191-202. |
| 5. | Castilla-Cortazar I, Prieto J, Urdaneta E, Pascual M, Nunez M, Zudaire E, Garcia M, Quiroga J, Santidrian S. Impaired intestinal sugar transport in cirrhotic rats: correction by low doses of insulin-like growth factor I. Gastroenterology. 1997;113:1180-1187. |
| 6. | Castilla-Cortazar I, Picardi A, Tosar A, Ainzua J, Urdaneta E, Garcia M, Pascual M, Quiroga J, Prieto J. Effect of insulin-like growth factor I on in vivo intestinal absorption of D-galactose in cirrhotic rats. Am J Physiol. 1999;276:G37-G42. |
| 7. | Castilla-Cortazar I, Garcia M, Quiroga J, Diez N, Diez-Caballero F, Calvo A, Diaz M, Prieto J. Insulin-like growth factor-I reverts testicular atrophy in rats with advanced cirrhosis. Hepatology. 2000;31:592-600. |
| 8. | Pascual M, Castilla-Cortazar I, Urdaneta E, Quiroga J, Garcia M, Picardi A, Prieto J. Altered intestinal transport of amino acids in cirrhotic rats: the effect of insulin-like growth factor-I. Am J Physiol Gastrointest Liver Physiol. 2000;279:G319-G324. |
| 9. | Cemborain A, Castilla-Cortazar I, Garcia M, Quiroga J, Muguerza B, Picardi A, Santidrian S, Prieto J. Osteopenia in rats with liver cirrhosis: beneficial effects of IGF-I treatment. J Hepatol. 1998;28:122-131. |
| 10. | Cemborain A, Castilla-Cortazar I, Garcia M, Muguerza B, Delgado G, Diaz-Sanchez M, Picardi A. Effects of IGF-I treatment on osteopenia in rats with advanced liver cirrhosis. J Physiol Biochem. 2000;56:91-99. |
| 11. | Castilla-Cortazar I, Aliaga-Montilla MA, Salvador J, Garcia M, Delgado G, Gonzalez-Baron S, Quiroga J, Prieto J. Insulin-like growth factor-I restores the reduced somatostatinergic tone controlling growth hormone secretion in cirrhotic rats. Liver. 2001;21:405-409. |
| 12. | Castilla-Cortazar I, Garcia M, Muguerza B, Quiroga J, Perez R, Santidrian S, Prieto J. Hepatoprotective effects of insulin-like growth factor I in rats with carbon tetrachloride-induced cirrhosis. Gastroenterology. 1997;113:1682-1691. |
| 13. | Mirpuri E, Garcia-Trevijano ER, Castilla-Cortazar I, Berasain C, Quiroga J, Rodriguez-Ortigosa C, Mato JM, Prieto J, Avila MA. Altered liver gene expression in CCl4-cirrhotic rats is partially normalized by insulin-like growth factor-I. Int J Biochem Cell Biol. 2002;34:242-252. |
| 14. | Garcia-Fernandez M, Castilla-Cortazar I, Diaz-Sanchez M, Navarro I, Puche JE, Castilla A, Casares AD, Clavijo E, Gonzalez-Baron S. Antioxidant effects of insulin-like growth factor-I (IGF-I) in rats with advanced liver cirrhosis. BMC Gastroenterol. 2005;5:7. |
| 15. | Muguerza B, Castilla-Cortazar I, Garcia M, Quiroga J, Santidrian S, Prieto J. Antifibrogenic effect in vivo of low doses of insulin-like growth factor-I in cirrhotic rats. Biochim Biophys Acta. 2001;1536:185-195. |
| 16. | Bailey SM, Cunningham CC. Contribution of mitochondria to oxidative stress associated with alcoholic liver disease. Free Radic Biol Med. 2002;32:11-16. |
| 17. | Bailey SM, Pietsch EC, Cunningham CC. Ethanol stimulates the production of reactive oxygen species at mitochondrial complexes I and III. Free Radic Biol Med. 1999;27:891-900. |
| 18. | Kwong LK, Sohal RS. Age-related changes in activities of mitochondrial electron transport complexes in various tissues of the mouse. Arch Biochem Biophys. 2000;373:16-22. |
| 19. | Kowaltowski AJ, Vercesi AE. Mitochondrial damage induced by conditions of oxidative stress. Free Radic Biol Med. 1999;26:463-471. |
| 20. | Cardoso SM, Pereira C, Oliveira R. Mitochondrial function is differentially affected upon oxidative stress. Free Radic Biol Med. 1999;26:3-13. |
| 21. | Kaplowitz N, Tsukamoto H. Oxidative stress and liver disease. Prog Liver Dis. 1996;14:131-159. |
| 22. | Kaplowitz N. Biochemical and cellular mechanisms of toxic liver injury. Semin Liver Dis. 2002;22:137-144. |
| 23. | Kiningham KK, Oberley TD, Lin S, Mattingly CA, St Clair DK. Overexpression of manganese superoxide dismutase protects against mitochondrial-initiated poly(ADP-ribose) polymerase-mediated cell death. FASEB J. 1999;13:1601-1610. |
| 24. | Cortopassi GA, Wong A. Mitochondria in organismal aging and degeneration. Biochim Biophys Acta. 1999;1410:183-193. |
| 25. | The Guiding Principles for Research Involving Animals. National Academy of Sciences. The National Institute of Health -NIH. 1991;. |
| 26. | Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81-128. |
| 27. | Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464-478. |
| 28. | O’Connor JE, Vargas JL, Kimler BF, Hernandez-Yago J, Grisolia S. Use of rhodamine 123 to investigate alterations in mitochondrial activity in isolated mouse liver mitochondria. Biochem Biophys Res Commun. 1988;151:568-573. |
| 29. | Hatefi Y, Haavik AG, Griffiths DE. Studies on the electron transfer system. XL. Preparation and properties of mitochondrial DPNH-coenzyme Q reductase. J Biol Chem. 1962;237:1676-1680. |
| 30. | Trounce I, Byrne E, Dennett X, Chen WW, Marzuki S. Affinity chromatography isolation of human cytochrome oxidase and small-scale Western immunoblot probing of the enzyme complex in mitochondrial cytopathy patients. Biochem Med Metab Biol. 1991;46:17-27. |
| 31. | Hoek JB, Cahill A, Pastorino JG. Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology. 2002;122:2049-2063. |
| 32. | Sastre J, Serviddio G, Pereda J, Minana JB, Arduini A, Vendemiale G, Poli G, Pallardo FV, Vina J. Mitochondrial function in liver disease. Front Biosci. 2007;12:1200-1209. |
| 33. | Jelski W, Chrostek L, Szmitkowski M. [Biochemical basic of alcoholic liver injury]. Pol Merkur Lekarski. 2006;21:376-380. |
| 34. | Gramenzi A, Caputo F, Biselli M, Kuria F, Loggi E, Andreone P, Bernardi M. Review article: alcoholic liver disease--pathophysiological aspects and risk factors. Aliment Pharmacol Ther. 2006;24:1151-1161. |
| 35. | Shukla SD, Aroor AR. Epigenetic effects of ethanol on liver and gastrointestinal injury. World J Gastroenterol. 2006;12:5265-5271. |
| 36. | Cunningham CC, Bailey SM. Ethanol consumption and liver mitochondria function. Biol Signals Recept. 2001;10:271-282. |
| 37. | Albano E. Alcohol, oxidative stress and free radical damage. Proc Nutr Soc. 2006;65:278-290. |
| 38. | Krahlenbuhl S, Reichen J, Zimmermann A, Gehr P, Stucki J. Mitochondrial structure and function in CCl4-induced cirrhosis in the rat. Hepatology. 1990;12:526-532. |
| 39. | Bras M, Queenan B, Susin SA. Programmed cell death via mitochondria: different modes of dying. Biochemistry (Mosc). 2005;70:231-239. |
| 40. | Tsujimoto Y. Cell death regulation by the Bcl-2 protein family in the mitochondria. J Cell Physiol. 2003;195:158-167. |
| 41. | Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383-424. |
| 42. | Gu Y, Wang C, Cohen A. Effect of IGF-1 on the balance between autophagy of dysfunctional mitochondria and apoptosis. FEBS Lett. 2004;577:357-360. |
| 43. | Leinninger GM, Russell JW, van Golen CM, Berent A, Feldman EL. Insulin-like growth factor-I regulates glucose-induced mitochondrial depolarization and apoptosis in human neuroblastoma. Cell Death Differ. 2004;11:885-896. |
| 44. | Kondo T, Kitano T, Iwai K, Watanabe M, Taguchi Y, Yabu T, Umehara H, Domae N, Uchiyama T, Okazaki T. Control of ceramide-induced apoptosis by IGF-1: involvement of PI-3 kinase, caspase-3 and catalase. Cell Death Differ. 2002;9:682-692. |
| 45. | Ness JK, Scaduto RC Jr, Wood TL. IGF-I prevents glutamate-mediated bax translocation and cytochrome C release in O4 + oligodendrocyte progenitors. Glia. 2004;46:183-194. |
| 46. | Conchillo M, de Knegt RJ, Payeras M, Quiroga J, Sangro B, Herrero JI, Castilla-Cortazar I, Frystyk J, Flyvbjerg A, Yoshizawa C. Insulin-like growth factor I (IGF-I) replacement therapy increases albumin concentration in liver cirrhosis: results of a pilot randomized controlled clinical trial. J Hepatol. 2005;43:630-636. |