Published online Apr 7, 2008. doi: 10.3748/wjg.14.2023
Revised: January 15, 2008
Published online: April 7, 2008
AIM: To investigate the role of pancreatic stellate cells (PSCs) and galectin-3 (GAL-3) in the proliferation and infiltration of pancreatic cancer cell line SW1990.
METHODS: Human pancreatic cancer cell line SW1990 and PSCs were cultured in vitro. Supernatant fluid of cultured PSCs and SW1990 cells was collected. Expression of GAL-3 in SW1990 cells and PSCs was detected by ELISA, RT-PCR and Western blotting. Proliferation of cultured PSCs and SW1990 cells was measured by 3-(4, 5-methylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay and flow cytometry. Infiltration of SW1990 cells was detected by a cell infiltration kit.
RESULTS: SW1990 cells expressed GAL-3 and this was up-regulated by the supernatant fluid of cultured PSCs. PSCs did not express GAL-3. SW1990 cells stimulated proliferation of PSCs via GAL-3. GAL-3 antibody inhibited SW1990 cell proliferation, while the supernatant fluid of PSCs stimulated proliferation of SW1990 cells through interaction with GAL-3 protein. The supernatant fluid of PSCs enhanced the invasiveness of SW1990 cells through interaction with GAL-3.
CONCLUSION: GAL-3 and PSCs were involved in the proliferation and infiltration process of pancreatic cancer cells.
-
Citation: Jiang HB, Xu M, Wang XP. Pancreatic stellate cells promote proliferation and invasiveness of human pancreatic cancer cells
via galectin-3. World J Gastroenterol 2008; 14(13): 2023-2028 - URL: https://www.wjgnet.com/1007-9327/full/v14/i13/2023.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.2023
Tumor desmoplasia, a process in which fibrous tissue (e.g., collagen, fibronectin and laminin) infiltrates and envelops neoplasia, is one of the representative histopathological findings in ductual adenocarcinoma of the pancreas. The desmoplastic reaction may contribute to the rapid progression, early metastasis, and a limited response to chemotherapy and radiotherapy of pancreatic carcinoma[1–5]. Some studies have confirmed that pancreatic cancer cells activate pancreatic stellate cells (PSCs) via transforming growth factor (TGF)-β and other cytokines[67]. Although pancreatic carcinoma cells are able to produce the fibrotic extracellular matrix (ECM) that surrounds carcinoma, most studies have indicated that the fibrotic ECM is mainly produced and secreted by PSCs[8].
Galectin-3 (GAL-3), is a member of the β-galactoside-binding protein family which recognizes the N-acetyllactosamine structure of various glycoconjugates[910]. Studies on hepatic stellate cells (HSCs) have shown that GAL-3 stimulates HSC DNA synthesis in a dose-dependent manner, but no report on the effect of GAL-3 on PSCs has been published[11]. Some studies have been carried out to evaluate the role of GAL-3 in carcinoma proliferation, infiltration and metastasis[12–18], but few of these were on pancreatic cancer. Some immunohistochemical studies have reported that GAL-3 is expressed in pancreatic cancer[1920]. It has been confirmed that laminin, one important component of the fibrotic ECM that surrounds pancreatic cancer, can be recognized by GAL-3, so GAL-3 may play an important role in the progression of pancreatic cancer.
SW1990 cells were cultured in 100 mL culture bottles containing Dulbecco’s modified Eagle’s medium (DMEM, Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco) 100 U/mL penicillin and 100 U/mL streptomycin at 37°C in a 5% CO2/air humidified atmosphere. PSCs were provided by our laboratory and were cultured in DMEM/F-12 supplemented with 10% FBS and antibiotics under the same conditions.
SW1990 cells and PSCs cultured in 100 mL bottles were washed in PBS and then incubated with serum-free DMEM (5 mL/bottle) for 24 h. Supernatants of cells were collected and filtered under sterilized conditions to remove cell debris, and were stored at -80°C. The concentration of GAL-3 protein in these supernatants was detected by ELISA (Bender Systems).
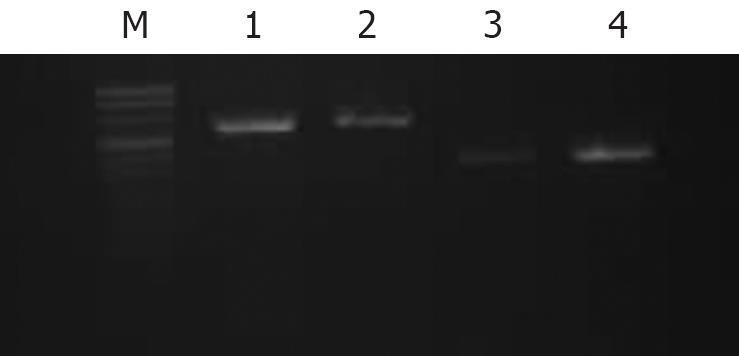
SW1990 cells were washed in PBS and incubated with serum-free DMEM for 24 h. The cells were divided into two groups, one was still incubated with serum-free medium, and the other was exposed to serum-free DMEM supplemented with 40% (v/v) supernatants of PSCs (SPSCs). Cells were harvested and counted 24 h later. Total RNA of 106 cells in each group was extracted according to the manufacturer’s instructions (TRIzol, Gibco-BRL, Rockville, MD, USA). The concentration and purity of RNA was determined by measuring the absorbance at 260 and 280 nm. After that, 1 &mgr;g total RNA in each group was reversed-transcribed to cDNA and amplified by RT-PCR (Gibco) according to the manufacturer’s instructions. For detection of GAL-3 mRNA, the following oligonucleotide primers were used: 5'-ATGATGCGTTATCTGGGTCT-3' and 3'-TATTGGACGGAAACGGAC-5'. The amplification reaction involved denaturation at 94°C for 5 min followed by 35 cycles of denaturation at 94°C for 1 min, annealing for 1 min at 58°C, and extension for 1 min 30 s at 97°C. Expression of GAL-3 by PSCs was also detected by RT-PCR.
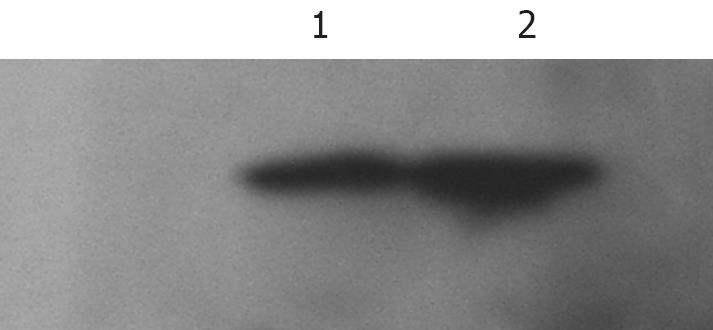
Two groups of SW1990 cells were prepared as mentioned for RT-PCR. SW1990 cells were centrifuged at 600 g for 10 min. The cell pellet was washed twice with ice-cold PBS, resuspended in 150 &mgr;L lysis buffer (1% Triton X-100 in 5 mmol/L Tris-HCl, pH 8.0, 15 mmol/L NaCl, 2 mmol/L PMSF). The fragmented cells were scraped and removed into a sterilized Eppendorf tube and conserved on ice for 20 min, then centrifuged at 12 000 r/min for 20 min, and unresolved debris was discarded. Proteins were transferred onto PVDF membranes using the wet transfer technique, and the membranes were incubated overnight with monoclonal mouse anti-human-GAL-3 antibodies (R&D) diluted 1:200 in TBS. After 1 h incubation with horseradish peroxidase-labeled secondary antibodies (goat anti-mouse IgM diluted 1:10 000 in TBS), GAL-3 was visualized with the ECL Western Blot Detection Kit (Gibco).
Cell growth experiments were performed using the 3-(4, 5-methylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay and were reconfirmed by cell cycle analysis, which was performed by flow cytometry. In the MTT assay, cells were seeded with medium that contained 10% FBS at a density of 6000 cells/well in 96-well plates, grown overnight, washed in PBS, and incubated with serum-free medium for 24 h. Cells were exposed to different concentration of SPSC (5%, 10%, 20% or 40%), supernatants of SW1990 cells (SSW; 5%, 10%, 20% or 40%), GAL-3 monoclonal antibody (GAL-3 MA; 10, 50 or 250 ng/mL, or 1.25 &mgr;g/mL), or recombinant human GAL-3 protein (5, 25, 125 or 625 ng/mL). Twenty-four hours later, MTT was added (50 &mgr;g/well) for 4 h. Formazan products were solubilized with DMSO, and the optical density was measured at 490 nm.
For flow cytometry, cells cultured in 100-mL culture bottles were washed in PBS and incubated with serum-free medium for 24 h. Cells were then exposed to 40% SPSC (for SW1990 cells), 40% SSW (for PSCs), 1 &mgr;g/mL GAL-3 MA or 100 ng/mL recombinant GAL-3 protein for 24 h. Cells were harvested and resuspended in fixation fluid at a density of 106/mL, then 1800 &mgr;L trypsin solution was added to the fixation fluid, followed by 1500 &mgr;L RNase solution. Several minutes later, 1500 &mgr;L propidium iodide solution was added, and 15 min later, cells were filtered, and the cell cycle was detected by FACSCaliber (Becton Dickinson).
Invasion assays were carried out following the manufacturer’s instructions of cell invasion assay kit (Chemicon International Inc., catalog: ECM550). For the invasion assay, we used a modified Boyden chamber. The chamber had two compartments divided by a polycarbonate filter (8 &mgr;m pore size), coated with a reconstituted basement membrane (ECMatrix solution). 3 × 105 SW1990 cells were added to each upper compartment, and chemoattractant fluid was added to the lower compartment (Group A: control group, serum-free medium in the upper compartment and medium containing 2% FBS in the lower compartment; Group B, serum-free medium containing 1 &mgr;g/mL GAL-3 MA in the upper compartment and medium containing 2% FBS in the lower compartment; Group C, serum-free medium in the upper compartment and medium containing 2% FBS and 40% SPSC in the lower compartment; Group D, serum-free medium containing 1 &mgr;g/mL GAL-3 MA in the upper compartment and medium containing 2% FBS and 40% SPSC in the lower compartment). After 48 h incubation, non-invading cells were removed by cotton-tipped swabs and the filters were stained in the staining solution for 20 min and rinsed several times in water and air dried. Three filters were used per group. The number of invading cells was counted in 10 random high-powered fields per filter under a Zeiss microscope.
The data were expressed as mean ± SD and compared by ANOVA and the bivariate correlate test. P < 0.05 was considered statistically significant.
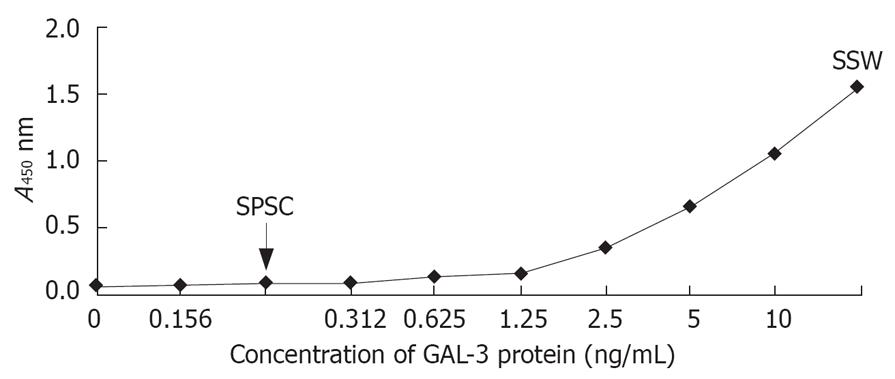
GAL-3 protein in SSWs and SPSCs was measured by ELISA kit (Figure 1). The concentration of GAL-3 in SSWs was above the upper limit of the ELISA kit detection range (10 ng/mL), which meant that SW1990 cells could secrete relatively large amounts of GAL-3 into the extracellular fluid. There was little GAL-3 in SPSCs.
Both RT-PCR (Figure 2) and Western blotting (Figure 3) confirmed that SW1990 cells expressed GAL-3, and the SPSCs up-regulated expression of GAL-3. PSCs showed no expression of GAL-3 according to RT-PCR and Western blotting.
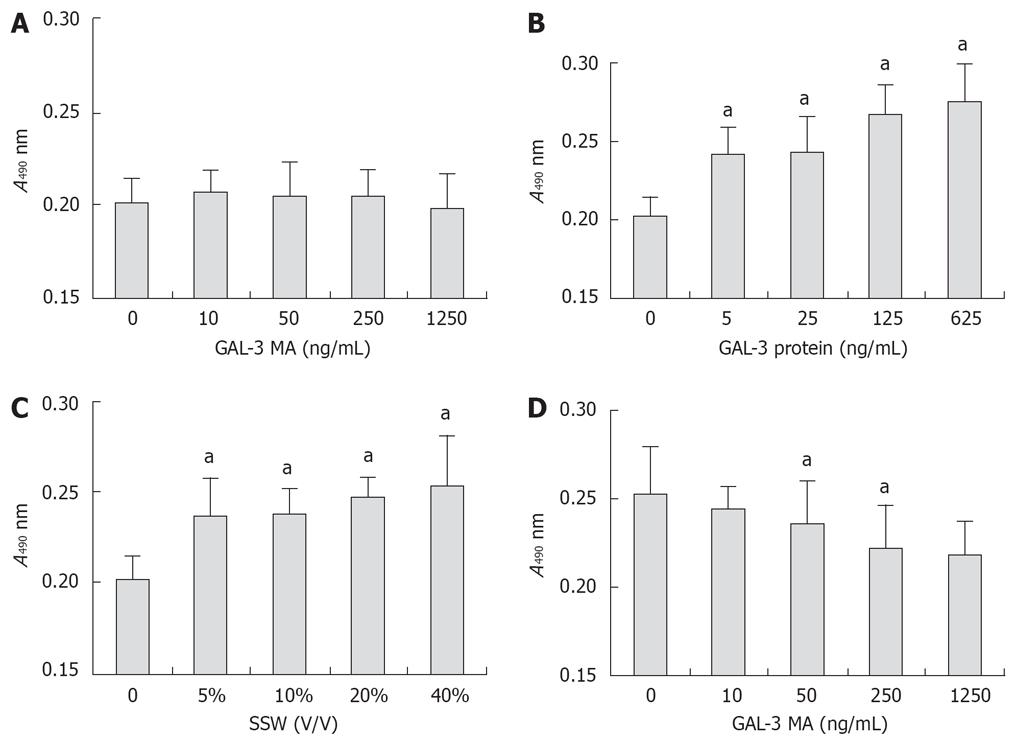
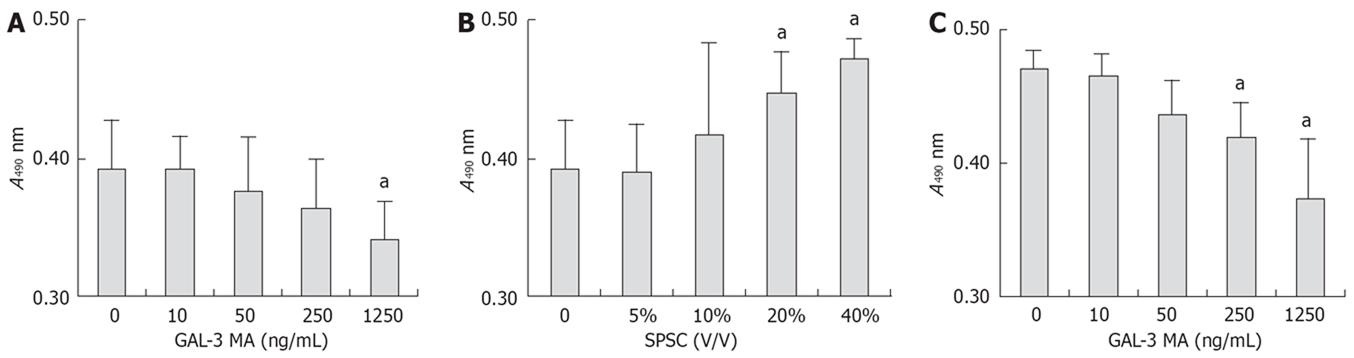
According to MTT assay (Figure 4), GAL-3 antibody had no effect on the proliferation of PSCs. SSW stimulated PSC proliferation and this was partly inhibited by GAL-3 MA, which suggested that the stimulatory effect of SSW on PSC proliferation was partly mediated via GAL-3. This was confirmed by the effect of recombinant GAL-3 protein on PSC proliferation. According to the GAL-3 ELISA kit, the concentration of GAL-3 in SSW was > 10 ng/mL, and in the MTT assay, 5 ng/mL GAL-3 was sufficient to significantly stimulate PSC proliferation, which meant that GAL-3 secreted by pancreatic cells played a role in PSC proliferation in pancreatic cancer.
PSC proliferation detected by flow cytometry is show in Table 1. The S-phase fraction of the control group was the lowest, that of group 4 was the highest, and group 2 had a higher S-phase fraction than group 3. This result was consistent with that of the MTT assay. PSCs are normal cells that grow very slowly, and have high demand on serum during culturing, so the S-phase fraction of all four groups was not very high.
| Group | Medium | G0 + G1 fraction | S phase fraction | G2/M fraction | S + G2/M fraction |
| 1 (Control) | Serum-free medium | 89.81 | 1.94 | 8.25 | 10.19 |
| 2 | Serum-free medium containing 40% SSW | 89.63 | 3.23 | 7.14 | 10.37 |
| 3 | Serum-free medium containing 40% SSW and 1 &mgr;g/mL GAL-3 antibody | 89.42 | 2.70 | 7.88 | 10.58 |
| 4 | Serum-free medium containing 100 ng/mL GAL-3 protein | 87.04 | 3.68 | 9.28 | 12.96 |
According to MTT assay (Figure 5), GAL-3 MA inhibited proliferation of SW1990 cells, and this was positively related to antibody concentration, which suggested that GAL-3 protein was involved in the proliferation process of SW1990 cells. SW1990 cells may increase their proliferation by paracrine or autocrine GAL-3 protein. SPSC stimulated proliferation of SW1990 cells. After GAL-3 MA was added to serum-free DMEM containing 40% SPSC, absorbance declined with the increase in antibody concentration, but it was still higher than that in groups that only used GAL-3 MA. There was a significant correlation between the effect of GAL-3 MA and SPSC on absorbance, which suggested that the stimulatory effect of SPSC on proliferation of SW1990 cells was partly related to GAL-3.
SW1990 cell proliferation detected by flow cytometry is show in Table 2. SW1990 cells are quickly proliferating cancer cells, and the S-phase fraction may not properly reflect their proliferation. Therefore, we use the total S phase plus G2/M phase fraction to measure their proliferation. Compared with the control group, GAL-3 MA distinctly inhibited their proliferation, SPSC obviously stimulated proliferation, and GAL-3 MA partly inhibited the SPSC-induced stimulation. This result was consistent with the results of the MTT assay.
| Group | Medium | G0 + G1 fraction | S phase fraction | G2/M fraction | S + G2/M fraction |
| 1 (control) | Serum-free DMEM | 71.80% | 16.76% | 11.45% | 28.20% |
| 2 | Serum-free DMEM containing 1 &mgr;g/mL GAL-3 MA | 79.28% | 13.87% | 6.85% | 20.72% |
| 3 | Serum-free DMEM containing 40% SPSC | 62.51% | 20.34% | 17.15% | 37.49% |
| 4 | Serum-free DMEM containing 40% SPSC and 1 &mgr;g/mL GAL-3 MA | 67.91% | 20.56% | 11.53% | 32.09% |
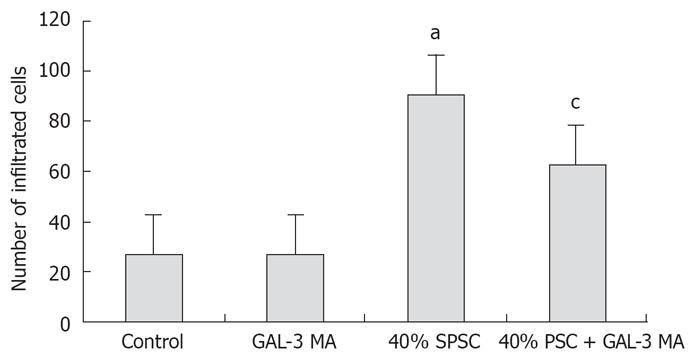
The results of cell invasion are shown in Figures 6 and 7. GAL-3 MA had no effect on invasion of SW1990 cells. SPSC stimulated invasion of SW1990 cells and this was partly inhibited by GAL-3 MA, which suggested that the stimulatory effect of SPSC on SW1990 cell invasion was partly mediated via GAL-3 expressed on the cells.
Our study confirmed that human pancreatic cancer cell line SW1990 expressed and secreted GAL-3 protein, and that GAL-3 MA inhibited proliferation of SW1990 cells, which suggests that cancer cells such as SW1990 can stimulate their proliferation by autocrine or paracrine GAL-3. However, more studies should be done to clarify this and to establish the signaling pathway through which GAL-3 stimulates proliferation of cancer cells.
Ductal adenocarcinoma of the pancreas is composed of infiltrating cancer cells surrounded by a predominant dense fibroblastic stroma. Previous studies have shown that the fibrotic ECM is mainly produced and secreted by PSCs, and pancreatic cancer cells activate PSCs via TGF-β and other cytokines such as basic fibroblast growth factor to increase production of ECM[8]. Our study, including MTT assay and flow cytometry, confirmed that pancreatic cancer cells stimulated proliferation of PSCs via GAL-3, but further studies should be performed to elucidate the stimulation mechanism of GAL-3 on the proliferation of PSCs. Our study also confirmed that PSCs, through interaction with GAL-3, stimulated pancreatic cancer cells to proliferate, but the exact mechanism needs to be further investigated. Our study confirmed that pancreatic cancer cells and PSCs stimulated proliferation of each other. This may be one of the important reasons for the rapid progression of pancreatic cancer, and preventing this counter-stimulation may be very important in slowing the progression of the disease. GAL-3 is involved in the counter-stimulation between pancreatic cancer cells and PSCs, and this may help us to establish new methods for treating pancreatic cancer.
It has been shown by many studies that GAL-3 plays an important role in the interaction between cells and ECM, and this interaction is very important for carcinoma infiltration and metastasis. Activated PSCs can produce and secrete a lot of ECM, including collagen, fibronectin and laminin. Our infiltration study confirmed that the SPSC stimulated the infiltration of pancreatic cancer cells through interaction with GAL-3, which suggests that PSCs play an important role in the early infiltration of pancreatic cancer. Since GAL-3 stimulated the proliferation of pancreatic cancer cells and PSCs, and was involved in neoplastic and ECM interaction, we suggest that it plays an important role in the infiltration and metastasis of pancreatic cancer.
Bachem et al[8] discovered pancreatic stellate cells (PSCs) in 1998. After that, several studies showed that there are activated PSCs around pancreatic cancer tissues, pancreatic cancer cells can activate PSCs, and activated PSCs can produce a lot of extracellular matrix (ECM), which lead to the desmoplasia reaction in pancreatic cancer. Galectin-3 (GAL-3) is a member of the β-galactoside-binding protein family which recognizes the N-acetyllactosamine structure of various glycoconjugates. Many studies have shown that GAL-3 plays a role in carcinoma proliferation, infiltration and metastasis. One study has also shown that pancreatic cancer may express GAL-3, but the role of GAL-3 in pancreatic cancer has not been investigated.
Previous studies have shown that pancreatic cancer cells can activate PSCs via cytokines such as TFG-β and platelet-derived growth factor, which are expressed by cancer cells, but few have discussed the role of activated PSCs in the progression of pancreatic caner. Bachem et al[8] have shown that GAL-1 can activate PSCs, and several others have shown that Gal-3 activates HSCs, but up till now, no study on the effect of GAL-3 on PSCs has been published.
This study showed that pancreatic cancer cells activated PSCs via GAL-3 expressed by cancer cells besides TGF-β and PDGF. It showed that activated PSCs promoted progress of pancreatic cancer by stimulating the proliferation and invasion of cancer cells. This study showed that there were complicated interactions between pancreatic cancer cells and PSCs. It also suggests that ECM and polysaccharides might play a role in the progress of pancreatic cancer, since GAL-3 is a lectin that recognizes the N-acetyllactosamine structure of various glycoconjugates.
This study provides a basis for future studies on the mechanisms of how GAL-3 activates PSCs, and how PSCs promote the proliferation and invasion of pancreatic cancer. It also provides a basis for future studies on treating pancreatic cancer through inhibition of GAL-3.
SW1990 is a commonly used human pancreatic cancer cell line in pancreatic cancer research, which was first derived from spleen metastasis of a grade 2 ductal pancreatic cancer in the early 1980s. PSCs are vitamin A-storage cells that resemble HSCs in the healthy pancreas and comprise approximately 4% of all pancreatic cells; they show a periacinar distribution. GAL-3 is an endogenous β-galactoside-binding protein that is expressed widely in normal and neoplastic cells.
This was a well-executed study. It explored the role of GAL-3 in the proliferation and invasion of pancreatic cancer cells in vitro, and showed that GAL-3 may play a role in the progression of pancreatic cancer.
| 1. | Koenig A, Mueller C, Hasel C, Adler G, Menke A. Collagen type I induces disruption of E-cadherin-mediated cell-cell contacts and promotes proliferation of pancreatic carcinoma cells. Cancer Res. 2006;66:4662-4671. |
| 2. | Edderkaoui M, Hong P, Vaquero EC, Lee JK, Fischer L, Friess H, Buchler MW, Lerch MM, Pandol SJ, Gukovskaya AS. Extracellular matrix stimulates reactive oxygen species production and increases pancreatic cancer cell survival through 5-lipoxygenase and NADPH oxidase. Am J Physiol Gastrointest Liver Physiol. 2005;289:G1137-G1147. |
| 3. | Miyamoto H, Murakami T, Tsuchida K, Sugino H, Miyake H, Tashiro S. Tumor-stroma interaction of human pancreatic cancer: acquired resistance to anticancer drugs and proliferation regulation is dependent on extracellular matrix proteins. Pancreas. 2004;28:38-44. |
| 4. | Vaquero EC, Edderkaoui M, Nam KJ, Gukovsky I, Pandol SJ, Gukovskaya AS. Extracellular matrix proteins protect pancreatic cancer cells from death via mitochondrial and nonmitochondrial pathways. Gastroenterology. 2003;125:1188-1202. |
| 5. | Shintani Y, Hollingsworth MA, Wheelock MJ, Johnson KR. Collagen I promotes metastasis in pancreatic cancer by activating c-Jun NH(2)-terminal kinase 1 and up-regulating N-cadherin expression. Cancer Res. 2006;66:11745-11753. |
| 6. | Lohr M, Schmidt C, Ringel J, Kluth M, Muller P, Nizze H, Jesnowski R. Transforming growth factor-beta1 induces desmoplasia in an experimental model of human pancreatic carcinoma. Cancer Res. 2001;61:550-555. |
| 7. | Yoshida S, Yokota T, Ujiki M, Ding XZ, Pelham C, Adrian TE, Talamonti MS, Bell RH Jr, Denham W. Pancreatic cancer stimulates pancreatic stellate cell proliferation and TIMP-1 production through the MAP kinase pathway. Biochem Biophys Res Commun. 2004;323:1241-1245. |
| 8. | Bachem MG, Schunemann M, Ramadani M, Siech M, Beger H, Buck A, Zhou S, Schmid-Kotsas A, Adler G. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology. 2005;128:907-921. |
| 9. | Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, Gitt MA, Hirabayashi J, Hughes C, Kasai K. Galectins: a family of animal beta-galactoside-binding lectins. Cell. 1994;76:597-598. |
| 10. | Barondes SH, Cooper DN, Gitt MA, Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994;269:20807-20810. |
| 11. | Maeda N, Kawada N, Seki S, Ikeda K, Okuyama H, Hirabayashi J, Kasai KI, Yoshizato K. Involvement of Galectin-1 and Galectin-3 in Proliferation and Migration of Rat Hepatic Stellate Cells in Culture. Comp Hepatol. 2004;3 Suppl 1:S10. |
| 12. | Allen HJ, Sucato D, Woynarowska B, Gottstine S, Sharma A, Bernacki RJ. Role of galaptin in ovarian carcinoma adhesion to extracellular matrix in vitro. J Cell Biochem. 1990;43:43-57. |
| 13. | Oda Y, Leffler H, Sakakura Y, Kasai K, Barondes SH. Human breast carcinoma cDNA encoding a galactoside-binding lectin homologous to mouse Mac-2 antigen. Gene. 1991;99:279-283. |
| 14. | Miyazaki J, Hokari R, Kato S, Tsuzuki Y, Kawaguchi A, Nagao S, Itoh K, Miura S. Increased expression of galectin-3 in primary gastric cancer and the metastatic lymph nodes. Oncol Rep. 2002;9:1307-1312. |
| 15. | Hittelet A, Legendre H, Nagy N, Bronckart Y, Pector JC, Salmon I, Yeaton P, Gabius HJ, Kiss R, Camby I. Upregulation of galectins-1 and -3 in human colon cancer and their role in regulating cell migration. Int J Cancer. 2003;103:370-379. |
| 16. | Song YK, Billiar TR, Lee YJ. Role of galectin-3 in breast cancer metastasis: involvement of nitric oxide. Am J Pathol. 2002;160:1069-1075. |
| 17. | Inufusa H, Nakamura M, Adachi T, Aga M, Kurimoto M, Nakatani Y, Wakano T, Miyake M, Okuno K, Shiozaki H. Role of galectin-3 in adenocarcinoma liver metastasis. Int J Oncol. 2001;19:913-919. |
| 18. | Moon BK, Lee YJ, Battle P, Jessup JM, Raz A, Kim HR. Galectin-3 protects human breast carcinoma cells against nitric oxide-induced apoptosis: implication of galectin-3 function during metastasis. Am J Pathol. 2001;159:1055-1060. |
| 19. | Schaffert C, Pour PM, Chaney WG. Localization of galectin-3 in normal and diseased pancreatic tissue. Int J Pancreatol. 1998;23:1-9. |
| 20. | Berberat PO, Friess H, Wang L, Zhu Z, Bley T, Frigeri L, Zimmermann A, Buchler MW. Comparative analysis of galectins in primary tumors and tumor metastasis in human pancreatic cancer. J Histochem Cytochem. 2001;49:539-549. |