Published online May 7, 2008. doi: 10.3748/wjg.14.2715
Revised: March 11, 2008
Published online: May 7, 2008
AIM: To assess the economics of various chemotherapeutic regimens for advanced gastric cancer (AGC), and to select the best cost-effective regimen for the common Chinese patients.
METHODS: Data source used in this study was the Chinese Biomedical Disk Database. Patients were diagnosed as AGC and any regimen was eligible. Outcome measures included median survival time (MST) and percentage of complete and partial response (CR+PR). Economic statistics was per capita direct medical cost (DMC) of a single cycle. TreeAge Pro Healthcare 2007 software was used to carry out cost-effectiveness and incremental cost-effectiveness analysis. Sensitivity analyses were applied by altering willingness-to-pay and annual discount rate, and also re-analyzed by excluding the studies with apparent heterogeneity.
RESULTS: Seven retrospective economics studies on 760 patients were included. 5-fluorouracil-based regimens were universal, and also some new agents were involved, such as docetaxel, paclitaxel, and oxaliplatin. By processing analysis, we could recommend etoposide, leucovorin and 5-fluorouracil (ELF) regimen as preference, with a DMC/MST ratio of 2543 RBM/11.7 mo and a DMC/CR+PR ratio of 2543 RMB/53.3%. Uracil-tegafur, etoposide and cisplatin (FEP) or 5-fluorouracil, adrimycin/epirubin and mitomycin (FAM) regimens could be regarded as optional first-line chemotherapy for AGC in common Chinese patients. With no regard for willingness-to-pay, the docetaxel, cisplatin and 5-fluorouracil (DCF) regimen could be chosen as either a first- or a second-line chemotherapy, with a DMC/CR+PR ratio of 9979 RMB/56.3%.
CONCLUSION: 5-fluorouracil regimens are still considered the mainstream for AGC, while new agents such as taxanes are optional. More randomized clinical trials are required before any mandatory recommendation of certain regimens for patients with AGC in China is made.
- Citation: Chen XZ, Jiang K, Hu JK, Zhang B, Gou HF, Yang K, Chen ZX, Chen JP. Cost-effectiveness analysis of chemotherapy for advanced gastric cancer in China. World J Gastroenterol 2008; 14(17): 2715-2722
- URL: https://www.wjgnet.com/1007-9327/full/v14/i17/2715.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2715
Gastric cancer is the fourth most common malignancy worldwide, and China is one of the countries with a high incidence of the disease[1]. It is one of the most common causes for cancer mortality and leads to approximately 160 000 deaths annually in China[2]. Surgery remains the only established curative treatment for this disease in resectable stages[3]. However, about 84% of patients with gastric cancer will have advanced disease, and their median survival time (MST) is only 3-4 mo if they are not treated with chemotherapy[4]. Chemotherapy for advanced gastric cancer (AGC) can improve either time-to-progression or MST, and are well tolerated[4]. However, recommended regimens are still controversial since their survival benefit appears marginal in some studies[3]. Additionally, in China, a developing country with a huge population, oncologists need to know which regimen is the best cost-effective for the common Chinese patients. The present study was to review the Chinese literature and to make an economic assessment of various regimens for AGC.
We searched the Chinese Biomedical Disk Database (CBMdisc) as the data source. The search strategy was (“gastric cancer” OR “gastric carcinoma” OR “gastric adenocarcinoma”) AND (“economics” OR “cost-effectiveness” OR “CEA”). There was no limitation to publication year. Two reviewers (Chen XZ and Jiang K) carried out selection and assessment independently.
Either prospective or retrospective controlled studies were eligible; but, case serial reports were excluded. Patients were diagnosed having gastric cancer by gastric endoscopy and biopsy or postoperative pathological test. The staging system used was either UICC or Japanese TNM classification. Patients with early gastric cancer (stage I) were excluded. Any regimen of chemotherapy was acceptable for the present analysis. All patients should receive at least two cycles of chemotherapy. Surgery was either resection or explorative laparotomy. Any other therapies were ineligible, such as radiotherapy and biotherapy. The MST (month) was used as the primary outcome measure, or/and percentage of complete and partial response (CR+PR, %) as the secondary outcome measure. The economic parameter and direct medical cost (DMC, RMB) of a single cycle should be available from literature. The criteria for CR/PR were the standard World Health Organization (WHO) tumor response criteria. DMC contained the expenditure of drugs, tests, treatment, nursing and hospitalization.
Basic information about studies was extracted by two reviewers (Chen XZ and Jiang K) independently, including publication year, city of the hospital, sample and demography, and details of regimens. The effectiveness data contained percentage of CR+PR and length of MST. The percentage of CR+PR was numerical data, and was accumulated as 100 × (Σni)/(ΣNi). The length of MST was parameter data, and was synthesized as [Σ(xiNi)]/(ΣNi). The abbreviations were respectively shown as (n) = number of patients reaching CR or PR criteria in any regimen of individual study, (N) = number of patients undertaking any regimen of individual study, (χ) = months of MST in any regimen of individual study, and (i) = number of included studies for any regimen. The economic data were DMC, and synthesized as [Σ(yiNi)]/(ΣNi). The abbreviations were shown as (y) = per capita DMC (¥, RMB) in a single cycle of any regimen of individual study, all of which were converted to the 2007 price by discount rate. The annual discount rate was assumed as 1% initially, and (n), (N) and (i) were the same as in the above formulas.
TreeAge Pro Healthcare 2007 software was used in modeling and analyses. Both cost-effectiveness analysis and incremental cost-effectiveness analysis were considered, with C/E ratio and incremental C/E (ΔC/ΔE) ratio calculated, respectively. The regimen of the lowest cost was selected as the common baseline for other regimens to refer to. For the thresholds, we enacted the expected MST as ≥ 9 mo, CR+PR percentage as ≥ 50%, and willingness-to-pay (WTP) for a single cycle as ≤ 3000 RMB (DMC/MST ratio ≤ 333.3 RMB, and DMC/CR+PR ratio ≤ 60 RMB). Once uncertainty was met in decision making, sensitivity analysis was carried out by alternating the willing-to-pay (WTP) from 1000 RMB to 10 000 RMB, or the discount rate from 1% to 10%, or even excluding the studies with a potential heterogeneity.
Seven studies on 760 patients were included in the present analysis[5–11]. All the included studies were retrospective case-control cost-effectiveness analyses. The detailed information is listed in Table 1. Eleven regimens were involved, eight of which were 5-fluorouracil-based and two were uracil-tegafur-based. One study included stages II and III mainly[5]. The components of reported DMC were different to some extent. One study, on capecitabine and cisplatin regimen versus oxaplatin and 5-fluorouracil regimen, was excluded for only overall cost of all cycles retrieved, but per capita DMC of a single cycle[12].
| Study and publication (yr) | Area of China | Design | Sample | Weight (%) | Regimens | Staging | Operation | Outcome measures | Median follow-up period | |||||
| I | II | III | IV | Effectiveness | Economics | |||||||||
| Dong[5] | Western-northern | Case-control study | 44 | 5.8 | Two cycles at least, and 3-4 wk for one cycle. | FAM | 0 | 7 | 9 | 0 | UR | (1) CR+PR (WHO tumor response criteria) (2) Median survival time | DMC contained: (1) Drugs (2) Tests (3) Treatment for ADR | UR |
| EAP | 0 | 5 | 6 | 2 | ||||||||||
| ELF | 0 | 10 | 4 | 1 | ||||||||||
| Yu[6] | Eastern-southern | Case-control study | 41 | 5.4 | Four cycles totally, and 28 d for one cycle. | FAM | 0 | 0 | 5 | 10 | None | (1) CR+PR (WHO tumor response criteria) (2) Median survival time | DMC contained: (1) Drugs (2) Treatment for ADR | UR |
| EAP | 0 | 0 | 3 | 8 | ||||||||||
| ELF | 0 | 0 | 5 | 9 | ||||||||||
| Ding[7] | Eastern-southern | Case-control study | 49 | 6.4 | Two cycles at least, and 3-4 wk for one cycle. | FAM | 0 | 1 | 8 | 9 | None | (1) CR+PR (WHO tumor response criteria) (2) Median survival time | DMC contained: (1) Drugs (2) Treatment for ADR | UR |
| EAP | 0 | 1 | 5 | 9 | ||||||||||
| ELF | 0 | 2 | 8 | 6 | ||||||||||
| Qian[8] | Eastern-southern | Case-control study | 226 | 29.7 | Two cycles at least, and 3 wk for one cycle. | FAM | Almost composed of stage IV as reported. | Unresectable disease, or palliative operation, or recurrence/metastases after curative operation. | (1) CR+PR (WHO tumor response criteria) (2) Median survival time | DMC contained: (1) Drugs (2) Treatment (3) Fee for berth | UR | |||
| UFTM | ||||||||||||||
| FEP | ||||||||||||||
| LFP/M | ||||||||||||||
| Yang[9] | Eastern-northern | Case-control study | 256 | 33.7 | Three cycles at least, and 3 wk for one cycle. | FAMTX | All unresectable newly diagnosed patients. | None | (1) CR+PR (2) Median survival time | DMC contained: (1) Drugs (2) Treatment for ADR | 26.9 mo | |||
| ECF | ||||||||||||||
| Zhou[10] | Eastern-southern | Case-control study | 48 | 6.3 | Two cycles at least, and 4 wk for one cycle. | TCF | UR | UR | (1) CR+PR (WHO tumor response criteria) (2) Time-to-progression | DMC contained: (1) Drugs (2) Tests (3) Treatment | UR | |||
| FOLFOX | ||||||||||||||
| Liu[11] | Eastern-southern | Case-control study | 96 | 12.6 | All six cycles. | DCF | 0 | 0 | 30 | 2 | UR | CR+PR (Chinese association of gastric cancer response criteria) | DMC contained: (1) Drugs (2) Treatment (3) Fee for berth | UR |
| ECF | 0 | 0 | 29 | 1 | ||||||||||
| FOLFOX | 0 | 0 | 31 | 3 | ||||||||||
The primary data about the clinical and economic outcomes were retrieved (Table 2), and the data were synthesized according to the method specified before (Table 3). The data about the overall survival were not enough to be analyzed. The MST of each regimen ranged from 6.1 to 11.7 mo, and CR+PR percentage from 21.0% to 56.3%. However, the MST data about the TCF, DCF and FOLFOX regimens with new chemotherapeutic agents were not reported. The per capita DMC of a single cycle ranged from 1756.95 RMB to 9979.00 RMB.
| Regimens studies | N | CR+PR | MST | DMC | |
| n | % | (mo) | (¥, RMB) | ||
| 5FU+ADM/EPI+MMC (FAM) | |||||
| Dong[5] | 16 | 7 | 43.8 | 6.0 | 2439.78 |
| Yu[6] | 15 | 6 | 40.0 | 13.5 | 1965.00 |
| Ding[7] | 18 | 7 | 38.9 | 11.5 | 1888.50 |
| Qian[8] | 35 | 12 | 34.3 | 11.5 | 1509.78 |
| DDP+VP-16+ADM/EPI (EAP) | |||||
| Dong[5] | 13 | 7 | 53.8 | 8.0 | 2952.42 |
| Yu[6] | 12 | 6 | 50.0 | 9.0 | 2705.00 |
| Ding[7] | 15 | 8 | 53.3 | 8.5 | 2170.00 |
| 5FU+VP-16+CF (ELF) | |||||
| Dong[5] | 15 | 7 | 46.7 | 9.0 | 3823.05 |
| Yu[6] | 14 | 8 | 57.1 | 13.0 | 1640.00 |
| Ding[7] | 1 | 9 | 56.3 | 13.0 | 1602.00 |
| UFT+MMC (UFTM) | |||||
| Qian[8] | 49 | 16 | 32.6 | 8.5 | 2322.80 |
| UFT+DDP+VP-16 (FEP) | |||||
| Qian[8] | 51 | 24 | 47.9 | 10.0 | 1902.90 |
| 5FU+CF+DDP+MMC (LFP/M) | |||||
| Qian[8] | 91 | 40 | 44.0 | 9.0 | 2907.76 |
| 5FU+CF+ADM+MTX (FAMTX) | |||||
| Yang[9] | UR | UR | 21.0 | 6.1 | 1705.28 |
| 5FU+EPI+DDP (ECF) | |||||
| Yang[9] | UR | UR | 46.0 | 8.7 | 1526.67 |
| Liu[11] | 30 | 12 | 40.0 | UR | 4158.00 |
| 5FU+DDP+PTX (TCF) | |||||
| Zhou[10] | 22 | 11 | 50.0 | UR | 2640.60 |
| 5FU+CF+DXL+DDP (DCF) | |||||
| Liu[11] | 32 | 18 | 56.3 | UR | 9979.00 |
| 5FU+CF+L-OHP (FOLFOX) | |||||
| Zhou[10] | 26 | 12 | 46.1 | UR | 1588.99 |
| Liu[11] | 34 | 18 | 52.9 | UR | 4498.00 |
| Regimens | Accumulated | Synthesized | Accumulated | Synthesized |
| n | MST (mo) | CR+PR (%) | DMC1 (¥, RMB) | |
| 5FU+ADM/EPI+MMC (FAM) | 84 | 10.8 | 38.1 | 1978.23 |
| 5FU+VP-16+CF(ELF) | 45 | 11.7 | 53.3 | 2543.12 |
| 5FU+CF+DDP+MMC (LFP/M) | 91 | 9.0 | 44.0 | 3056.08 |
| 5FU+CF+ADM+MTX (FAMTX) | UR | 6.1 | 21.0 | 1756.95 |
| 5FU+EPI+DDP (ECF) | UR | 8.7 | 44.42 | 2276.432 |
| 5FU+DDP+PTX (TCF) | 22 | UR | 50.0 | 2667.01 |
| 5FU+CF+DXL+DDP (DCF) | 32 | UR | 56.3 | 9979.00 |
| 5FU+CF+L-OHP (FOLFOX) | 60 | UR | 50.0 | 3244.31 |
| UFT+MMC (UFTM) | 49 | 8.5 | 32.7 | 2441.29 |
| UFT+DDP+VP-16(FEP) | 51 | 10.0 | 47.1 | 1999.97 |
| DDP+VP-16+ADM/EPI (EAP) | 40 | 8.5 | 52.5 | 2790.23 |
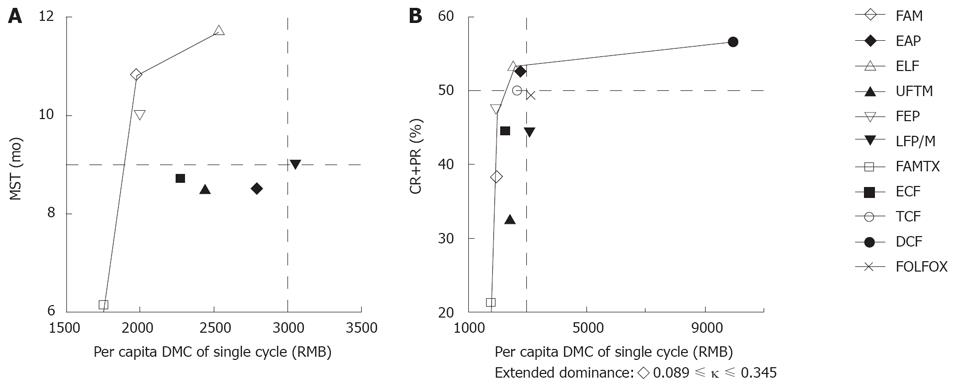
The C/E ratios of MDC/MST and DMC/CR+PR were calculated, respectively (Table 4). The ELF regimen had the longest MST (11.7 mo), and the DCF regimen had the highest percentage of CR+PR (56.3%). The FAM regimen was of the lowest DMC/MST ratio (183.17 RMB for per 1-mo survival), and the FEP regimen was of the lowest DMC/CR+PR ratio (42.46 RMB for per 0.01 probability of CR+PR). The FAMTX regimen with the lowest per capita DMC in a single cycle was selected as the common baseline for other regimens to refer to. Incremental cost-effectiveness analysis showed that FAM and ELF regimens were not dominated in the DMC/MST analysis. The FEP, ELF and DCF regimens were not dominated in the DMC/CR+PR analysis (Table 4 and Figure 1). According to our previous enacted thresholds, the considerable regimens included ELF, FAM, FEP, LFP/M, EAP, TCF and DCF, but only ELF regimen was conformed to all the thresholds.
| Regimens | Cost (¥, RMB) | Incr cost (¥, RMB) | Eff | Incr Eff | C/E (¥, RMB) | Incr C/E (ICER) (¥, RMB) |
| DMC/MST analyses | ||||||
| 5Fu+CF+ADM+MTX (FAMTX)1 | 1756.95 | - | 6.1 | - | 288.02 | Baseline |
| 5Fu+ADM/EPI+MMC (FAM) | 1978.23 | 221.28 | 10.8 | 4.7 | 183.17 | 47.08 |
| UFT+DDP+VP-16 (FEP) | 1999.97 | 21.74 | 10.0 | -0.8 | 200.00 | Dominated |
| 5Fu+EPI+DDP (ECF) | 2276.43 | 298.20 | 8.7 | -2.1 | 261.66 | Dominated |
| UFT+MMC (UFTM) | 2441.29 | 463.06 | 8.5 | -2.3 | 287.21 | Dominated |
| 5Fu+VP-16+CF (ELF) | 2543.12 | 564.89 | 11.7 | 0.9 | 217.36 | 627.66 |
| DDP+VP-16+ADM/EPI (EAP) | 2790.23 | 247.11 | 8.5 | -3.2 | 328.26 | Dominated |
| 5FU+CF+DDP+MMC (LFP/M) | 3056.08 | 512.96 | 9.0 | -2.7 | 339.56 | Dominated |
| DMC/CR+PR analyses | ||||||
| 5Fu+CF+ADM+MTX (FAMTX)1 | 1756.95 | - | 21.0 | - | 83.66 | Baseline |
| 5Fu+ADM/EPI+MMC (FAM) | 1978.23 | 221.28 | 38.1 | 17.1 | 51.92 | Extended dominance |
| UFT+DDP+VP-16 (FEP) | 1999.97 | 21.74 | 47.1 | 9 | 42.46 | 2.42 |
| 5Fu+EPI+DDP (ECF) | 2276.43 | 276.46 | 44.4 | -2.7 | 51.27 | Dominated |
| UFT+MMC (UFTM) | 2441.29 | 441.32 | 32.7 | -14.4 | 74.66 | Dominated |
| 5Fu+VP-16+CF (ELF) | 2543.12 | 543.15 | 53.3 | 6.2 | 47.71 | 87.6 |
| 5FU+DDP+PTX (TCF) | 2667.01 | 123.89 | 50.0 | -3.3 | 53.34 | Dominated |
| DDP+VP-16+ADM/EPI (EAP) | 2790.23 | 247.11 | 52.5 | -0.8 | 53.15 | Dominated |
| 5FU+CF+DDP+MMC (LFP/M) | 3056.08 | 512.96 | 44.0 | -9.3 | 69.46 | Dominated |
| 5FU+CF+L-OHP (FOLFOX) | 3244.31 | 701.19 | 50.0 | -3.3 | 64.89 | Dominated |
| 5FU+CF+DXL+DDP (DCF) | 9979.00 | 7435.88 | 56.3 | 3.0 | 177.25 | 2478.63 |
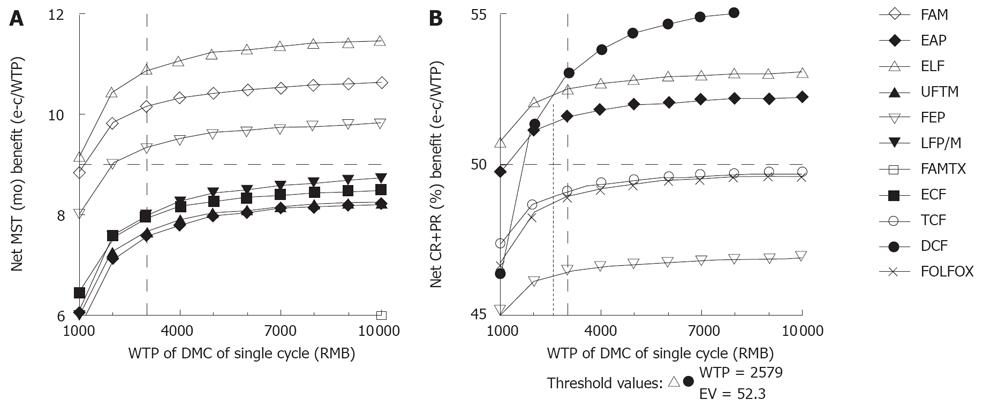
When altering the willingness-to-pay (WTP), if we want to get the longest survival time, the ELF regimen was selected without doubt, while if we want to get the best tumor response probability, the ELF regimen was selected on WTP below 2579.00 RMB, while the DCF regimen was selected beyond 2579.00 RMB (Figure 2). Additionally, the FAM, FEP and EAP regimens were conformed to the previous enacted thresholds, but inferior to the ELF and DCF regimens.
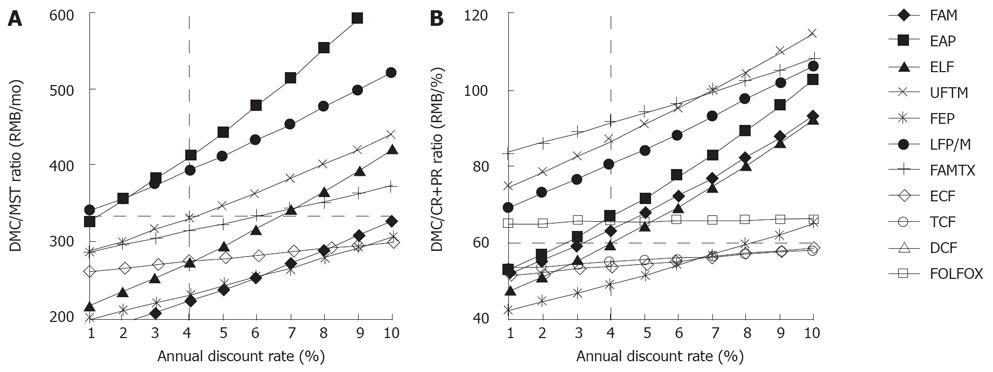
When altering the annual discount rate from 1% to 10%, the targeted EAP, FAM, ELF and FEP regimens were rejected once the annual discount rate was more than 1%, 3%, 4% and 8%, respectively, according to the previous enacted thresholds (Figure 3). The ECF and TCF regimens were not rejected based on these sensitivity analyses. If considering the discount rate as an annual increase in Chinese consumption price index, i.e. 1.0%-3.9% from 1999 to 2005[13], we assumed that the discount rate increased from 1.0% to 4.0%. Thus, only ELF, FEP, ECF and TCF regimens were not rejected in this aspect.
Additionally, further sensitivity analyses were carried out by excluding those studies with a potential heterogeneity (Table 5). After excluding the two studies contaminated with stage II disease[57] on the FAM, EAP and ELF regimens, re-analysis was carried out, showing that the FAM and ELF regimens were not dominated.
| Regimens | Excluding studies contaminated with stage II diseases | Excluding studies from poorly developing areas of China | Excluding studies published before the last 5 years | Excluding the studies weighted more than 25% of overall sample | ||||||||
| Cost | Eff | ICER | Cost | Eff | ICER | Cost | Eff | ICER | Cost | Eff | ICER | |
| Sensitivity analyses of DMC/MST ratio (RMB/mo) | ||||||||||||
| 5FU+ADM/EPI+MMC (FAM) | 1749.101 | 12.1 | Baseline | 1822.06 | 11.9 | DT | - | - | - | 2257.821 | 10.3 | Baseline |
| 5FU+VP-16+CF (ELF) | 1775.88 | 13.0 | 29.76 | 1744.781 | 13.0 | Baseline | - | - | - | 2543.12 | 11.7 | 203.79 |
| 5FU+CF+DDP+MMC (LFP/M) | 3056.08 | 9.0 | DT | 3056.08 | 9.0 | DT | - | - | - | - | - | - |
| 5FU+CF+ADM+MTX (FAMTX) | 1756.95 | 6.1 | DT | - | - | - | 1756.951 | 6.1 | Baseline | - | - | - |
| 5FU+EPI+DDP (ECF) | 2276.43 | 8.7 | DT | - | - | - | 2276.43 | 8.7 | 199.8 | - | - | - |
| 5FU+DDP+PTX (TCF) | - | - | - | - | - | - | - | - | - | - | - | - |
| 5FU+CF+DXL+DDP (DCF) | - | - | - | - | - | - | - | - | - | - | - | - |
| 5FU+CF+L-OHP (FOLFOX) | - | - | - | - | - | - | - | - | - | - | - | - |
| UFT+MMC (UFTM) | 2441.29 | 8.5 | DT | 2441.29 | 8.5 | DT | - | - | - | - | - | - |
| UFT+DDP+VP-16 (FEP) | 1999.97 | 10.0 | DT | 1999.97 | 10.0 | DT | - | - | - | - | - | - |
| DDP+VP-16+ADM/EPI (EAP) | 2929.13 | 9.0 | DT | 2594.35 | 8.7 | DT | - | - | - | 2790.23 | 8.5 | DT |
| Sensitivity analyses of DMC/CR+PRratio (RMB/%) | ||||||||||||
| 5FU+ADM/EPI+MMC (FAM) | 1749.101 | 36.0 | Baseline | 1822.06 | 36.8 | DT | - | - | - | 2257.821 | 40.8 | Baseline |
| 5FU+VP-16+CF (ELF) | 1775.88 | 57.1 | 1.27 | 1744.78 1 | 56.7 | Baseline | - | - | - | 2543.12 | 53.3 | 22.82 |
| 5FU+CF+DDP+MMC (LFP/M) | 3056.08 | 44.0 | DT | 3056.08 | 44.0 | DT | - | - | - | - | - | - |
| 5FU+CF+ADM+MTX (FAMTX) | 1756.95 | 21.0 | DT | - | - | - | 1756.951 | 21.0 | Baseline | - | - | - |
| 5FU+EPI+DDP (ECF) | 2276.43 | 44.4 | DT | 4158.00 | 40.0 | DT | 2276.43 | 44.4 | 22.2 | 4158.00 | 40 | DT |
| 5FU+DDP+PTX (TCF) | 2667.01 | 50.0 | DT | 2667.01 | 50.0 | DT | 2667.01 | 50.0 | 69.75 | 2667.01 | 50 | DT |
| 5FU+CF+DXL+DDP (DCF) | 9979.00 | 56.3 | DT | 9979.00 | 56.3 | DT | 9979.00 | 56.3 | 1160.63 | 9979.00 | 56.3 | 2478.63 |
| 5FU+CF+L-OHP (FOLFOX) | 3244.31 | 50.0 | DT | 3244.31 | 50.0 | DT | 3244.31 | 50.0 | DT | 3244.31 | 50 | DT |
| UFT+MMC (UFTM) | 2441.29 | 32.7 | DT | 2441.29 | 32.7 | DT | - | - | - | - | - | - |
| UFT+DDP+VP-16 (FEP) | 1999.97 | 47.1 | DT | 1999.97 | 47.1 | DT | - | - | - | - | - | - |
| DDP+VP-16+ADM/EPI (EAP) | 2929.13 | 50.0 | DT | 2594.35 | 51.9 | DT | - | - | - | 2790.23 | 52.5 | DT |
Considering the economic gap between northern and southern areas, or western and eastern areas of China, we excluded the two studies on FAM, EAP, ELF, FAMTX and ECF regimens conducted in relatively poor developing areas[59], and the cost-effectiveness was re-analyzed, showing that only ELF regimen was not dominated.
If only the recent studies published within 5 years (2003-2007)[9–11] were included, the re-analysis of FAMTX, ECF, TCF, DCF and FOLFOX regimens showed that ECF, TCF and DCF regimens were not dominated. However, the incremental cost-effectiveness analysis found that paclitaxel regimen was paid more than 69.75 RMB per cycle to gain a higher 0.01 probability of CR+PR more than ECF regimen, and docetaxel regimen was paid 1160.63 RMB more than TCF regimen. However, the outcome data about MST were insufficient in recent studies.
If the studies weighted more than 25% of the overall included participants influencing the results most were excluded[89], the FAM, ELF and DCF regimens were not dominated. The docetaxel regimen was more expensive to gain a higher CR+PR rate than FAM and ELF regimens.
Finally, if our previous enacted thresholds were used to assess each regimen in excluding sensitivity analysis (Table 5), the results in CR+PR aspect indicated that only TCF regimen conformed to the threshold limitation, and with no regard for publication date, EAP or ELF regimen was acceptable. Besides, in MST aspect, regimens with new agents were lack of outcome data. With no regard for publication date, either FAM or ELF regimen was better in improving the median survival according to the threshold limitation.
In conclusion, although FAM or FEP regimen was the best cost-effective choice, ELF regimen trended to be paid more attention to gaining a better response and survival outcome. The taxanes regimen was also attractive in the case of a high level of willingness-to-pay.
The present cost-effectiveness analysis was based on 7 Chinese retrospective economic case-control studies with a limited sample, which might contain apparent observation bias. The level of evidence source was low (level 3b according to Oxford standard)[14], and there was a potential heterogeneity in some aspects. One study[5] half patients had stage II disease, and another study[7] was also contaminated with patients of the disease at stage II (Table 1). Moreover, although all the studies reported the information about DMC, the detailed items, such as fee counted in some studies, were slightly different, but not in the others (Table 1). The homogeneity in participants should also be questioned, because some studies included patients with recurrence/metastases after curative operation for the disease, while others were newly diagnosed as AGC. Therefore, these factors might remarkably influence the validity of the present analysis.
To our knowledge, there is no standard chemotherapy protocol for AGC worldwide. However, 5-fluorouracil-based regimens have been recommended as a standard modality of chemotherapy for gastric cancer[13]. Cisplatin plus 5FU (FP) or etoposide (EP) in the treatment of several types of cancer, including AGC, has been widely used in the treatment of cancer due to their synergistic activity in vitro[15]. Based on the current available evidence, 5FU-based regimens are universally used in the treatment of Chinese patients, and uracil-tegafur-based regimens are also optional. In the present study, the ELF and FAM regimens had a longer MST of over 10 mo. The ECF regimen was recommended for AGC in NCCN guideline, which appears to improve MST and quality of life[1]. However, the results of studies in China are compromising and less cost-effective. Thus, we cannot simply transplant the Western experience to the common Chinese patients, and the ECF regimen requires more prospective randomized controlled trials to confirm its efficacy for the common Chinese patients.
Moreover, some new agents have been applied to the treatment of AGC, such as paclitaxel, docetaxel, oxaliplatin and capecitabine, usually used as second-line regimens. The present literature review found that regimens with the new agents would get a relatively higher CR+PR percentage, but increased the DMC. A retrospective study showed that capecitabine (Xeloda) plus cisplatin regimen could reach a CR+PR percentage of 64.3%[12]. Capecitabine is considered a promising agent for the treatment of gastric cancer. Phase III trials of capecitabine-based regimens, comparing its efficacy and safety with those of parenteral 5FU-based regimens in the first-line metastatic setting, are important[16]. It was reported that oxaliplatin regimens also have a good efficacy and acceptable safety profile in AGC[17]. It has been shown that oxaliplatin, folinic acid, 5-fluorouracil and irinotecan (COFFI) regimen could reach a 67% CR+PR rate in AGC[17], while FOLFOX-4 regimen as a first-line therapy for elderly patients could reach 31%[18], suggesting that irinotecan might play a positive role in the treatment of AGC, and other studies reported that irinotecan, 5-fluorouracil and folinic acid (FOLFIRI) regimen is a very promising and useful treatment of AGC[1920].
Based on the available data, we could recommend ELF regimen as the preference, and FAM or FEP regimen as a first- or a second-line therapy for AGC in common Chinese patients. With no regard for willingness-to-pay, the DCF regimen can be chosen as either the first-line chemotherapy or the second-line chemotherapy. A recent study showed that combined docetaxel, cisplatin and fluorouracil (DCF) regimen not only significantly improves clinical benefit, quality of life, disease progression and overall survival compared with CF regimen[21]. Besides, paclitaxel regimen is also considered an active and well tolerated therapy for AGC[22]. Generally, the TCF regimen is recommended as a second- or a third-line therapy for AGC, with a tolerable and acceptable toxicity profile[2324]. It was reported that PTX plus 5FU as a first-line chemotherapy can prolong the MST and 1-year survival rate[25]. However, another study reported that only 27% of patients with measurable disease have achieved a MST of 26 wk[26]. Thus, chemotherapy with docetaxel appears to be promising in the treatment of AGC, while chemotherapy with paclitaxel needs further confirmation[27]. Based on the availability of agents, physician/patient expectations and the “standard” regimens will disappear in the future oncology fields of individualized therapy[28].
In conclusion, 5-fluorouracil regimens, especially ELF regimens, are still recommended as the mainstream for AGC in China, while some new agents are optional, such as taxanes. More randomized clinical trials are required before mandatory recommendation for certain regimens for patients with AGC in China is made.
Gastric cancer is the forth common malignancy worldwide, and China is one of the countries with a high incidence of the disease. About 84% of patients with Gastric cancer will progress to its advanced stage in about 84% of patients. If they do not receive chemotherapy, their median survival time (MST) is only 3-4 mo. Studies have found that chemotherapy for Advanced gastric cancer (AGC) might improve the MST and can be well tolerated. However, recommendation for regimens is still controversial, since the survival appears marginal in some studies.
In China, a developing country with a huge population, oncologists need to know which regimen is the best cost-effective for the common Chinese patients. The present paper reviews the Chinese literatures and makes an economics assessment of various regimens for AGC.
Some new agents have been applied to the treatment of AGC, such as paclitaxel, docetaxel, oxaliplatin and capecitabine, usually used as the second-line regimen drugs. The present literature review found that regimens with new agents could get a relatively higher CR+PR percentage while increasing the direct medical cost (DMC).
In any case, regimens with 5-fluorouracil are still considered the mainstream for the treatment of AGC, while new agents are optional, such as taxane. More randomized clinical trials are required before mandatory regimen recommendations are made for patients with AGC in China.
AGC is an adenocarcinoma of the stomach at a more advanced stage than T1 stage, i.e. the primary lesion penetrates into tissues beyond the mucosal layer, which can be divided as a locally advanced or metastatic disease. 5-fluorouracil (5FU), a chemotherapeutic agent used to treat several types of cancer, including digestive, head and neck cancers, can prevent cells from making DNA and RNA to disrupt the growth of cancer cells. Taxanes are a group of chemotherapeutic agents including paclitaxel (PTX) and docetaxel (DXL), which are also able to prevent the growth of cancer cells.
This paper reports a health economics assessment of various chemotherapeutic regimens for AGC, and the authors selected the best cost-effective regimen for common Chinese patients. Although they concluded that more randomized clinical trials are required before any mandatory recommendation of certain regimens is made for patients with AGC in China, this paper offers a lot of important information.
| 1. | National Comprehensive Cancer Network. Clinical practice guidelines in oncology: gastric cancer. 2007;. |
| 2. | Chen XM, Chen GY, Wang ZR, Zhu FS, Wang XL, Zhang X. Detection of micrometastasis of gastric carcinoma in peripheral blood circulation. World J Gastroenterol. 2004;10:804-808. |
| 3. | Hu JK, Li CM, Chen XZ, Chen ZX, Zhou ZG, Zhang B, Chen JP. The effectiveness of intravenous 5-fluorouracil-containing chemotherapy after curative resection for gastric carcinoma: A systematic review of published randomized controlled trials. J Chemother. 2007;19:359-375. |
| 4. | Rivera F, Vega-Villegas ME, Lopez-Brea MF. Chemotherapy of advanced gastric cancer. Cancer Treat Rev. 2007;33:315-324. |
| 5. | Dong YL, Li R. Cost-effectiveness analysis of three chemotherapy regimens for gastric cancer. Zhongguo Yaoxue Zazhi. 1999;34:627-628. |
| 6. | Yu LX, Lv ZC. Cost-effectiveness analysis of three chemotherapy regimens for gastric cancer. Zhongguo Yiyuan Yaoxue Zazhi. 1999;19:552-553. |
| 7. | Ding WX, Zhai ZY. Cost-effectiveness analysis of three chemotherapy regimens for gastric cancer. Zhenjiang Yixueyuan Xuebao. 2000;10:89-90. |
| 8. | Qian SS, Qin LR. Cost-effectiveness analysis of four chemotherapy regimens for advanced gastric cancer. Yaowu Liuxingxue Zazhi. 2002;11:250-251. |
| 9. | Yang L, Cui CX, Wang JW. Health economics assessment of chemotherapy for gastric cancer. Zhongguo Quanke Yixue. 2004;7:1640-1641. |
| 10. | Zhou T, Yang QL, Ling Y. Cost-effectiveness analysis of two new chemotherapeutic agent containing regimens for advanced gastric cancer. Shiyong Linchuang Yiyao Zazhi. 2006;10:28-29. |
| 11. | Liu YX, Zhang J, Chen SX, Li JW. Cost-effectiveness analysis of three chemotherapeutic schemes for advanced gastric cancer. Yaoxue Shijian Zazhi. 2007;25:117-120. |
| 12. | Yang Q, Chen DS. Cost-effectiveness analysis of three chemotherapy regimens for gastric cancer. Zhongguo Yiyuan Yaoxue Zazhi. 2007;27:230-232. |
| 13. | Liu SH, Chen QS. Predictive goal of regulation of the residents’ consumer price index in China. Jing Ji Shi. 2005;10:50-52. |
| 14. | Phillips B, Ball C, Sackett D, Badenoch D, Straus S, Haynes B, Dawes M; Oxford Centre for Evidence-based Medicine Levels of Evidence (May 2001). Oxford: Centre for Evidence-Based Medicine. . |
| 15. | Icli F, Celik I, Aykan F, Uner A, Demirkazik A, Ozet A, Ozguroglu M, Tas F, Akbulut H, Firat D. A randomized Phase III trial of etoposide, epirubicin, and cisplatin versus 5-fluorouracil, epirubicin, and cisplatin in the treatment of patients with advanced gastric carcinoma. Turkish Oncology Group. Cancer. 1998;83:2475-2480. |
| 16. | Ajani J. Review of capecitabine as oral treatment of gastric, gastroesophageal, and esophageal cancers. Cancer. 2006;107:221-231. |
| 17. | Chiesa MD, Buti S, Tomasello G, Negri F, Buononato M, Brunelli A, Lazzarelli S, Brighenti M, Donati G, Passalacqua R. A pilot phase II study of chemotherapy with oxaliplatin, folinic acid, 5-fluorouracil and irinotecan in metastatic gastric cancer. Tumori. 2007;93:244-247. |
| 18. | Nardi M, Azzarello D, Maisano R, Del Medico P, Giannicola R, Raffaele M, Zavettieri M, Costarella S, Falzea A. FOLFOX-4 regimen as fist-line chemotherapy in elderly patients with advanced gastric cancer: a safety study. J Chemother. 2007;19:85-89. |
| 19. | Beretta E, Di Bartolomeo M, Buzzoni R, Ferrario E, Mariani L, Gevorgyan A, Bajetta E. Irinotecan, fluorouracil and folinic acid (FOLFIRI) as effective treatment combination for patients with advanced gastric cancer in poor clinical condition. Tumori. 2006;92:379-383. |
| 20. | Kim SG, Oh SY, Kwon HC, Lee S, Kim JH, Kim SH, Kim HJ. A phase II study of irinotecan with bi-weekly, low-dose leucovorin and bolus and continuous infusion 5-fluorouracil (modified FOLFIRI) as salvage therapy for patients with advanced or metastatic gastric cancer. Jpn J Clin Oncol. 2007;37:744-749. |
| 21. | Ajani JA, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, Rodrigues A, Fodor M, Chao Y, Voznyi E. Clinical benefit with docetaxel plus fluorouracil and cisplatin compared with cisplatin and fluorouracil in a phase III trial of advanced gastric or gastroesophageal cancer adenocarcinoma: the V-325 Study Group. J Clin Oncol. 2007;25:3205-3209. |
| 22. | Lokich JJ, Sonneborn H, Anderson NR, Bern MM, Coco FV, Dow E, Oliynyk P. Combined paclitaxel, cisplatin, and etoposide for patients with previously untreated esophageal and gastroesophageal carcinomas. Cancer. 1999;85:2347-2351. |
| 23. | Kim YH, Shin SW, Kim BS, Kim JH, Kim JG, Mok YJ, Kim CS, Rhyu HS, Hyun JH, Kim JS. Paclitaxel, 5-fluorouracil, and cisplatin combination chemotherapy for the treatment of advanced gastric carcinoma. Cancer. 1999;85:295-301. |
| 24. | Yamaguchi K, Nakagawa S, Yabusaki H, Nashimoto A. Combination chemotherapy with 5-fluorouracil, cisplatin and paclitaxel for pretreated patients with advanced gastric cancer. Anticancer Res. 2007;27:3535-3539. |
| 25. | Ninomiya M, Kondo K, Matsuo K, Hirabayashi N, Kojima H, Kobayashi M, Kawamura S, Ando T, Musha N, Konno H. Multicenter phase II trial of combination chemotherapy with weekly paclitaxel and 5-fluorouracil for the treatment of advanced or recurrent gastric carcinoma. J Chemother. 2007;19:444-450. |
| 26. | Kim YH, Shin SW, Kim BS, Kim JH, Kim JG, Mok YJ, Kim CS, Rhyu HS, Hyun JH, Kim JS. Paclitaxel, 5-fluorouracil, and cisplatin combination chemotherapy for the treatment of advanced gastric carcinoma. Cancer. 1999;85:295-301. |
| 27. | Roth AD, Ajani J. Docetaxel-based chemotherapy in the treatment of gastric cancer. Ann Oncol. 2003;14 Suppl 2:ii41-ii44. |
| 28. | Ajani JA. Standard of care for gastric cancer based on meta-analysis? Treading on thin ice or it is very nice! J Clin Oncol. 2006;24:5473-5474; author reply 5474-5476. |