Published online Apr 21, 2008. doi: 10.3748/wjg.14.2329
Revised: February 26, 2008
Published online: April 21, 2008
AIM: To investigate whether human hepatocytes could proliferate after transplantation to normal immunocompetent rats treated with 2-acetaminofluorene or Retrorsine and partial hepatectomy.
METHODS: L02 hepatocyte-tolerant Sprague-Dawley rats were injected with Retrorsine, 2-acetaminofluorene or normal saline. L02 hepatocytes were then transplanted via the spleen. Human albumin and its mRNA, specific proliferating cell nuclear antigen (PCNA), L02 hepatocyte dynamic distribution, number density and area density of PCNA-positive cells in the liver were determined.
RESULTS: All the examined indicators were not significantly different between the rats treated with 2-acetaminofluorene and normal saline, which was not the case with rats treated with Retrorsine. A dynamic distribution of L02 hepatocytes in the rat liver was detected from wk 1 to mo 6 after transplantation in the Retrorsine group and from wk 1 to 10 in the 2-acetaminofluorene group. Human albumin and its mRNA were detected from wk 2 to mo 6 in the Retrorsine group and from wk 1 to 8 in the 2-acetaminofluorene group. Specific human PCNA was detected in the rat liver from wk 2 to mo 6 in the Retrorsine group and from wk 2 to 6 in the 2-acetaminofluorene group. Human albumin and its mRNA contents as well as the number of PCNA positive cells reached a peak at wk 4.
CONCLUSION: L02 human hepatocytes could not proliferate significiantly after transplantation to the normal, immunocompetent rats treated with 2-acetaminofluorene. L02 human hepatocytes can survive for 10 wk after transplantation and express human albumin for 8 wk. L02 human hepatocytes can proliferate and express human albumin for 6 mo after transplantation to the rats treated with Retrorsine. The chimeric L02 human hepatocytes, which then underwent transplantation into tolerant rats, were normal in morphogenesis, biochemistry and function.
- Citation: Lin H, Mao Q, Wang YM, Jiang L. Proliferation of L02 human hepatocytes in tolerized genetically immunocompetent rats. World J Gastroenterol 2008; 14(15): 2329-2337
- URL: https://www.wjgnet.com/1007-9327/full/v14/i15/2329.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2329
An animal model of rats with chimeric human livers has been created with human hepatocytes being transplanted into rat livers. These rats can be used to identify hepatitis virus with the problem of species-specificity being resolved[1]. With immunodeficiency[2] or induced immunotolerance rats[3], an animal model of rats with chimeric human liver infected with Hepatitis B virus can be established. An animal model of tolerant rats with chimeric human liver can be applied in pathogenesis, antiviral therapy and the development of vaccines[4]. However, human hepatocytes transplanted into rats can survive only a limited time. The lifetime of transplanted hepatocytes must be prolonged to establish a persistent animal model. With an exogenous growth stimulus, such as partial hepatectomy, the recipient hepatocytes can not only regenerate but the transplanted hepatocytes can also proliferate[5]. If restraining the regeneration of recipient hepatocytes with 2-acetaminofluorene (2-AAF), Retrorsine(Rts), dipin, furan, or 3'5-diethoxycarbonyl-1,4-dihydrocollidine (DDC)[6–8], followed by an exogenous growth stimulus such as carbon tetrachloride(CCl4) or partial hepatectomy (PH)[9–11], the proliferation of transplanted hepatocytes can be improved significantly. We investigate whether human hepatocytes could repopulate after transplantation to rats with a normal immune system treated with 2-acetaminofluorene (2-AAF) or Retrorsine (Rts) and partial hepatectomy (PH).
L02 Human hepatocytes were injected into the peritoneal cavities of fetal Sprague-Dawley rats to induce immune tolerance to L02 human hepatocytes. The 2- or 3-wk rats were injected with 2-AAF or Rts. The L02 human hepatocytes stained with 1,1’-Dioctadecyl-3, 3, 3’, or 3’-tetramethylindocarbocyanine perchlorate (DiI) were transplanted into the spleen via intrasplenic injection. The survival and proliferation of human L02 hepatocytes in the rat liver were analyzed by an immunofluorescence method, reverse transcription-polymerase chain reaction (RT-PCR), immunohistochemical study, DiI fluorescence tracing and computer-aided image analysis.
Nine Sprague-Dawley rats at reproductive age were purchased from the Third Military Medical University. Rats at gestational age 15-17 d and weighing 250-300 g were used. They were maintained on an alternating 12-h light/dark cycle with food and water available ad libitum. All studies were conducted under protocols approved by the Laboratory Animal Resource Center of the Third Military Medical University, China.
L02 hepatocytes, the isolated normal human hepatocytes, which have not been immortalized, were purchased from Shanghai Institute of Cell Biology of Chinese Academy of Science. L02 hepatocytes were digested with pancreatin, centrifuged twice at 1100 r/min for 3 min, and then resuspended. Hepatocytes were counted using a blood cell counting chamber and cell viability was assessed by trypan blue exclusion assay. Cells with viability above 80% were used in the study.
Induction of immunotolerance of fetal rats: Sprague-Dawley rats at gestational age 15-17 d were etherized, their abdomens were cut open along the linea alba abdominis, and their uterus was exposed. 50 &mgr;L of human L02 hepatocytes suspension (1 × 108 cells) were slowly injected into the abdominal cavities of the 8-12 fetal rats in utero using a 1 mL-syringe, and then the abdominal walls of the pregnant rats were sutured layer by layer. Pregnant rats were fed till spontaneous delivery.
Drug solution preparation: The drug solution was prepared as described elsewhere[12]. Retrorsine (Sigma Company) was added to distilled water at 10 mg/mL and titrated to pH 2.5 with 1 mol/L HCl. The solution was neutralized with 1 mol/L NaOH, and NaCl was added for a final concentration of 6 mg/mL retrorsine and 0.15 mol/L NaCL (pH 7). The working solution was used immediately after preparation. 2-acetaminofluorene was dissolved in M400 polyethylene glycol.
Animal grouping and drug injection: Pregnant rats were randomized to three groups, namely, the experimental (2AAF and Rts) group and the control group, and 30 fetal rats were assigned to each group. For the Rts group, immunotolerant rats were intraperitoneally injected with retrorsine (30 mg/kg/rat) 3 and 5 wk post birth. For the 2AAF group, immunotolerant rats were intraperitoneally injected with 2-acetaminofluorene (30 mg/kg/rat) 2 wk post birth, once every other day, total 4 injection. For the control group, immunotolerant rats were intraperitoneally injected with physiologic saline and the other procedures were the same as the experimental group. 7 wk post birth, rats of the Rts group and 3 wk post birth, rats of the 2AAF group and the control group were etherized and underwent 2/3 hepatectomy and transplantation of 100 &mgr;L of DiI-labeled human L02 hepatocyte suspension (> 1012 cells/L) through the spleen.
DiI fluorescence staining: The method was described previously[13]. Five &mgr;L of 1 mmol/L Vybrant DiI (Sigma Company) cell-labeling solution dissolved in 100% ethanol was added to 1 mL of culture medium. Cells were stained for 40 min at 37°C in a CO2 incubator. Labeled cells were centrifuged three times by 1100 revolutions per minute (r/min) × 3 min and washed with phosphate-buffered saline (PBS). They were then counted by trypan blue exclusion assay and suspended at 1.0 × 1012/L with PBS. Cells were observed and photographed under the fluorescence microscope (Olympus Company) with a rhodamine filter.
L02 hepatocytes transplantation: 3 or 7-wk-old fetal rats of the experimental group were etherized. The abdomen was cut open along the linea alba abdominis and the spleen was exposed. 100 &mgr;L of DiI-stained L02 hepatocyte suspension was slowly injected along the mesenterial edge into the splenic body and tail. The injection site was pressed gently to prevent hemorrhage or effusion.
Specimen collection: Specimens were collected one week after transplantation, and then at wk 2, 4, 6, 8 and mo 4 and 6. Specimens were collected from four rats at each collection point. Under anesthetization, rats underwent partial hepatectomy, and fresh liver tissue was subjected to frozen sectioning. Sections were observed under the fluorescence microscope with a rhodamine filter. Some tissue was fixated in 40 g/L neutral formalin, embedded in paraffin and sectioned. After surgery, rats were fed as usual.
DiI fluorescence tracing: L02 hepatocytes were stained with DiI and observed under the fluorescence microscope with a rhodamine filter before transplantation. Following L02 hepatocytes transplantation, fresh rat liver tissue was subjected to frozen sectioning and was also observed under the fluorescence microscope.
Detection of human albumin in liver tissue by immunofluorescence method: Fresh liver tissue was subjected to frozen sectioning with a thickness of 4-8 &mgr;m. The sections were placed at room temperature for 30 min and fixated at 4°C in acetone for 10 min. Unspecific staining was blocked at < 37°C with 100 mL/L blocking serum for 20 min. The sections were then added to rat anti-human albumin monoclonal antibody (Sigma Company, 1:400) and kept at 4°C overnight. They were subsequently added to fluorescence labeled a secondary antibody, ie., Fluorescein isothiocyanate (FITC)-labeled goat anti-rat IgG (Beijing Zhongshan Company, 1:100), and incubated at 37°C in darkness for 45 min. The sections were mounted with 900 mL/L buffering glycerol and observed under a laser confocal microscope (Swiss Zeiss Company). For negative control, the primary antibody was replaced by PBS.
Immunohistochemical detection of human PCNA in the liver tissue: Streptavidin peroxidase conjunction (S-P) method was used. In brief, fresh liver tissue was fixated in 40 g/L neutral formalin, embedded in paraffin and sectioned, baked at 60°C for 1 to 2 h, and underwent deparaffinage. The sections were added with PBS containing 0.5 g/L Triton × 100 and incubated at room temperature for 5 min. Then the sections were incubated with 3% hydrogen peroxide at 37°C for 20 min to block endogenous peroxidase. The sections were incubated with specific monoclonal antibody against human proliferating cell nuclear antigen (PCNA) (Chemicon Company, 1:200) or non-specific monoclonal antibody against PCNA (Beijing Dingguo Company, 1:200, against vertebrate PCNA) was added and incubated at 4°C overnight, then polymer boost and horseradish peroxidase-labeled goat anti-rat IgG multimer (Beijing Zhongshan Company) was added. The next steps included 3,3’-Diaminobenzidine (DAB) coloration, after-stain with hematoxylin, mounting with neutral resin, and observation under a light microscope. For negative control, the primary antibody was replaced by PBS.
Cell image analysis: The LeicaQWin image analysis system (Germany Leica Company) was used. Five areas were randomly selected in each section at the magnitude of 100 and the number density (cell/&mgr;m2) and area density (&mgr;m2/&mgr;m2) of PCNA-positive cells were calculated.
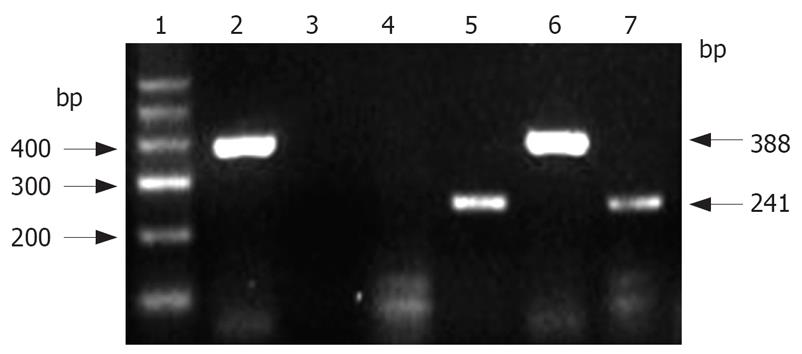
RT-PCR detection of human albumin mRNA in the rat liver tissue: This step was carried out according to the instructions for use of the Trizol RNA extraction kit. The two-step method using RT-PCR kit (BioDev Company) was adopted for reverse transcription and amplification. Primers were signed and synthesized by Takara Company (Dalian, China). Human albumin mRNA primers: forward primer Hs: 5’-TCGACAACGGCTCCGGCAT-3’, reverse primer Ha: 5’-AAGGTGTGGTGCCAGATTTTC-3’, length of the amplified fragment: 241 bp; normal rat albumin mRNA primers: forward primer Rs: 5’-CGGTTTAGGGACTTAGGAGAACAGC-3’, reverse primer Ra: 5’-ATAGTGTCCCAGAAAGCTGGTAGGG-3’, length of the amplified fragment: 388 bp. Amplification was carried out by using the following cycle: initial denaturation at 94°C for 5 min, denaturation at 94°C for 50 s, reassociation at 55°C for 50 s, extension at 72°C for 1 min (32 cycles), and a final extension step at 72°C for 8 min. To exclude false positive results, negative control was set by amplification without AMV reverse transcriptase. The products were subjected to 1.5% agarose gel electrophoresis, ethidium bromide staining and image acquisition using a gel scanner (America Bio-Rad Company).
Data were expressed as mean ± SD. Paired t-tests were adopted to compare group differences using SPSS 10.0.
Anesthetic overdose caused one death in the experimental (2AAF) group and two deaths in the control group. The remaining rats survived.
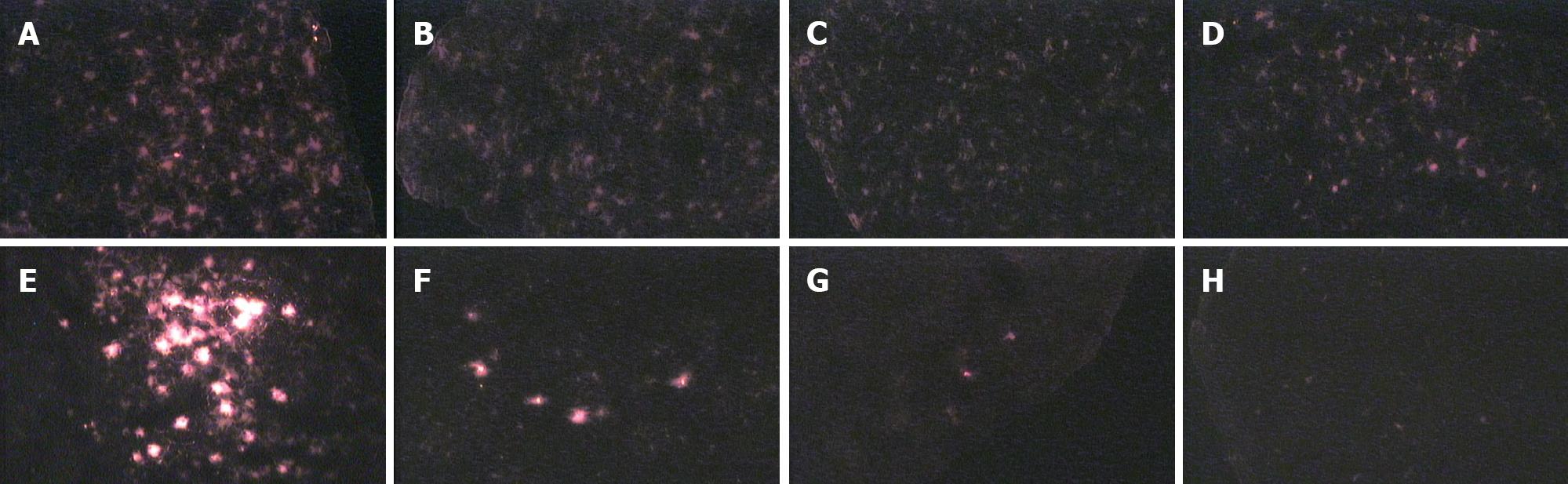
DiI-stained cells gave off red fluorescence seen through a green filter. They were round or elliptical, and evenly stained, and their nuclei could not be differentiated from cytoplasm. At wk 1, 2, 4, 6 and 8 and mo 4 and 6, the distribution of L02 hepatocytes in the rat liver was dynamically observed under the fluorescence microscope in the Rts group. Transplanted L02 hepatocytes distributed in the rat liver parenchyma and weak fluorescence dispersed evenly from wk 1 to mo 4. At mo 6, the number of cells with fluorescence and fluorescence intensity began to decrease, and fluorescence distributed evenly in patches. There was no significant difference between the 2AAF and the control group. Transplanted L02 hepatocytes first distributed in mass in the portal area of the host rat, and then scattered to liver parenchyma. Compared to the Rts group, at the same time point, the number of cells with fluorescence was significantly reduced in the 2AAF or the control group. The number of cells with fluorescence decreased over time and the fluorescence intensity was also weakened over time. Cell fluorescence persisted till week 10 (Figure 1).
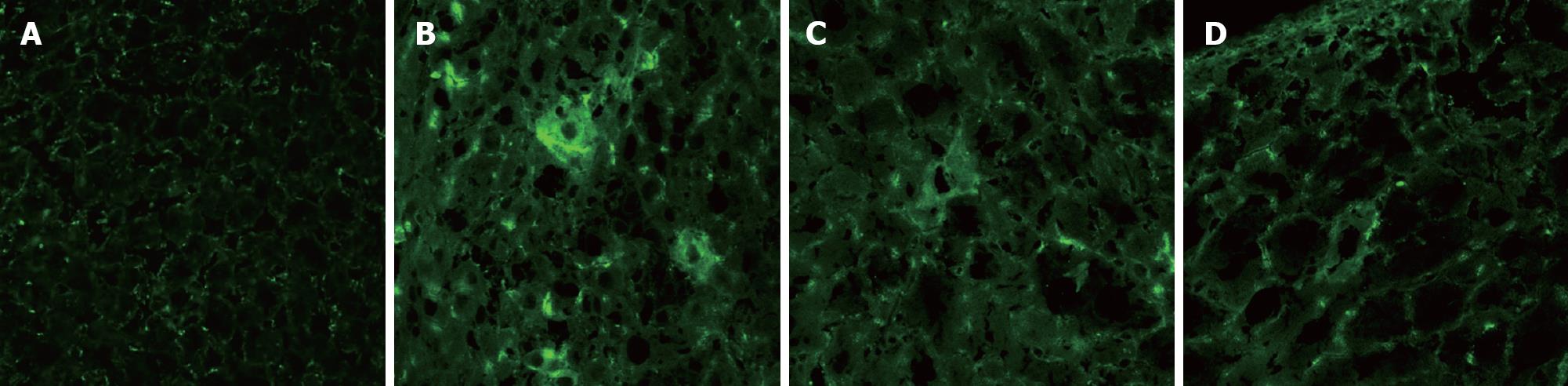
FITC was used to label the secondary antibody, and the positive cell cytoplasm gave off green fluorescence under the laser confocal microscope through the blue filter, and there was a distinct boundary between positive cells and adjacent ones. In the Rts group, at wk 2, 4, 6 and 8 and mo 4 and 6, human albumin was detected, with most cells giving off green fluorescence at week 4. In the 2AAF and the control group, human albumin was detected at wk 2, 4, 6 and 8, but not in 4 rats throughout 10 wk. With time, there was more human albumin at the same time point in the Rts group than in the 2AAF and the control group. In the Rts group liver parenchyma exhibited mild to moderate destruction of hepatic cord, inflammatory cell filtration of the portal area and dispersed enlarged hepatocytes with enlarged nuclei. However, in the 2AAF and the control group these phenomena were not significant. Hepatic fibrosis or liver necrosis was present in neither group (Figure 2).
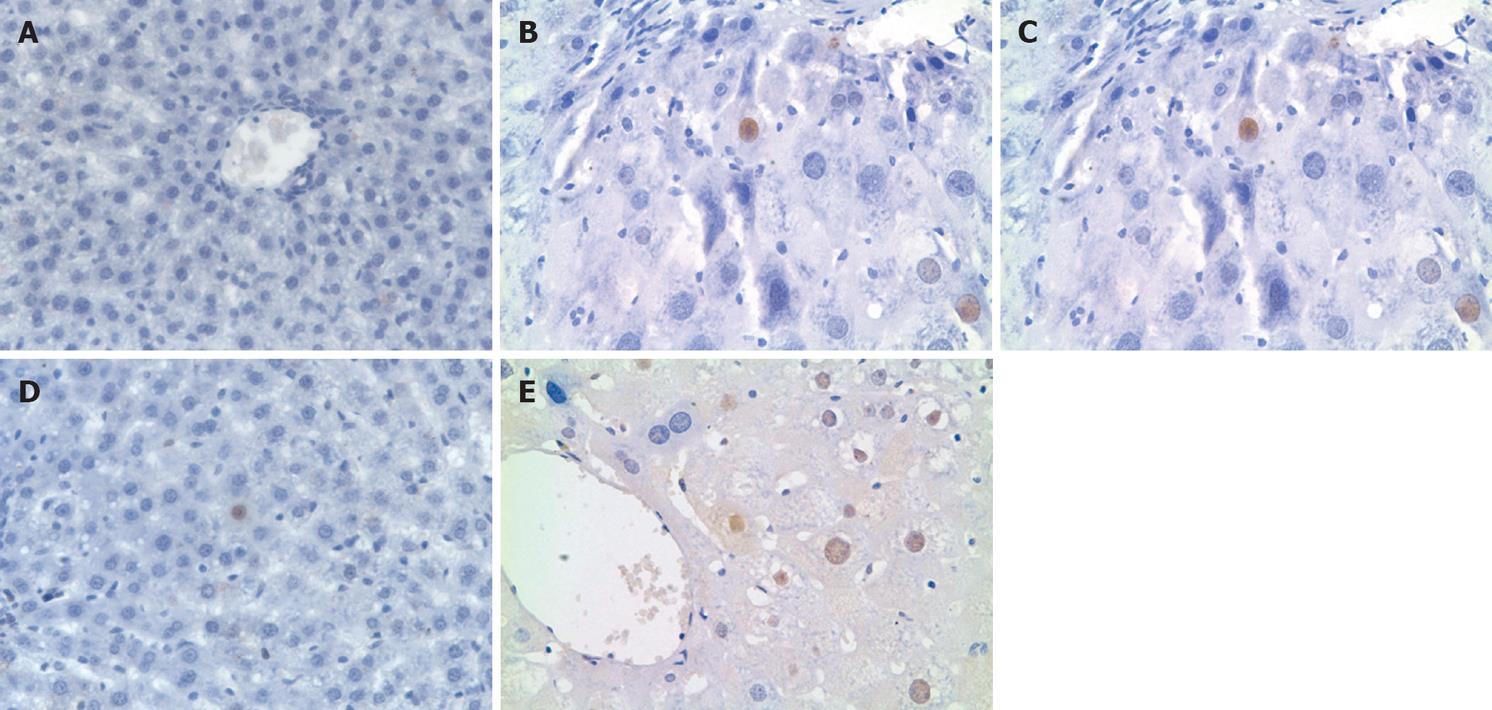
Human PCNA-positive nuclei appeared brown after DAB coloration (Figure 3). The primary antibody was specifically against human PCNA, and there was no human PCNA detected in the rat liver tissue 1 wk after transplantation. Human PCNA was detected in the Rts group at wk 2, 4, 6 and 8, and mo 4 and 6, with the most detected at wk 4. Human PCNA then gradually decreased , however, still appeared at mo 6. In the 2AAF and the control group, human PCNA was detected at wk 2, 4 and 6, with the most detected at wk 4. With a primary antibody not specifically against human PCNA, human PCNA was detected in the rat liver tissue 1 wk after transplantation. In the Rts group, a great amount of non-specific human PCNA was detected at wk 1, 2, 4, 6 and 8, and mo 4 and 6, with no decrease in the number of PCNA-positive nuclei. In the 2AAF and the control group, a great amount of non-specific human PCNA was detected at wk 1, 2, 4, 6 and 8, with no changes in the number of PCNA-positive nuclei.
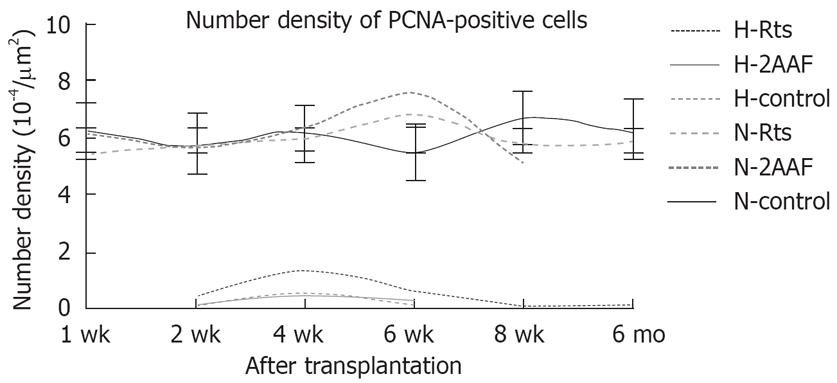
Number density of PCNA-positive cells at various time points were calculated by computer-aided image analysis and were then subjected to statistical analysis. Using the primary antibody specifically against human PCNA, there were significant differences in the number or area density between the Rts and the control group. There were no significant differences between the 2AAF and the control group. While using the primary antibody not specifically against human PCNA, there were no significant differences between either groups (Table 1). The curve chart of number density of PCNA-positive cells is presented in Figure 4.
| Index | Subgroup | 1 wk | 2 wk | 4 wk | 6 wk | 8 wk | 6 mo | |
| Number density | Human PCNA | H-Rts | 0.47 ± 0.08a | 1.33 ± 0.34a | 0.56 ± 0.38a | 0.08 ± 0.03 | 0.11 ± 0.07 | |
| (10-4&mgr;m2/&mgr;m2) | H-2AAF | 0.14 ± 0.03 | 0.43 ± 0.04 | 0.26 ± 0.08 | ||||
| H-control | 0.10 ± 0.07 | 0.46 ± 0.04 | 0.14 ± 0.04 | |||||
| Nonspecific PCNA | N-Rts | 5.53 ± 1.12 | 5.68 ± 0.89 | 6.02 ± 0.87 | 6.78 ± 1.03 | 5.77 ± 0.68 | 5.95 ± 1.73 | |
| N-2AAF | 6.26 ± 1.07 | 5.79 ± 0.75 | 6.32 ± 0.84 | 7.58 ± 1.17 | 5.18 ± 1.03 | |||
| N-Control | 6.20 ± 0.85 | 5.77 ± 1.18 | 6.16 ± 1.02 | 5.46 ± 1.03 | 6.73 ± 0.58 | 6.28 ± 0.73 | ||
| Area density | Human PCNA | H-Rts | 2.14 ± 0.22a | 7.85 ± 1.46a | 3.54 ± 1.63a | 0.58 ± 0.72 | 1.24 ± 0.04 | |
| (10-4&mgr;m2/&mgr;m2) | H-2AAF | 1.58 ± 0.27 | 2.36 ± 0.26 | 1.17 ± 0.22 | ||||
| H-Control | 1.47 ± 0.04 | 2.94 ± 0.64 | 2.37 ± 0.48 | |||||
| Nonspecific PCNA | N-Rts | 74.8 ± 1.06 | 69.3 ± 2.84 | 45.3 ± 3.38 | 77.3 ± 4.84 | 84.7 ± 2.67 | 73.7 ± 5.38 | |
| N-2AAF | 54.6 ± 2.56 | 49.9 ± 3.37 | 52.7 ± 4.46 | 47.5 ± 3.58 | 51.4 ± 3.27 | |||
| N-Control | 84.2 ± 6.84 | 67.5 ± 2.73 | 66.3 ± 4.48 | 74.2 ± 5.73 | 83.3 ± 4.83 | 66.3 ± 5.74 |
Human albumin mRNA was detected at wk 2, 4, 6 and 8, and mo 4 and 6 in the Rts group and at wk 2, 4, 6 and 8 in the 2AAF and the control group. Human albumin mRNA band (241 bp) and rat albumin mRNA band (388 bp) were detected by RT-PCR in the rat liver tissue using relevant human and rat albumin mRNA primers (Figure 5).
Rodent animal models especially play an important role in the development of hepatology. The hepatitis virus has species-specificity and, susceptible hosts are confined to higher grade primates, such as humans, macaques and chimpanzees[14] for various reasons. With immunodeficient or induced immunotolerant rats, rat animal models with chimeric human liver infected with hepatitis virus can be established. During embryonic development, the immune system is not mature, and tolerance of exogenous antigens can occur due to “T lymphocyte clonal deletion”[15]. Hence, animal models for graft tolerance may be established by induction of fetal rat immunotolerance against human hepatocytes by using human fetal hepatocytes and then transplantation of human hepatocytes into normal rat liver[16]. An animal model of tolerant rats with chimeric human hepatocytes was established based on the normal immune system, and the chimeric cells in the rat liver were normal human hepatocytes, which are susceptible to many hepatitis-causing agents. Therefore, this rat liver model may be used for studying known hepatitis-causing agents such as HBV, HCV, and HEV virus as well as unknown hepatitis-causing agents.
With the use of labeling technique, transplanted hepatocytes can be easily differentiated from host hepatocytes under the fluorescence microscope or by flow cytometry, and the function, turnover, distribution and survival of transplanted hepatocytes can be monitored directly. We used the lipophilic fluorescent dye DiI to label the whole cell. DiI fluorescence attenuated slowly. It was reported that DiI-labeled neurons can give off fluorescence for more than 4 wk in vitro and maintain activity for more than 9 mo in vivo. Fluorescence did not attenuate significantly or diffuse to adjacent neurons[17]. In this study, DiI fluorescence tracing and immunohistochemical study demonstrated that transplanted L02 hepatocytes first migrated to the distal portal area and liver sinuses in mass in host rat liver, then to liver parenchyma and were then scattered. Under the action of retrorsine, scattered L02 hepatocytes began to proliferate in clusters. In the Rts group, L02 hepatocytes scattered evenly with weak fluorescence during wk 1 after transplantation, suggesting that these cells migrated to the portal area and underwent cell division immediately after migrating to liver parenchyma, leading to weakened fluorescence. In the 2AAF and control groups, the fluorescence intensity was relatively high at the same time point, but the number of cells with fluorescence was significantly small compared to the experimental group, and both were gradually decreased, suggesting less cell divisions. The results demonstrated that retrorsine can significantly promote transplanted L02 hepatocytes to proliferate, but 2AAF cannot. After integrated into the parenchymal plates, transplanted hepatocytes exhibited albumin and enzyme expression and metabolism of biliary. It was observed that there was full reconstitution of gap junction and biliaral struction between the transplanted and host hepatocytes 5 d after transplantation. This means that successful integration needs at least 5 d[18]. In our study, the cells with fluorescence were detected 1 wk after transplantation but human albumin and mRNA were not detected, suggesting that transplanted L02 hepatocytes did not have the ability of human albumin exhibition and proliferation yet, though had been transferred and intergrated into the parenchymal plates. Cell fluorescence lasted for more than 6 mo in the Rts group and 8 wk in the 2AAF and control groups, confirming that DiI attenuates very slowly. In the 2AAF and control groups, disappearance of fluorescence was not due to attenuation but to limited proliferating capability of transplanted cells and decreases in cell viability.
In this study, by using immunofluorescence and RT-PCR, we detected human albumin and human albumin mRNA at wk 2, 4, 6 and 8, and mo 4 and 6 in the rat liver tissue with chimeric human hepatocytes in the Rts group and at wk 2, 4, 6 and 8 in the 2AAF and control groups. This further confirms the presence and biological function of human L02 cells in the chimeric rat liver. Immunological rejection normally happened 3 wk after transplantation[19]. So we could conclude that the dissolution of transplanted cells was not due to immunological rejection with the host, but to limited proliferating ability of transplanted hepatocytes[20]. The presence of human L02 hepatocytes persisted significantly longer in the Rts group than in the 2AAF and control groups. Hepatic fibrosis or necrosis was observed in neither group, suggesting that retrorsine promotes L02 hepatocyte proliferation in the rat liver tissue, and that hepatotoxicity of retrorsine does not affect the biochemical function of transplanted cells. In the Rts group, destruction of liver cord, remarkable inflammatory cell filtration and hepatocytes with enlarged nuclei in liver parenchyma were observed; however, these phenomena were not obvious in the 2AAF and control groups. These findings reflected the integration of transplanted hepatocytes and host cells as well as gradual proliferation and replacement of transplanted cells. The enlarged hepatocytes in rats exposed to retrorsine are interpreted to result from endoreduplication of host cells, which are able to duplicate DNA but cannot proceed through mitosis. Consistent with this interpretation, formation of enlarged cells is dependent on the presence of a stimulus for cell division. The hepatocytes of normal size and appearance could originate either from transplanted or host hepatocytes that were able to withstand the inhibitory effect of retrorsine or from progenitor cells that are unaffected by retrorsine and can differentiate into seemingly normal hepatocytes[21]. Laconi et al found that since total weight of liver and DNA content did not exceed the normal range during the proliferation of transplanted cells, the transplanted cells not only simply coexist with host cells but replace them[22]. Besides retrorsine, transplanted cells per se may also accelerate the replacement of host cells.
Retrorsine and 2/3 hepatectomy (Rts/PH) in combination are frequently used to stimulate proliferation of the transplanted hepatocytes. A two-thirds hepatectomy stimulates chimeric liver regeneration and increases the rate of host cell renewal. Retrorsine potently and persistently blocks the cell cycle of host hepatocytes, but does not affect the cell cycle of transplanted hepatocytes, resulting in stronger growth vigor of transplanted hepatocytes. However, the precise mechanism involved remains unclear. It was shown that compared to injection of retrorsine, injection of a great number of hepatocytes (> 1 × 108 cells) into the spleen, repeated transplantation of hepatocytes or induction of liver toxicity before cell transplantation influenced hepatocyte proliferation mildly[23]. Although the toxicity of retrorsine may lead to hepatocyte necrosis, it did not affect hepatocyte proliferation, thus shedding light on efficient proliferation of transplanted hepatocytes and the clinical application of retrorsine.
Retrorsine is a pyrrolidine alkaloid derived from Senecio plants, which may potently and persistently block the cell cycle of proliferating host hepatocytes, while not affecting the cell cycle of proliferating transplanted hepatocytes, thus enhancing the selective growth of transplanted hepatocytes. Despite a short half life period, retrorsine may suppress hepatocyte proliferation for weeks and even months[24]. Retrorsine mainly blocks phases S and G2 of the liver cell cycle; that is, it blocks cell mitosis. Moreover, it may prevent hepatocytes from entering phase S, binding to DNA, and blocking DNA synthesis[25]. Other DNA-binding agents such as diethylnitrosamine cannot maintain the proliferation of transplanted hepatocytes, indicating the peculiarity of alkaloid[22]. Laconi et al found that in the absence of exogenous growth stimuli, such as partial hepatectomy, retrorsine treatment may give rise to results similar to those of a combination of retrorsine treatment and partial hepatectomy, suggesting that besides maintenance of selective growth vigor of transplanted hepatocytes, there may be another mechanism of retrorsine action[26]. Gordon et al investigated the mechanism by which retrorsine causes elimination of irreversibly damaged giant hepatocytes, finding that retrorsine can block liver cell mitosis and results in the accumulation of cells at late phase S and phase G2, and induces apoptosis and cell injury depending on the relative levels of the proapoptotic protein Bax and the anti-apoptotic protein Bcl-xl[27].
2AAF is a hepatotoxic drug that blocks DNA synthesis and suppresses proliferation of host hepatocytes, finally activating the proliferation of oval cells[2829]. In our study, we investigated whether 2AAF can promote the proliferation of transplanting human hepatocytes in the tolerant rat liver.
The computer-aided image analysis system has been widely used to analyze immunohistochemical images and calculate the number, number density, area, and area density of transplanted hepatocytes, maximum cell diameter and other morphological parameters. In our study, the number density and area density of PCNA positive cells at various time points were calculated by computer-aided image analysis and were then subjected to statistical analysis. It was found that, using the primary antibody specifically against human PCNA, human PCNA was observed at wk 2, 4 and 6 both in the 2AAF and control group, showing that human L02 cells in proliferative phase survived in the rat livers.
Image analysis revealed that there were not significant differences in the number density and area density between both groups, suggested that 2AAF could not promote the proliferation of transplanted cells. In both groups, the highest number of proliferating L02 hepatocytes was observed at wk 4, showing the strongest proliferating capability at wk 4. The proliferating L02 hepatocytes disappeared at wk 8, but fluorescence tracing and immunohistochemical results showed that transplanted hepatocytes still survived and owned some functions at wk 8. Image analysis revealed that there were significant differences in the number density and area density between both groups, suggesting that retrorsine significantly promoted the proliferation of transplanted cells. Human PCNA was observed at wk 2, 4, 6 and 8, and mo 4 and 6 in the Rts group and at wk 2, 4 and 6 in the control group, and human PCNA persisted significantly longer in the Rts group than in the control group, suggesting that retrorsine can inhibit the proliferation of host hepatocytes and promote the activation of oval cells and the proliferation of transplanted hepatocytes. Zheng et al postulated that regeneration of severely injured liver following retrorsine treatment may first involve the proliferation and differentiation of transplanted adult hepatocytes, and then the proliferation and differentiation of mono- or bi-potent precursor cells[30].
In the Rts group, the highest number of proliferating L02 hepatocytes was observed at wk 4, with significantly more cells in the Rts group than in the control group, and following this the number gradually dropped. At mo 4 after transplantation, there was a small number of positive cells. This number was similar to that at mo 6, suggesting that the high-level differentiation and proliferation of L02 hepatocytes in the rat liver persisted till mo 4, and L02 hepatocytes still replicated at low levels during late stages. Zheng et al transplanted DPPIV + hepatocytes into DPPIV-rats subjected to retrorsine treatment and partial hepatectomy, and they found that DNA replication and cell division were the most frequent at mo 1 and then subsided, and that a platform of cell proliferation was reached at mo 3, but with DNA replication remaining at a low level[30]. Our findings are largely in agreement with their results.
Using a primary antibody not specifically against human PCNA, there were no significant differences in the persisting time and number of proliferating L02 hepatocytes between the experimental (Rts or 2AAF) groups and the control group. Non-specific PCNA presented in large amounts after wk 1, demonstrated that the proliferation of rat hepatocytes was remarkable and persistent following partial hepatectomy. Seventy percent hepatectomy is frequently used to reproduce the liver regeneration models in rats. Transplanted hepatocytes accounted for a minority of the cell population in the chimeric liver and partial hepatectomy caused the proliferation of just a few L02 cells. Moreover, even if retrorsine enhanced the proliferation of transplanted cells, specific human PCNA-positive cells were substantially fewer than non-specific PCNA-positive cells at the same time point. Non-specific PCNA was observed at wk 1, while specific human PCNA was not. Retrorsine permitted DNA synthesis in the host hepatocytes and mainly blocked hepatocytes at late phase S and phase G2; that is, it blocked cell mitosis and inhibited the proliferation of host hepatocytes. Through this mechanism[25], hepatocytes with enlarged nuclei emerged following retrorsine treatment, partially because of replication and synthesis of DNA in host hepatocytes and failure of these cells to undergo mitosis, which indicates that selective proliferation of transplanted hepatocytes in retrorsine treated animals is dependent, at least in part, on the persistent cell cycle block imposed by retrorsine on host cells. 2AAF blocked DNA synthesis and suppressed proliferation of host hepatocytes, and mainly promoted the proliferation of oval cells. But in our study, 2AAF did not promote the proliferation of transplanted L02 hepatocytes.
Retrorsine promoted the proliferation of L02 hepatocytes in rat liver with chimeric human hepatocytes and prolonged cell survival, and thus a stable, persistent animal model was achieved. The study sheds light on the usefulness of rat liver models with chimeric human hepatocytes; the animal model based on the normal immune system may open up a new avenue of study on the immune response and pathogenetic mechanism of viral infection, and antiviral therapy as well as the development of potent vaccines.
An animal model of rats with chimeric human liver is that human hepatocytes are transplanted into rat liver. But human hepatocytes transplanted into rats just can survive a limited time. The live time of transplanted hepatocytes must be prolonged to establish a persistent animal model.
Retrorsine, or other hepatotoxicity drugs and 2/3 hepatectomy in combination are frequently used to stimulate proliferation of the transplanted hepatocytes. 2/3 hepatectomy stimulates chimeric liver regeneration and increases the rate of host cell renewal. Retrorsine potently and persistently blocks the cell cycle of host hepatocytes, but does not affect the cell cycle of transplanted hepatocytes, resulting in stronger growth vigor of transplanted hepatocytes. 2AAF is a hepatotoxicity drug to block DNA synthesis and suppress proliferation of host hepatocytes, finally activated the proliferation of oval cells.
The animal model of tolerant rats with chimeric human hepatocytes we established was based on the normal immune system, and the chimeric cells in the rat liver were normal human hepatocytes. With the use of DiI labeling technique, transplanted hepatocytes can be easily differentiated from host hepatocytes under the fluorescence microscope, and the function, turnover, distribution and survival of transplanted hepatocytes can be monitored directly. The survival and proliferation of human L02 hepatocytes in the rat liver were analyzed by immunofluorescence method, reverse transcription-polymerase chain reaction (RT-PCR), immunohistochemical study, and computer-aided image analysis.
The chimeric cells in the rat liver were normal human hepatocytes, which are susceptible to many hepatitis-causing agents. Therefore, this rat liver model may be used for studying known hepatitis-causing agents such as HBV, HCV, and HEV virus and unknown hepatitis-causing agents. The animal model of tolerant rats with chimeric human liver can be applied in pathogenesis, antiviral therapy and development of vaccine.
An animal model of rats with chimeric human liver is that human hepatocytes are transplanted into rat liver. With induced immunotolerance rats, it can establish animal model of rats with chimeric human liver infected with Hepatitis B virus, which can be applied in pathogenetic mechanism of viral infection, antiviral therapy and so on.
There is interest in humanized rats and mice for studies of human metabolism and transplantation. In this paper rat livers are repopulated with human hepatocytes after chemical treatment with retrorsine and partial hepatectectomy.
| 1. | Zhu Y, Yamamoto T, Cullen J, Saputelli J, Aldrich CE, Miller DS, Litwin S, Furman PA, Jilbert AR, Mason WS. Kinetics of hepadnavirus loss from the liver during inhibition of viral DNA synthesis. J Virol. 2001;75:311-322. |
| 2. | Mercer DF, Schiller DE, Elliott JF, Douglas DN, Hao C, Rinfret A, Addison WR, Fischer KP, Churchill TA, Lakey JR. Hepatitis C virus replication in mice with chimeric human livers. Nat Med. 2001;7:927-933. |
| 3. | Wu CH, Ouyang EC, Walton CM, Wu GY. Human hepatocytes transplanted into genetically immunocompetent rats are susceptible to infection by hepatitis B virus in situ. J Viral Hepat. 2001;8:111-119. |
| 4. | Sass DA, Shakil AO. Fulminant hepatic failure. Gastroenterol Clin North Am. 2003;32:1195-1211. |
| 5. | Mizuguchi T, Mitaka T, Katsuramaki T, Hirata K. Hepatocyte transplantation for total liver repopulation. J Hepatobiliary Pancreat Surg. 2005;12:378-385. |
| 6. | Fandrich F, Ruhnke M. Stem cells and liver replacement. Med Klin (Munich). 2003;98 Suppl 2:18-22. |
| 7. | Witek RP, Fisher SH, Petersen BE. Monocrotaline, an alternative to retrorsine-based hepatocyte transplantation in rodents. Cell Transplant. 2005;14:41-47. |
| 8. | Menthena A, Deb N, Oertel M, Grozdanov PN, Sandhu J, Shah S, Guha C, Shafritz DA, Dabeva MD. Bone marrow progenitors are not the source of expanding oval cells in injured liver. Stem Cells. 2004;22:1049-1061. |
| 9. | Dahlke MH, Popp FC, Bahlmann FH, Aselmann H, Jager MD, Neipp M, Piso P, Klempnauer J, Schlitt HJ. Liver regeneration in a retrorsine/CCl4-induced acute liver failure model: do bone marrow-derived cells contribute? J Hepatol. 2003;39:365-373. |
| 10. | Guha C, Deb NJ, Sappal BS, Ghosh SS, Roy-Chowdhury N, Roy-Chowdhury J. Amplification of engrafted hepatocytes by preparative manipulation of the host liver. Artif Organs. 2001;25:522-528. |
| 11. | Okumoto K, Saito T, Haga H, Hattori E, Ishii R, Karasawa T, Suzuki A, Misawa K, Sanjo M, Ito JI. Characteristics of rat bone marrow cells differentiated into a liver cell lineage and dynamics of the transplanted cells in the injured liver. J Gastroenterol. 2006;41:62-69. |
| 12. | Avril A, Pichard V, Bralet MP, Ferry N. Mature hepatocytes are the source of small hepatocyte-like progenitor cells in the retrorsine model of liver injury. J Hepatol. 2004;41:737-743. |
| 13. | Han MZ, Zhou YN, Zhao XQ, Li FD, Han GP, Zhao RB, Wen J. Use DiI as a tracer in mouse bone marrow cells transplantation via portal vein. Chin J Organ Transplant. 2003;24:251-255. |
| 14. | Dandri M, Burda MR, Gocht A, Torok E, Pollok JM, Rogler CE, Will H, Petersen J. Woodchuck hepatocytes remain permissive for hepadnavirus infection and mouse liver repopulation after cryopreservation. Hepatology. 2001;34:824-833. |
| 15. | Kim HB, Shaaban AF, Milner R, Fichter C, Flake AW. In utero bone marrow transplantation induces donor-specific tolerance by a combination of clonal deletion and clonal anergy. J Pediatr Surg. 1999;34:726-729; discussion 729-730. |
| 16. | Wu GY, Konishi M, Walton CM, Olive D, Hayashi K, Wu CH. A novel immunocompetent rat model of HCV infection and hepatitis. Gastroenterology. 2005;128:1416-1423. |
| 17. | Vidal-Sanz M, Villegas-Perez MP, Bray GM, Aguayo AJ. Persistent retrograde labeling of adult rat retinal ganglion cells with the carbocyanine dye diI. Exp Neurol. 1988;102:92-101. |
| 18. | Koenig S, Stoesser C, Krause P, Becker H, Markus PM. Liver repopulation after hepatocellular transplantation: integration and interaction of transplanted hepatocytes in the host. Cell Transplant. 2005;14:31-40. |
| 19. | Song E, Chen J, Antus B, Su F, Wang M, Exton MS. Adenovirus-mediated Bcl-2 gene transfer inhibits apoptosis and promotes survival of allogeneic transplanted hepatocytes. Surgery. 2001;130:502-511. |
| 20. | Oertel M, Rosencrantz R, Chen YQ, Thota PN, Sandhu JS, Dabeva MD, Pacchia AL, Adelson ME, Dougherty JP, Shafritz DA. Repopulation of rat liver by fetal hepatoblasts and adult hepatocytes transduced ex vivo with lentiviral vectors. Hepatology. 2003;37:994-1005. |
| 21. | Dabeva MD, Laconi E, Oren R, Petkov PM, Hurston E, Shafritz DA. Liver regeneration and alpha-fetoprotein messenger RNA expression in the retrorsine model for hepatocyte transplantation. Cancer Res. 1998;58:5825-5834. |
| 22. | Laconi E, Laconi S. Principles of hepatocyte repopulation. Semin Cell Dev Biol. 2002;13:433-438. |
| 23. | Laconi E, Oren R, Mukhopadhyay DK, Hurston E, Laconi S, Pani P, Dabeva MD, Shafritz DA. Long-term, near-total liver replacement by transplantation of isolated hepatocytes in rats treated with retrorsine. Am J Pathol. 1998;153:319-329. |
| 24. | Guo D, Fu T, Nelson JA, Superina RA, Soriano HE. Liver repopulation after cell transplantation in mice treated with retrorsine and carbon tetrachloride. Transplantation. 2002;73:1818-1824. |
| 25. | Laconi S, Curreli F, Diana S, Pasciu D, De Filippo G, Sarma DS, Pani P, Laconi E. Liver regeneration in response to partial hepatectomy in rats treated with retrorsine: a kinetic study. J Hepatol. 1999;31:1069-1074. |
| 26. | Laconi S, Pillai S, Porcu PP, Shafritz DA, Pani P, Laconi E. Massive liver replacement by transplanted hepatocytes in the absence of exogenous growth stimuli in rats treated with retrorsine. Am J Pathol. 2001;158:771-777. |
| 27. | Gordon GJ, Coleman WB, Grisham JW. Bax-mediated apoptosis in the livers of rats after partial hepatectomy in the retrorsine model of hepatocellular injury. Hepatology. 2000;32:312-320. |
| 28. | Paku S, Dezso K, Kopper L, Nagy P. Immunohistochemical analysis of cytokeratin 7 expression in resting and proliferating biliary structures of rat liver. Hepatology. 2005;42:863-870. |
| 29. | Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168-1170. |
| 30. | Zheng YW, Ohkohchi N, Taniguchi H. Quantitative evaluation of long-term liver repopulation and the reconstitution of bile ductules after hepatocellular transplantation. World J Gastroenterol. 2005;11:6176-6181. |