Published online Apr 21, 2008. doi: 10.3748/wjg.14.2430
Revised: January 22, 2008
Published online: April 21, 2008
AIM: To investigate whether malignant esophageal stromal tumors contain PAS-positive patterned matrix-associated vascular channels, which are lined by tumor cells, but not vascular endothelial cells. That is vasculogenic mimicry (VM) independent of tumor angiogenesis.
METHODS: Thirty-six tissue samples of malignant esophageal stromal tumors were analyzed. Tissue sections were stained for Vascular endothelial growth factor (VEGF), CD31 and periodic acid Schiff (PAS). The level of VEGF, the microvascular density (MVD) and the vasculogenic mimicry density (VMD) were determined.
RESULTS: PAS-positive patterned matrix-associated vascular channels were detected in 33.3% (12/36) of tumor samples. Within these patterned channels, red blood cells were found. The level of VEGF and the MVD in tumors containing patterned channels were significantly higher than those in tumors not containing patterned channels (P < 0.05). At the same time, the malignant degree of tumors was higher, the proportions of tumors containing patterned channels were not only more, but also in the each kind of tumors containing patterned channels.
CONCLUSION: In malignant esophageal stromal tumors, a VM mechanism causes some tumor cells to deform themselves and secrete extracellular matrix; thus, PAS-positive patterned matrix-associated vascular channels appear and supplying blood to the tumors to sustain their growth and metastasis.
- Citation: Zhao H, Gu XM. Study on vasculogenic mimicry in malignant esophageal stromal tumors. World J Gastroenterol 2008; 14(15): 2430-2433
- URL: https://www.wjgnet.com/1007-9327/full/v14/i15/2430.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2430
Vasculogenic mimicry is defined by the phenomenon whereby plastic malignant tumor cells transform and excrete extracellular matrix, thus mimicking the blood transport channel formation of normal vascular tissues[1]. Vasculogenic mimicry (VM) is a totally new pattern of tumor angiogenesis, which differs from normal angiogenesis remarkably. Tumors containing VM show such biological behaviors as higher malignancy, non-directional or bi-directional, rapid proliferation, and high incidence of metastasis by a vascular route[2]. VM was first discovered in a human uvea malignant melanoma by Maniotis[1] in 1999. In recent years, it has been reported that VM mainly exists in bi-directional malignant tumors, such as malignant melanomas in other sites, synovial sarcomas, mesotheliosarcomas, sarcoma epithelioides and acinus rhabdomyosarcomas[2]; it was also found in inflammatory breast cancers, inflammatory liver cancers, prostate cancers and ovary cancers[3]. Based on the features of VM discussed above, with regard to the fact gastrointestinal stromal tumors are a group of non-directional tumors, commonly vascular stromal tumors arising from the gastrointestinal mesenchymal cells (Cajal cells), we selected esophageal stromal tumors to investigate the existence of VM. To date, similar studies on the relationship between VM and malignant esophageal stromal tumors have not been reported broadly.
Among paraffin samples collected from 1997 to 2000 in Shandong University, Qilu Hospital, 36 samples of malignant esophageal stromal tumors with follow-up documents were selected and observed. Eighteen of these showed low malignancy, while the others were highly malignant. Combined with a review of the history, they were diagnosed once again and stratified into low malignancy and high malignancy groups based on the degree of differentiation and mitosis count, and the existence of necrosis.
At least 4 sections were obtained from each sample, and these were stained for VEGF, CD31, and PAS by immunohistochemistry as described previously[2]. The glass slides were silicificated, and the cover slides were soaked and washed with 50 mL of 75% ethanol containing one drop of concentrated hydrochloric acid.
The streptavidin peroxidase method was used. Mouse monoclonal anti-human VEGF and CD31 antibodies, caprine-anti-mouse IgG and DAB developer were all purchased from Zhongshan BioTechnologies Company. The periodic acid Schiff (PAS) reagents were prepared in our laboratory. Three samples of gastric mucous adenocarcinomas were stained as a control group. All of the mucous locations were stained cherry red, which proved the reliability of the quality of the PAS reagents.
The level of VEGF was evaluated using a stereological grid counting method. CD31-and PAS-stained sections were observed using a 200 times objective on a light microscope; the positive CD31 and PAS staining images were taken as measurement objects. Ten fields of vision were selected randomly to assess the microvascular density (MVD) and the vasculogenic mimicry density (VMD); the average of the 10 counts was taken as the ultimate expression of MVD and VMD.
Statistical analyses were performed using software from SPSS for Windows 10.0. The data was analyzed with a matched paired rank sum test. P < 0.05 was taken to indicate statistical significance.
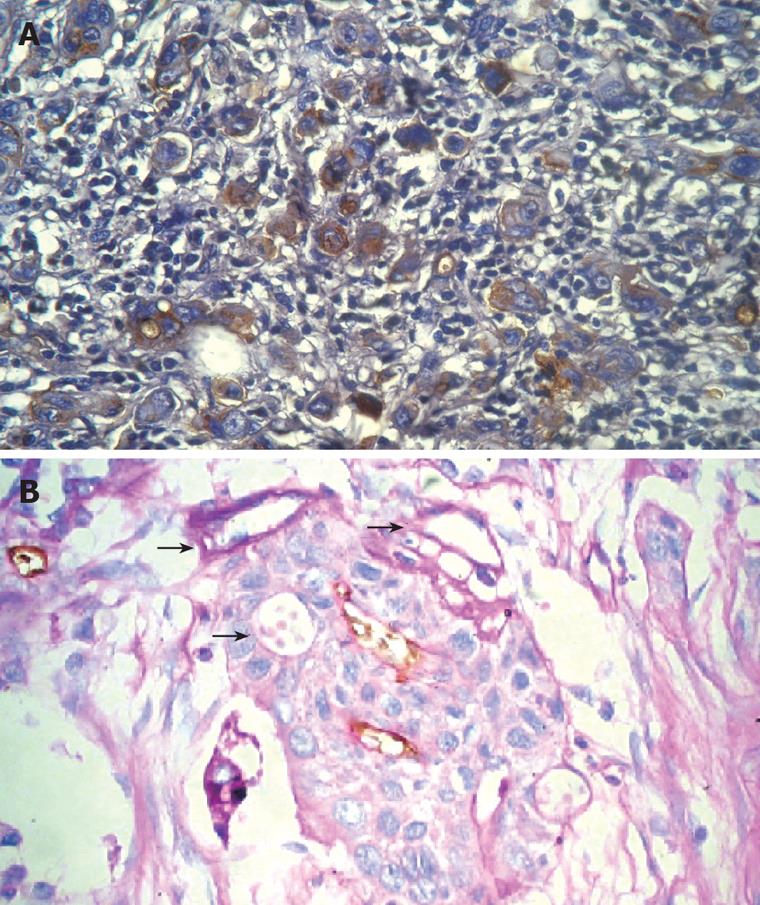
Based on CD31 and PAS staining, CD31-negative, PAS-positive vascular-like patterns, which indicated VM, could be seen in 33.3% (12/36) of malignant esophageal stromal tumors. VM could be found in 20.0% (4/20) of low malignancy tumors and 50.0% (8/16) of highly malignant tumors; this difference in the level of VM was statistically significant. Analysis of clinical data revealed that the 5 years survival rate of tumors expressing VM was 8.3 % (1/12), which is much less than that of tumors not expressing VM (41.7%; 10/24).
The PAS-positive human uvea melanomas identified by Maniotis showed linear, parallel linear, cruciform, half-moon, annuliform, and lattice forms[1]. All of the forms talked about above were also found in malignant esophageal stromal tumors. Besides, red blood cells were also found in the angiogenesis mimicry (Figure 1).
The levels of VEGF as well as MVD in tumors including VM were less than those in tumors that did not include VM (P = 0.002, P < 0.0001, respectively; Table 1). With increased tumor malignancy, the levels of VEGF, as well as MVD and VMD increased gradually, all of which showed statistical significance between high and low malignancy tumors (P = 0.047, P = 0.002, P = 0.037, respectively). Moreover, among low malignancy tumors, MVD was greater than VMD. However, the opposite was true of highly malignant tumors (Table 2).
| Existence of VM | Samples | VEGF | MVD |
| Yes | 12 | 91.49 ± 29.12 | 45.84 ± 13.81 |
| No | 24 | 128.39 ± 18.45 | 76.92 ± 14.62 |
| t value | 3.874 | 5.972 | |
| P value | 0.002 | 0 |
| Malignancy | Samples | VEGF | MVD | VMD |
| Low | 4 | 45 ± 19 | 15 ± 8 | 38 ± 25 |
| High | 8 | 128 ± 42 | 81 ± 17 | 122 ± 39 |
| F value | 4.1262 | 12.139 | 5.826 | |
| P value | 0.047 | 0.002 | 0.037 |
Maniotis et al firstly reported the phenomenon of VM in 1999, providing a primary description and explanation. They found in human uvea malignant melanoma new blood transport channels, the walls of which were formed by metamorphoses from the malignant melanoma and its matrix. Because the structure of these channels was similar to that of the normal vasculature, it was called angiogenesis mimicry. VM channels, co-existing with the normal vasculature, formed the microcirculation system concomitantly in tumors, and provided blood supply for their growth. Light microscopic, electron microscopic and immunohistochemical investigations of endothelial cells confirmed that the walls of VM channels consisted of tumor cells, not vascular endothelial cells. However, neither necrosis nor fibrosis was found in areas containing VM channels. PAS staining showed that a layer of PAS-positive basal membrane was found outside of the VM channels, while confocal laser scanning microscopy revealed that red blood cells could be seen in VM channels. then they found in tumor cells three dimensional cultures vascular reticulate structure, reproduced the former discovery and confirmed the blood cells in VM channels. Taking advantage of macromolecular contrast agents, dynamic MRI angiography revealed VM channels communicating with the normal vasculature in inflammatory breast cancers[3]. The blood flow was also found based on such technique. Genetic array analysis showed that highly invasive melanoma cells showed plasticity and a polyergic embryoid genetic phenotype; thus, they could mimic endothelial cells, forming VM channels that were strikingly different from normal vascular channels. VM channels had the features of embryoid invasion, which were commonly found in invasive tumors and related to the invasion and prognosis of tumors[5].
Our study selected 36 malignant esophageal stromal tumors. Based on CD31 and PAS staining, VM was observed. With increasing malignancy of tumors, the proportion of VM tumors increased gradually; the level of VEGF, as well as MVD and VMD also increased gradually, showing positive correlation tendency with the presence of VM. The possible causes of this are as follows. The higher the malignancy, the more rapidly tumor cells divide and proliferate, the more immature their differentiation, and thus, the plasticity they show. If tumors grow too rapidly, the current vascular would not be able to provide sufficient nutrition and oxygen; thus, hypoxia would induce the expression of VEGF and further tumor angiogenesis, concomitant with the high expression of CD31 or MVD. If the tumor angiogenesis still could not synchronize with the rapid growth of tumors, partial tumor cells may then deform and excrete extracellular matrix, mimicking the normal vascular structure, forming a blood transport system, and communicating with the normal vasculature, all of which ensure the blood supply to new tumor cells[5]. At the same time, it could also be appreciated that when angiogenesis did not synchronize with the growth of tumors and VM is not found in some part of malignant gastrointestinal stromal tumors, necrosis or fibrosis is an inevitable event[1–5]. Our study also observed that, on the inner walls of a minority of VM channels, CD31 was expressed; this could be explained by the assumptions that few tumor cells on the inner walls of VM channels have potent plasticity, could mimic the activity of endothelial cells, and have the capacity to excreting CD31 molecules. However, it remains to be determined whether the VM phenomenon is just an emergent mechanism when the tumor cells are in the “condition of hunger”, which serves to solve the shortage of blood supply resulting from the rapid growth of tumor cells, temporarily, and is then replaced by normal vasculature formed by endothelial cells with the passage of time.
Analysis of clinical data revealed tumors that formed more VM channels were usually found in patients showing early metastasis, poor prognosis, and shorter life span. The formation of VM channels may be explained as follows. VM channels and normal vasculature form the tumor's microcirculation concomitantly and communicate with the body's circulation system; thus, the tumor becomes rich in vasculature. The inner walls of VM channels consist of metamorphosed tumor cells, which structures are crumbly than normal vascular, all of which serve to the tumor cells' blood route metastasis[6]. Recent research[7] showed more highly invasive malignant melanomas express more protein tyrosine kinase (PTK), and immunofluorescence showed that protein tyrosine kinase phosphorylation was centered specifically in the area where VM channels formed. Yet the PTK signal transduction path has a close relationship with various intracellular growth factors, overexpression of which could facilitate the growth, differentiation, proliferation, adhesion and metastasis of tumor cells. Thus, it could be concluded PTK overexpression has a close relationship with the formation of VM channels and the blood metastasis of tumor cells. That study also confirmed the capacity of inhibitors of PTK to decrease VM in malignant tumors. Currently, the inhibitor of PTK imatinib has been extensively used in the clinical treatment of gastrointestinal stromal tumors, primarily being targeted toward inhibiting continuous activation of Kit PTK caused by the mutation of oncogene c-Kit in GIST, thus further decreasing the division and proliferation of tumor cells[8]. However, the capacity of inhibitors of PTK to decrease VM could be one of the reasons why such drugs could be successfully used in the treatment of GIST. It was also reported[9] that MMP (matrix metalloproteinase) inhibitor, PI-3K (phosphatidylinositol-3 kinase) inhibitor, PSMA (prostate-specific membrane antigen) inhibitor, tetrocycalamycin (CMT-3) modified chemically, and an antibody against laminin or laminin 5γ2 strand antisense oligonucleotides have a role in the inhibition of VM.
The phenomenon of VM deepens our knowledge about the mechanisms underlying tumors' blood supply and blood metastasis. However, study of VM in tumors is still at the early stage, and further research and discussion on various problems is required. It is reasonable to believe that with increased knowledge of its underlying mechanism, inhibiting VM is sure to provide a new target in the treatment of various neoplasms, including malignant esophageal stromal tumors.
Many studies have declared vasculogenic mimicry as a new pattern of tumor angiogenesis, which differs from the normal angiogenesis remarkably. Studies on the relationship between vasculogenic mimicry (VM) and malignant esophageal stromal tumors have not been reported broadly.
This study was designed to investigate whether malignant esophageal stromal tumors contain PAS-positive patterned matrix-associated vascular channels.
In malignant esophageal stromal tumors, VM enables tumors to establish additional blood supply to sustain their growth and metastasis
The authors investigated the relationship of VM and malignant esophageal stromal tumors. It is a very interesting study.
| 1. | Maniotis AJ, berg R, Hess A, Seftor EA, Gardner LM, Pe'er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Pathol. 1999;155:739-752. |
| 2. | Hao X, Sun B, Zhang S, Zhao X. [Microarray study of vasculogenic mimicry in bi-directional differentiation malignant tumor]. Zhonghua Yixue Zazhi. 2002;82:1298-1302. |
| 3. | Dupuy E, Hainaud P, Villemain A, Bodevin-Phedre E, Brouland JP, Briand P, Tobelem G. Tumoral angiogenesis and tissue factor expression during hepatocellular carcinoma progression in a transgenic mouse model. J Hepatol. 2003;38:793-802. |
| 4. | Miettinen M, Lasota J. Gastrointestinal stromal tumors--definition, clinical, histological, immunohistochemical, and molecular genetic features and differential diagnosis. Virchows Arch. 2001;438:1-12. |
| 5. | Shirakawa K, Kobayashi H, Heike Y, Kawamoto S, Brechbiel MW, Kasumi F, Iwanaga T, Konishi F, Terada M, Wakasugi H. Hemodynamics in vasculogenic mimicry and angiogenesis of inflammatory breast cancer xenograft. Cancer Res. 2002;62:560-566. |
| 6. | Cai XS, Jia YW, Mei J, Tang RY. Tumor blood vessels formation in osteosarcoma: vasculogenesis mimicry. Chin Med J (Engl). 2004;117:94-98. |
| 7. | Hess AR, Seftor EA, Gardner LM, Carles-Kinch K, Schneider GB, Seftor RE, Kinch MS, Hendrix MJ. Molecular regulation of tumor cell vasculogenic mimicry by tyrosine phosphorylation: role of epithelial cell kinase (Eck/EphA2). Cancer Res. 2001;61:3250-3255. |
| 8. | DeMatteo RP. The GIST of targeted cancer therapy: a tumor (gastrointestinal stromal tumor), a mutated gene (c-kit), and a molecular inhibitor (STI571). Ann Surg Oncol. 2002;9:831-839. |
| 9. | Hess AR, Seftor EA, Seftor RE, Hendrix MJ. Phosphoinositide 3-kinase regulates membrane Type 1-matrix metalloproteinase (MMP) and MMP-2 activity during melanoma cell vasculogenic mimicry. Cancer Res. 2003;63:4757-4762. |