Published online Apr 21, 2008. doi: 10.3748/wjg.14.2406
Revised: March 4, 2008
Published online: April 21, 2008
AIM: To compare the antisecretory activity and plasma drug concentrations of a single oral dose of 10 mg lafutidine, a novel H2 receptor antagonist, with those of the proton pump inhibitor lansoprazole (LPZ) 30 mg.
METHODS: Ten volunteers without H pylori infection participated in this crossover study comparing lafutidine 10 mg with LPZ 30 mg. Intragastric pH was monitored for 6 h in all participants, and blood samples were collected from four randomly selected individuals after single-dose administration of each drug.
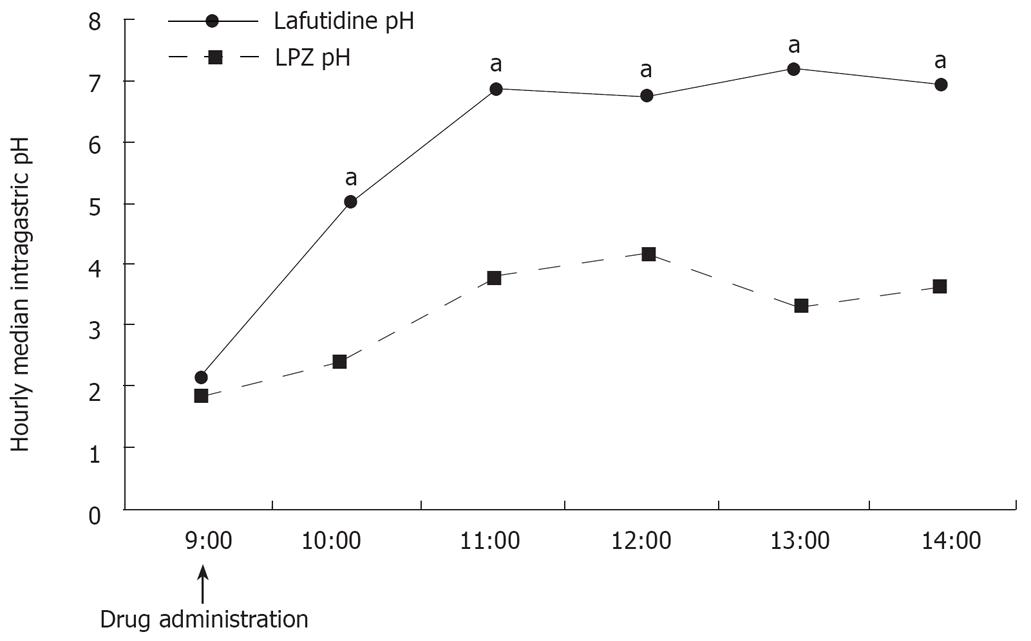
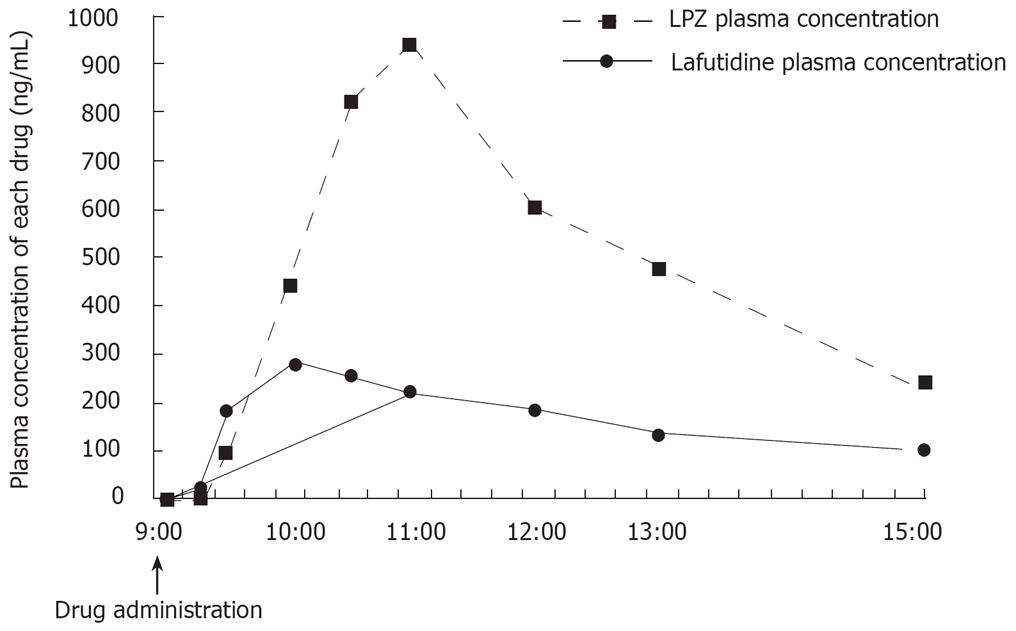
RESULTS: The median intragastric pH was significantly higher in individuals who received lafutidine 10 mg than in those who received LPZ 30 mg 2, 3, 4, 5, and 6 h after administration. Maximal plasma drug concentration was reached more promptly with lafutidine 10 mg than with LPZ 30 mg.
CONCLUSION: In H pylori-negative individuals, gastric acid secretion is more markedly inhibited by lafutidine than by LPZ.
- Citation: Yamagishi H, Koike T, Ohara S, Horii T, Kikuchi R, Kobayashi S, Abe Y, Iijima K, Imatani A, Suzuki K, Hishinuma T, Goto J, Shimosegawa T. Stronger inhibition of gastric acid secretion by lafutidine, a novel H2 receptor antagonist, than by the proton pump inhibitor lansoprazole. World J Gastroenterol 2008; 14(15): 2406-2410
- URL: https://www.wjgnet.com/1007-9327/full/v14/i15/2406.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2406
Gastroesophageal reflux disease (GERD) commonly occurs in the western countries[12], and its prevalence is now increasing in Japan[34]. Recently, Ohara et al[3] have shown that 42.2% of Japanese adults experience heartburn, similar to the rate of 42.4% reported in western studies[1]. Gastric acid has an important role in the pathogenesis of GERD. Suppression of gastric acid secretion is the most common therapeutic approach, and more effective and promptly acting treatments are required.
Two types of potent gastric acid-suppressing agents, proton pump inhibitors (PPIs) and histamine H2 receptor antagonists (H2RAs), are widely used to treat GERD. PPIs such as lansoprazole (LPZ), rabeprazole, and omeprazole, the most potent acid inhibitors available, are often used for first-line treatment. Controlled studies have demonstrated that PPIs are far more effective than H2RAs in patients with GERD[5–9]. In the treatment of reflux esophagitis, H2RAs have a number of disadvantages compared to PPIs, including shorter lasting efficacy and the development of tachyphylaxis, both limiting routine use[10]. In contrast, PPIs are highly effective, and produce profound and sustained inhibition of gastric acid secretion, making these agents the mainstay of treatment for GERD.
GERD has a high rate of relapse. The rising use of PPI therapy on demand has raised issues regarding efficacy. Several studies have demonstrated that on demand therapy with PPIs provides an alternative to continuous treatment in patients with non-severe GERD[1112]. However, pH monitoring studies[13–15] have shown that PPIs require 2 d to 3 d to inhibit acid secretion efficiently. In contrast, H2RAs potently and promptly suppress gastric acid secretion[1617]. In this respect, H2RAs might have advantage over PPIs, especially on the first day of treatment, i.e., when used on demand, for GERD. Furthermore, concerning the characteristics of GERD in Japan, it is important to distinguish the significant difference of acid secretion in Japanese patients when compared with that of western countries[1819]. Acid secretion among Japanese patients is lower compared to that of western population irrespectively of the status of H pylori infection. Moreover, endoscopic studies[34] have shown that GERD is mild in most Japanese patients. H2RAs are thus sometimes used for the treatment of mild-to-moderate GERD in Japan.
Lafutidine is a newly synthesized H2RA. Previous studies have shown that lafutidine promptly inhibits gastric acid secretion not only at night but also during the day[16], in contrast to other conventional H2RAs. Since patients with GERD often have symptoms during the day, we evaluated lafutidine in this study.
Few studies[17] have examined the correlation between intragastric pH and blood drug concentrations in the early phase (1-6 h) after a single dose of H2RAs or PPIs. The acid inhibitory activity of PPIs depends significantly on cytochrome P450 (CYP) 2C19 genotype, as well as on intrinsic pharmacokinetic and pharmacodynamic characteristics and dosing schemes[2021]. CYP2C19 genotypes were therefore determined for all participants in this study.
The major aim of this study was to compare the antisecretory activity of a single oral dose of 10 mg lafutidine (H2RA) with that of a single dose of 30 mg LPZ (PPI). We also examined the correlation between intragastric pH and plasma drug concentrations during the early phase (1-6 h) after single-dose administration.
Ten healthy male volunteers aged between 24 years and 48 years (mean, 28.7 years) and weighing 55 kg to 86 kg (mean, 68.6 kg) were included. Nobody of them had a history of gastrointestinal or hepatobiliary disease or of H pylori eradication therapy. None were receiving regular medication. All volunteers gave written informed consent. The study protocol was approved by the ethical committee of Tohoku University Graduate School of Medicine.
H pylori infection was diagnosed by the 13C-urea breath test[22]. A total of 10 H pylori-negative volunteers were invited and agreed to participate in this study.
After obtaining informed consent, a venous blood sample was collected from all participants. DNA was extracted from the nuclei of venous white blood cells. Genetic mutations were analyzed by either the polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method[23] or by the TaqMan PCR amplification method (Applied Biosystems Japan, Chiba, Japan[24]). On the basis of point mutations in exons 4 and 5, CYP2C19 gene status can be classified as homo-extensive metabolizer (homo-EM), hetero-extensive metabolizer (hetero-EM), or poor metabolizer (PM)[25–27]. Homo-EM has wild type alleles (wt/wt) without any mutation in exons 5 or 4; PM has mutated alleles (m1/m2) with mutations in both exons 5 and 4 (m1/m2, m1/m1, or m2/m2); and hetero-EM has a mutated allele in either exon 5 or 4 (wt/m1 or wt/m2).
All subjects (homo-EM = 3, hetero-EM = 6, PM = 3) participated in an open-label crossover study with lafutidine 10 mg or LPZ 30 mg. They were randomly assigned to receive a single oral dose of lafutidine 10 mg tablets or LPZ 30 mg capsule at a fixed time. A washout period of at least 14 d intervened between the two study periods. Intragastric pH was monitored for 6 h after drug administration. To monitor intragastric pH, a pH electrode was inserted transnasally and positioned fluoroscopically in the gastric corpus, approximately 10 cm below the esophagogastric junction. Intragastric pH was measured at 10-second intervals by means of a portable pH meter attached to a glass pH electrode (Chemical Instrument, Tokyo, Japan). The pH electrode was calibrated before each recording, using standard buffers of pH 1.68, 4.01, and 6.86. The pH data were analyzed using a commercially available software (Chemical Instrument). No food was allowed, and 100 mL of tap water was allowed only when participants felt thirsty. All subjects were instructed to remain upright; normal daily activities were not restricted.
To study the correlation between intragastric pH and plasma drug concentrations, blood samples were randomly collected from four individuals (No. 3, 5, 6, 7) in heparinized tubes before and 0.25, 0.5, 1, 1.5, 2, 3, 4, and 6 h after drug administration. Blood samples were immediately centrifuged at 3000 r/min for 10 min. All samples were stored at -20°C until assay. Plasma LPZ levels were measured by high performance liquid chromatography/tandem mass spectrometry[2829]. This method requires only 20 &mgr;L of serum and is a simple procedure. Analytes and the internal standard (lansoprazole deuterium derivatives) were separated using a mobile phase of acetonitrile/1 mmol/L ammonium formate (140/60, mL/L) on a C18 analytical column and analyzed in the selected reaction-monitoring (SRM) mode. Detection limit was 500 fg/20 &mgr;L.
Plasma lafutidine concentrations were determined by high-performance liquid chromatography (HPLC), using 2-phenyl-1H-benzimidazol as an internal standard (IS). A 1 mL plasma sample added to 0.5 mL of 1 N NaOH and 0.05 mL of IS (20 &mgr;g/mL) was mixed with 5 mL of n-hexane/dichloromethane (1:1). The mixture was shaken and then centrifuged at 3000 r/min at 5°C for 5 min. The organic phase was dried under a stream of nitrogen. The residue was dissolved in 0.2 mL of 10 mmol/L phosphate buffer (pH 6.1):acetonitrile (77:23), and the solution was injected into an HPLC system (Waters-2690) with a YMC-Pack Pro C18 column (4.6 i.d. × 150 mm, 5 &mgr;m), and a flow rate of 1 mL/min. UV absorbance was quantified at 230 nm. Detection limit was 5 ng/mL.
Intragastric pH is expressed as median values (ranges). Differences in between groups were assessed with the Wilcoxon signed-rank test. P values < 0.05 were considered to be statistical significant.
All 10 volunteers (all men; mean age, 28.7 years) completed the study according to the protocol. There were no adverse events. Three subjects were homo-EMs, 4 were hetero-EMs, and 3 were PM (Table 1).
| Subject | CYP2C19 | Age | Height (cm) | Body weight (kg) | BMI |
| 1 | Homo-EM | 23 | 171 | 70 | 23.9 |
| 2 | Homo-EM | 26 | 170 | 58 | 20.1 |
| 3 | Homo-EM | 28 | 169 | 60 | 21.0 |
| 4 | Hetero-EM | 22 | 165 | 60 | 22.0 |
| 5 | Hetero-EM | 28 | 170 | 80 | 27.6 |
| 6 | Hetero-EM | 40 | 172 | 75 | 25.3 |
| 7 | Hetero-EM | 44 | 181 | 82 | 25.0 |
| 8 | PM | 23 | 172 | 60 | 20.2 |
| 9 | PM | 23 | 175 | 55 | 18.0 |
| 10 | PM | 30 | 174 | 86 | 28.4 |
Median intragastric pH values during the first 6 h after the administration of each drug are shown in Figure 1. The median intragastric pH was significantly higher with lafutidine 10 mg than with LPZ 30 mg 2 h, 3 h, 4 h, 5 h, and 6 h after drug administration.
Mean plasma drug concentrations during the first 6 h after treatment are shown in Figure 2. The time to peak plasma concentration (Tmax) was shorter with lafutidine 10 mg (1 h) than with LPZ 30 mg (2 h)
PPIs and H2RAs are potent agents widely used for the treatment of GERD. Recently, the frequency of GERD has been increasing in Japan. Endoscopic studies have shown that the overall prevalence of reflux esophagitis among Japanese adults is 14% to 16%[34].
The increasing use of on-demand PPI therapy has raised various issues regarding efficacy. On-demand therapy has been reported an alternative to continuous treatment in patients with mild-to-moderate GERD who have frequent symptomatic relapses[1112]. Although many clinicians regard PPIs to be superior to H2RA in terms of continuous gastric acid suppression, a systematic review[13] of the efficacy of PPIs for heartburn relief during the first 1 d to 2 d of therapy found that symptoms were completely relieved for the entire day in about 30% of patients after their first dose. In contrast, H2RAs potently and quickly suppress gastric acid secretion[17] and may thus have advantages over PPIs, especially for the on-demand treatment of GERD.
The incidence of atrophic gastritis in the general population is estimated to be higher in Japan than in western countries[3031], whereas gastric acid levels are generally lower in Japan[1819]. Moreover, endoscopic studies[34] have reported that most Japanese patients have nonerosive reflux disease or mild forms of GERD. Consequently, some Japanese patients use H2RAs rather than PPIs for the management of mild-to-moderate GERD. Against this background, we compared the H2RA lafutidine with LPZ, one of the most widely used PPIs for the treatment of GERD in Japan.
In this study, lafutidine 10 mg was associated with a significantly prompter rise in intragastric pH and stronger inhibition of gastric acid secretion than was LPZ 30 mg during the early period (1-6 h) after administration of a single oral dose of either drug. Moreover, analysis of blood samples collected from randomly selected subjects showed that lafutidine 10 mg produced a significantly faster prompter rise in the plasma drug concentration than did LPZ 30 mg. These findings suggest that lafutidine 10 mg is especially useful for the on-demand treatment of acid-related symptoms in patients with mild GERD because of its prompter onset of action. However, we must consider the fact that H2RA have a number of disadvantages as compared with PPI, including a shorter duration of action and the development of tachyphylaxis, limiting routine use[10].
Our results are attributed to the different mechanisms of action of PPIs and H2RAs. PPIs are absorbed in the small intestine and transported via the systemic circulation to gastric parietal cells, where they bind to the proton pump and potently inhibit gastric acid secretion[32]. Some time is required for the PPIs to accumulate in parietal cells and then inhibit acid secretion. H2RAs are absorbed in the small intestine, reach gastric cells via the systemic circulation, and then directly and rapidly bind to gastric cell histamine receptors, resulting in immediate inhibition of gastric acid secretion.
Inhibition of gastric acid secretion by PPIs is known to significantly depend on CYP2C19 genotype status, as well as on intrinsic pharmacokinetic and pharmacodynamic characteristics and dosing schemes[2021]. PPIs, such as LPZ, omeprazole, and pantoprazole, are mainly metabolized by CYP2C19 in the liver. As stated above, CYP2C19 genotypes are classified into the three groups: homo-EM, hetero-EM, and PM. Plasma PPI levels and intragastric pH values during PPI treatment are lowest in homo-EM, followed by hetero-EM, and highest in PM[23–25]. Although the subjects this study included 3 PMs, lafutidine 10 mg was associated with a prompter rise in median intragastric pH during the early period (1-6 h) after administration of a single oral dose, as compared with LPZ 30 mg.
In conclusion, lafutidine 10 mg has a prompter onset of action than LPZ 30 mg in the early phase (1-6 h) after administration of a single oral dose. Lafutidine may thus offer advantages over LPZ for the on-demand treatment of GERD.
The prevalence of Gastroesophageal reflux disease (GERD) symptoms is now increasing in Japan. Concerning the characteristics of GERD in Japan, it is important to distinguish the significant difference of acid secretion in Japanese patients when compared with that of patients from western countries. Acid secretion among the Japanese individuals lower when compared to that of the western population irrespectively of H pylori infection status. In Japan, histamine H2 receptor antagonists (H2RAs) are thus sometimes used for the treatment of mild-to-moderate GERD.
To compare lafutidine 10 mg (H2RAs) to LPZ 30 mg (proton pump inhibitors: PPI) in the antisecretory activity and blood drug concentration in the immediate early period after administration of a single dose.
Few studies have reported the effect at the early post-administration phase (1-6 h) of single dose of H2RA and PPI.
We clearly state that lafutidine 10 mg has a prompter onset of action than LPZ 30 mg in the early phase (1-6 h) after administration of a single oral dose. It is reported that rapid acid suppression is important for effective pain relief at the onset of treatment in GERD patients. Thereby, our results show that lafutidine offers advantages over LPZ for the on-demand treatment of GERD.
This is a nice clear study. The authors ascertained the effectiveness of lafutidine compared with LPZ in the elevation of intragastric pH in the immediate early period after a single oral administration.
| 1. | Locke GR 3rd, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ 3rd. Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448-1456. |
| 2. | Stanghellini V. Three-month prevalence rates of gastrointestinal symptoms and the influence of demographic factors: results from the Domestic/International Gastroenterology Surveillance Study (DIGEST). Scand J Gastroenterol Suppl. 1999;231:20-28. |
| 3. | Ohara S, Kouzu T, Kawano T, Kusano M. [Nationwide epidemiological survey regarding heartburn and reflux esophagitis in Japanese]. Nippon Shokakibyo Gakkai Zasshi. 2005;102:1010-1024. |
| 4. | Fujimoto K, Iwakiri R, Okamoto K, Oda K, Tanaka A, Tsunada S, Sakata H, Kikkawa A, Shimoda R, Matsunaga K. Characteristics of gastroesophageal reflux disease in Japan: increased prevalence in elderly women. J Gastroenterol. 2003;38 Suppl 15:3-6. |
| 5. | Bate CM, Keeling PW, O'Morain C, Wilkinson SP, Foster DN, Mountford RA, Temperley JM, Harvey RF, Thompson DG, Davis M. Comparison of omeprazole and cimetidine in reflux oesophagitis: symptomatic, endoscopic, and histological evaluations. Gut. 1990;31:968-972. |
| 6. | Feldman M, Harford WV, Fisher RS, Sampliner RE, Murray SB, Greski-Rose PA, Jennings DE. Treatment of reflux esophagitis resistant to H2-receptor antagonists with lansoprazole, a new H+/K(+)-ATPase inhibitor: a controlled, double-blind study. Lansoprazole Study Group. Am J Gastroenterol. 1993;88:1212-1217. |
| 7. | Gough AL, Long RG, Cooper BT, Fosters CS, Garrett AD, Langworthy CH. Lansoprazole versus ranitidine in the maintenance treatment of reflux oesophagitis. Aliment Pharmacol Ther. 1996;10:529-539. |
| 8. | Farley A, Wruble LD, Humphries TJ. Rabeprazole versus ranitidine for the treatment of erosive gastroesophageal reflux disease: a double-blind, randomized clinical trial. Raberprazole Study Group. Am J Gastroenterol. 2000;95:1894-1899. |
| 9. | Vigneri S, Termini R, Leandro G, Badalamenti S, Pantalena M, Savarino V, Di Mario F, Battaglia G, Mela GS, Pilotto A. A comparison of five maintenance therapies for reflux esophagitis. N Engl J Med. 1995;333:1106-1110. |
| 10. | Fujisawa T, Adachi K, Komazawa Y, Mihara T, Azumi T, Katsube T, Furuta K, Kazumori H, Kinoshita Y. Helicobacter pylori infection prevents the occurrence of the tolerance phenomenon of histamine H2 receptor antagonists. Aliment Pharmacol Ther. 2004;20:559-565. |
| 11. | Bour B, Staub JL, Chousterman M, Labayle D, Nalet B, Nouel O, Pariente A, Tocque E, Bonnot-Marlier S. Long-term treatment of gastro-oesophageal reflux disease patients with frequent symptomatic relapses using rabeprazole: on-demand treatment compared with continuous treatment. Aliment Pharmacol Ther. 2005;21:805-812. |
| 12. | Bour B, Staub JL, Chousterman M, Labayle D, Nalet B, Nouel O, Pariente A, Tocque E, Bonnot-Marlier S. Long-term treatment of gastro-oesophageal reflux disease patients with frequent symptomatic relapses using rabeprazole: on-demand treatment compared with continuous treatment. Aliment Pharmacol Ther. 2005;21:805-812. |
| 13. | McQuaid KR, Laine L. Early heartburn relief with proton pump inhibitors: a systematic review and meta-analysis of clinical trials. Clin Gastroenterol Hepatol. 2005;3:553-563. |
| 14. | Saitoh T, Fukushima Y, Otsuka H, Hirakawa J, Mori H, Asano T, Ishikawa T, Katsube T, Ogawa K, Ohkawa S. Effects of rabeprazole, lansoprazole and omeprazole on intragastric pH in CYP2C19 extensive metabolizers. Aliment Pharmacol Ther. 2002;16:1811-1817. |
| 15. | Pantoflickova D, Dorta G, Ravic M, Jornod P, Blum AL. Acid inhibition on the first day of dosing: comparison of four proton pump inhibitors. Aliment Pharmacol Ther. 2003;17:1507-1514. |
| 16. | Koike T, Ohara S, Sehine H, Kawamura M, Abe Y, Inomata Y, Iijima K, Imatani A, Shimosegawa T. Effect of Helicobacter pylori status on intragastric pH during administration of lafutidine or famotidine. Hepatogastroenterology. 2007;54:1280-1284. |
| 17. | Inamori M, Togawa J, Iwasaki T, Ozawa Y, Kikuchi T, Muramatsu K, Chiguchi G, Matsumoto S, Kawamura H, Abe Y. Early effects of lafutidine or rabeprazole on intragastric acidity: which drug is more suitable for on-demand use? J Gastroenterol. 2005;40:453-458. |
| 18. | Haruma K, Kamada T, Kawaguchi H, Okamoto S, Yoshihara M, Sumii K, Inoue M, Kishimoto S, Kajiyama G, Miyoshi A. Effect of age and Helicobacter pylori infection on gastric acid secretion. J Gastroenterol Hepatol. 2000;15:277-283. |
| 19. | Feldman M, Cryer B, McArthur KE, Huet BA, Lee E. Effects of aging and gastritis on gastric acid and pepsin secretion in humans: a prospective study. Gastroenterology. 1996;110:1043-1052. |
| 20. | Shirai N, Furuta T, Moriyama Y, Okochi H, Kobayashi K, Takashima M, Xiao F, Kosuge K, Nakagawa K, Hanai H. Effects of CYP2C19 genotypic differences in the metabolism of omeprazole and rabeprazole on intragastric pH. Aliment Pharmacol Ther. 2001;15:1929-1937. |
| 21. | Horai Y, Kimura M, Furuie H, Matsuguma K, Irie S, Koga Y, Nagahama T, Murakami M, Matsui T, Yao T. Pharmacodynamic effects and kinetic disposition of rabeprazole in relation to CYP2C19 genotypes. Aliment Pharmacol Ther. 2001;15:793-803. |
| 22. | Ohara S, Kato M, Asaka M, Toyota T. Studies of 13C-urea breath test for diagnosis of Helicobacter pylori infection in Japan. J Gastroenterol. 1998;33:6-13. |
| 23. | De Morais SM, Wilkinson GR, Blaisdell J, Meyer UA, Nakamura K, Goldstein JA. Identification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in Japanese. Mol Pharmacol. 1994;46:594-598. |
| 24. | Heid CA, Stevens J, Livak KJ, Williams PM. Real time quantitative PCR. Genome Res. 1996;6:986-994. |
| 25. | Chang M, Tybring G, Dahl ML, Gotharson E, Sagar M, Seensalu R, Bertilsson L. Interphenotype differences in disposition and effect on gastrin levels of omeprazole--suitability of omeprazole as a probe for CYP2C19. Br J Clin Pharmacol. 1995;39:511-518. |
| 26. | Furuta T, Shirai N, Sugimoto M, Nakamura A, Okudaira K, Kajimura M, Hishida A. Effect of concomitant dosing of famotidine with lansoprazole on gastric acid secretion in relation to CYP2C19 genotype status. Aliment Pharmacol Ther. 2005;22:67-74. |
| 27. | Pearce RE, Rodrigues AD, Goldstein JA, Parkinson A. Identification of the human P450 enzymes involved in lansoprazole metabolism. J Pharmacol Exp Ther. 1996;277:805-816. |
| 28. | Oliveira CH, Barrientos-Astigarraga RE, Abib E, Mendes GD, da Silva DR, de Nucci G. Lansoprazole quantification in human plasma by liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;783:453-459. |
| 29. | Huang J, Xu Y, Gao S, Rui L, Guo Q. Development of a liquid chromatography/tandem mass spectrometry assay for the quantification of rabeprazole in human plasma. Rapid Commun Mass Spectrom. 2005;19:2321-2324. |
| 30. | Kawaguchi H, Haruma K, Komoto K, Yoshihara M, Sumii K, Kajiyama G. Helicobacter pylori infection is the major risk factor for atrophic gastritis. Am J Gastroenterol. 1996;91:959-962. |
| 31. | Mihara M, Haruma K, Kamada T, Komoto K, Yoshihara M, Sumii K, Kajiyama G. The role of endoscopic findings for the diagnosis of Helicobacter pylori infection: evaluation in a country with high prevalence of atrophic gastritis. Helicobacter. 1999;4:40-48. |
| 32. | Sachs G, Shin JM, Briving C, Wallmark B, Hersey S. The pharmacology of the gastric acid pump: the H+,K+ ATPase. Annu Rev Pharmacol Toxicol. 1995;35:277-305. |