Published online Mar 14, 2008. doi: 10.3748/wjg.14.1491
Revised: January 25, 2008
Published online: March 14, 2008
AIM: To examine the growth inhibitory effects of Phyllanthus emblica (P. emblica) and Terminalia bellerica (T. bellerica) extracts on human hepatocellular carcinoma (HepG2), and lung carcinoma (A549) cells and their synergistic effect with doxorubicin or cisplatin.
METHODS: HepG2 and A549 cells were treated with P. emblica and T. bellerica extracts either alone or in combination with doxorubicin or cisplatin and effects on cell growth were determined using the sulforhodamine B (SRB) assay. The isobologram and combination index (CI) method of Chou-Talalay were used to evaluate interactions between plant extracts and drugs.
RESULTS: P. emblica and T. bellerica extracts demonstrated growth inhibitory activity, with a certain degree of selectivity against the two cancer cell lines tested. Synergistic effects (CI < 1) for P. emblica/doxorubicin or cisplatin at different dose levels were demonstrated in A549 and HepG2 cells. The T. bellerica/cisplatin or doxorubicin also showed synergistic effects in A549 and HepG2 cells. In some instances, the combinations resulted in antagonistic effects. The dose reduction level was different and specific to each combination and cell line.
CONCLUSION: The growth inhibitory activity of doxorubicin or cisplatin, as a single agent, may be modified by combinations of P. emblica or T. bellerica extracts and be synergistically enhanced in some cases. Depending on the combination ratio, the doses for each drug for a given degree of effect in the combination may be reduced. The mechanisms involved in this interaction between chemotherapeutic drugs and plant extracts remain unclear and should be further evaluated.
-
Citation: Pinmai K, Chunlaratthanabhorn S, Ngamkitidechakul C, Soonthornchareon N, Hahnvajanawong C. Synergistic growth inhibitory effects of
Phyllanthus emblica andTerminalia bellerica extracts with conventional cytotoxic agents: Doxorubicin and cisplatin against human hepatocellular carcinoma and lung cancer cells. World J Gastroenterol 2008; 14(10): 1491-1497 - URL: https://www.wjgnet.com/1007-9327/full/v14/i10/1491.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.1491
Cancer is the third leading cause of death worldwide, preceded by cardiovascular and infectious diseases. Doxorubicin and cisplatin represent the current standard chemotherapeutic drugs for treatment of cancers. Although there are many therapeutic strategies including chemotherapy to treat cancer, high systemic toxicity and drug resistance limit the successful outcomes in most cases. Accordingly, several new strategies are being developed to control and treat cancer. One such approach could be a combination of an effective phytochemicals with chemotherapeutic agents, which when combined, would enhance efficacy while reducing toxicity to normal tissues.
The notion has evolved that natural products frequently exert a valuable role in broadening the scope of disease intervention strategies used by drug designers. Several herbs and plants with diversified pharmacological properties are known to be rich sources of chemical constituents that may have potential for the prevention and/or treatment of several human cancers[1–4].
P. emblica L. (Euphorbiaceae) known as emblic myrobalan, a shrub or tree, is widely used in folk medicine in Southeast Asia. This plant exhibits a variety of pharmacological effects including anti-inflammatory, anti-pyretic, anti-oxidant, anti-carcinogenic, and anti-mutagenic effects[5–7]. The active principles or extracts of P. emblica have demonstrated anti-proliferative effects in several cancer cell lines both in vitro and in vivo[78]. The anti-tumor activity of P. emblica extract was attributed to its ability to interfere with cell cycle regulation via the inhibition of cdc 25 phosphatase and partial inhibition of cdc 2 kinase activity[8].
T. bellerica (Combretaceae), known as belleric myrobalan, is a large deciduous tree, common to the plains and lower hills of Southeast Asia. This plant exhibits several pharmacological effects including anti-bacterial, anti-malarial, anti-fungal, anti-HIV, anti-oxidant, and anti-mutagenic effects[9–12]. T. bellerica extract exhibited anti-proliferative effects in several cancer cell lines including Shiongi 115, breast cancer MCF-7, prostate cancer PC-3 and DU-145 cells[13]. Phytochemical studies have shown that T. bellerica contains a variety of chemical components, including gallic acid[1415]. Furthermore, recent studies have demonstrated the cytotoxic activity of gallic acid in human leukemia HK-63, HOS-1 cell, HSC-2, and HL-60 cell lines[16–19]. Gallic acid has also been shown to induce apoptotic cell death in HSC-2 and HL-60 cells[1618].
These findings prompted us to test the anti-proliferative activity of P. emblica and T. bellerica extracts on human lung carcinoma A549, and human hepatocellular carcinoma HepG2 cell lines, and to investigate whether it can synergize the inhibitory action of doxorubicin and cisplatin, the standard chemotherapeutic drugs.
The dried fruits of T. bellerica and P. emblica were collected from Srakeaw and Nan Provinces in northeastern and northern Thailand, respectively. The voucher specimens were identified and kept by Associate Professor Dr. Noppamas Soonthornchareon, Department of Pharma-cognosy, Faculty of Pharmacy, Mahidol University. The dried fruits of medicinal plant were obtained and extracted with boiling water. Then, the aqueous solution was separated from the plant residues by filtration. After that, the aqueous solution was spray-dried. For testing, the extracts were dissolved in media and diluted to the desired concentrations.
Doxorubicin and Cisplatin (Platinol) were purchased from Ebewe (Austria) and Bristol-Myers Squibb (USA), respectively. The drugs were dissolved in water for injection at a concentration of 2 mg/mL. Both drugs were kept in aliquots at 4°C. Serial dilutions of these drugs were performed in culture media, immediately before each experiment, in order to obtain the required final concentrations.
The human lung carcinoma (A549) cell was kindly provided by the Chulabhorn Research Institute. The human hepatocellular carcinoma (HepG2) cell (ATCC HB-8065) was obtained from the National Cancer Institute, Bangkok, Thailand. The HepG2, and A549 cells were cultured in RPMI 1640 medium (Hyclone, Logan, UT, USA). Both cell lines were supplemented with 10% heat inactivated fetal bovine serum (Hyclone), 100 U/mL penicillin and 100 &mgr;g/mL streptomycin (Gibco BRL, Grand Island, NY). All cell lines were maintained at 37°C in a 5% CO2 incubator and the media were changed twice weekly.
The sulforhodamine B (SRB) assay was performed to assess growth inhibition using a colorimetric assay, which estimates cell number indirectly by staining total cellular protein with the dye SRB[20].
Briefly, 100 &mgr;L/well of cell suspensions (0.5-2.0 × 105 cells/mL) were seeded in 96-well microtiter plates and incubated at 37°C to allow for cell attachment. After 24 h, the cells were treated with the extract by adding 100 &mgr;L/well of each concentration in triplicate to obtain a final concentration of 0.8, 4, 12.5, 25, 50, 100, 200, 400, and 1000 &mgr;g/well for the extracts; 0.029, 0.058, 0.116, 0.174, and 0.348 &mgr;g/mL for the doxorubicin; and, 0.1, 0.2, 0.5, 1.0, 2.5, and 5.0 &mgr;g/mL for the cisplatin.
The plates were incubated for 1 h (d 0) and 72 h (d 3) at 37°C. At the end of each exposure time, the medium was removed. The cells were fixed with 20% (w/v) trichloroacetic acid (TCA, Fluka, Buchs, Switzerland) at 4°C for 1 h, stained for 30 min with 0.4% (w/v) SRB (Sigma, St. Louis, MO, USA) dissolved in 1% acetic acid (Sigma) for 30 min, and washed four times with 1% acetic acid. The protein-bound dye was solubilized with 10 mmol/L Tris base, pH 10 (Sigma).
The absorbance (OD) of each well was read on an ELISA plate reader (Amersham, Buckinghamshire, UK) at 492 nm. Percentage of cell survival was calculated using the formula: Percentage cell survival = [(OD test sample at d 3 - OD d 0)/(OD control at d 3 - OD d 0)] × 100.
Dose-response curves were plotted, and 50% growth inhibitory concentrations of extracts or drugs (IC50) were calculated through computation with the CalcuSyn software program (Biosoft, Cambridge, UK). By comparing the growth inhibitory effect with normal cells, the selectivity of the extract was expressed as the extract shown to be toxic to specific types of cancer cell lines.
Combination assays were performed using appropriate concentrations of P. emblica and T. bellerica extracts (4, 8, 16, 24, and 48 &mgr;g/mL for A549 cells, and 25, 50, 75, 100, and 200 &mgr;g/mL for HepG2 cells) with appropriate concentrations of doxorubicin (0.029, 0.058, 0.116, 0.174, and 0.348 &mgr;g/mL) or cisplatin (0.1, 0.2, 0.3, 0.4, and 0.8 &mgr;g/mL). Cells treated with the same final concentrations of the extracts or chemotherapeutic drugs alone were also examined. Cell growth inhibition was determined using the SRB assay, as previously described. In the assessment of synergism, the combination index (CI) method (i.e., additivity or antagonism) according to Chou and Talalay was used[21].
The CIs were calculated by the Chou-Talalay equation[21], which takes into account both the potency (Dm or IC50) and shape of the dose-effect curve. The general equation for the classic isobologram (CI = 1) is given by: CI = (D)1/(Dx)1 + (D)2/(Dx)2 (A) where (Dx)1 and (Dx)2 in the denominators are the doses (or concentrations) of D1 (drug #1, for example, the extract) and D2 (drug #2, for example, the doxorubicin) alone that gives x% inhibition, whereas (D)1 and (D)2 in the numerators are the doses of D1 and D2 in combination that also inhibits x% (i.e., isoeffective). The (Dx)1 and (Dx)2 can be readily calculated from the Median-effect equation of Chou et al[22]: Dx = Dm [fa/(1 - fa)]1/m (B) where Dx is the median-effect dose obtained from the anti-log of the X-intercept of the median-effect plot, X-log (D) versus, Y = log [fa/(1 - fa)], or Dm = 10-(Y-intercept)/m, fa is the fraction affected by dose D (e.g., 0.5 if cell growth is inhibited by 50%) and m is the slope of the median-effect plot. From (Dm)1, and (Dx)2 and D1 + D2, an isobologram can be constructed based on Eq. A: as CI < 1 indicates synergism; CI = 1 indicates an additive effect; and CI > 1 indicates antagonism.
For conservative, mutually nonexclusive isobolograms of two agents, a third term, (D)1(D)2/(Dx)1(Dx)2, is added to Eq. A. For simplicity, the third term is usually omitted, and thus the mutually exclusive assumption or classic isobologram is indicated. In this study, the CI values obtained from classic (mutually exclusive) calculations are given.
The dose-reduction index (DRI) defines the extent (folds) of dose reduction possible in a combination, for a given degree of effect, compared with the dose of each drug alone: (DRI)1 = (Dx)1/(D)1 and (DRI)2 = (Dx)2/(D)2. The relationship between DRI and CI is, therefore, expressed as: CI = (Dx)1/(D)1 + (Dx)2/(D)2 = 1/(DRI)1 + 1/(DRI)2
The data were analyzed using the SPSS software version 11.0. The IC50 of plants against cancer cells were compared with IC50 of normal cells and calculated by the Student’s t-test. Differences were considered significant at P < 0.01.
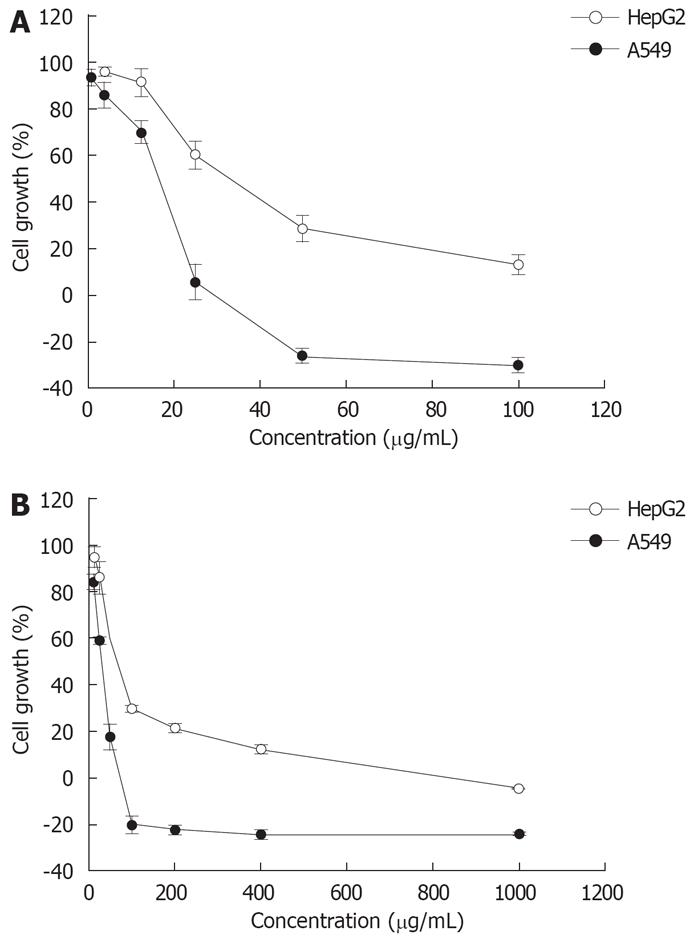
The effects of P. emblica and T. bellerica extracts on the proliferation of two cancer cell lines and Vero cells were determined using the SRB assay. All cell lines were growth-inhibited in a dose-dependent manner after exposure to the plant extracts (Figure 1). The IC50 values for P. emblica extract ranged from 12.18 ± 5.83 to 157.86 ± 14.90 &mgr;g/mL and for T. bellerica extract ranged from 27.07 ± 2.19 to 238.70 ± 8.45 &mgr;g/mL (Table 1).
When the activities of P. emblica and T. bellerica extracts against the cancer cell lines were compared with that against Vero cells and expressed as the ratio of IC50 values, the P. emblica extract had significantly different ratios of 13.0 and 5.2 against the A549 and HepG2 cells (P < 0.01), respectively. The T. bellerica extract also showed significantly different ratios of 8.8 and 2.9 against the A549 and HepG2 cells (P < 0.01), respectively (Table 1). It appears these two plant extracts were selectively toxic against the two cancer cell lines tested, motivating further work to determine the combination effect of these plant extracts with chemotherapeutic drugs.
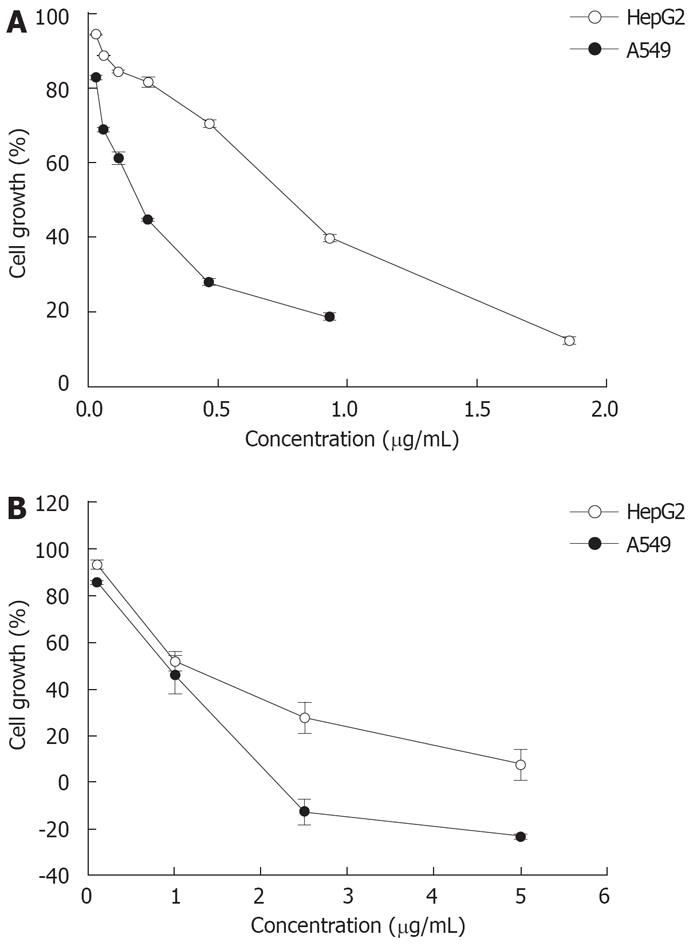
Using the SRB assay, the effects of doxorubicin and cisplatin on the proliferation of two cancer cell lines were determined. All cell lines were growth-inhibited in a dose-dependent manner (Figure 2). The IC50 values for doxorubicin vs cisplatin ranged from 0.170 ± 0.006 to 0.511 ± 0.025 &mgr;g/mL vs 1.04 ± 0.21 to 1.05 ± 0.18 &mgr;g/mL, respectively (Table 2).
| Drug | IC50 value (&mgr;g/mL ± SD) | |
| A549 | HepG2 | |
| Doxorubicin | 0.170 ± 0.006 | 0.511 ± 0.025 |
| Cisplatin | 1.04 ± 0.21 | 1.05 ± 0.18 |
The combination effects of P. emblica and T. bellerica extracts with doxorubicin and cisplatin in the A549 and HepG2 cell lines, as represented by the DRI, the CI and the dose-effect levels of cell growth inhibition (IC50-IC90), are summarized in Table 3.
| Cell line | Single extracts, drugs and combinations | Parameters | CI value at | DRI value at | ||||||
| Dm (&mgr;g/mL) | m | r | IC50 | IC75 | IC90 | IC50 | IC75 | IC90 | ||
| A549 | P. emblica | 36.75 | 0.71 | 0.99 | 2.67 | 3.90 | 5.67 | |||
| Doxorubicin | 0.164 | 0.78 | 0.99 | 1.64 | 2.07 | 2.60 | ||||
| (D)1 + (D)2 (4:0.029) | 13.75 + 0.100 | 0.93 | 0.99 | 0.98 | 0.74 | 0.56 | ||||
| T. bellerica | 17.78 | 1.57 | 0.86 | 1.26 | 0.85 | 0.57 | ||||
| Doxorubicin | 0.169 | 0.87 | 0.99 | 1.66 | 1.95 | 2.30 | ||||
| (D)1 + (D)2 (4:0.029) | 14.11 + 0.102 | 1.00 | 1.00 | 1.40 | 1.69 | 2.18 | ||||
| P. emblica | 18.75 | 0.75 | 0.95 | 1.65 | 2.63 | 4.19 | ||||
| Cisplatin | 0.596 | 0.99 | 0.96 | 2.09 | 2.33 | 2.59 | ||||
| (D)1 + (D)2 (40:1) | 11.38 + 0.285 | 1.09 | 0.98 | 1.08 | 0.81 | 0.62 | ||||
| T. bellerica | 30.36 | 0.95 | 0.97 | 1.76 | 1.87 | 1.99 | ||||
| Cisplatin | 1.228 | 1.36 | 1.00 | 2.85 | 2.15 | 1.62 | ||||
| (D)1 + (D)2 (40:1) | 17.26 + 0.431 | 1.00 | 1.00 | 0.92 | 1.00 | 1.12 | ||||
| HepG2 | P. emblica | 157.82 | 2.65 | 0.98 | 1.47 | 1.26 | 1.07 | |||
| Doxorubicin | 0.584 | 1.04 | 0.96 | 4.69 | 7.58 | 12.23 | ||||
| (D)1 + (D)2 (25:0.029) | 107.23 + 0.124 | 1.92 | 0.94 | 0.89 | 0.93 | 1.02 | ||||
| T. bellerica | 150.34 | 3.11 | 0.95 | 1.37 | 1.07 | 0.84 | ||||
| Doxorubicin | 0.569 | 1.04 | 0.95 | 4.48 | 7.09 | 11.24 | ||||
| (D)1 + (D)2 (25:0.029) | 109.66 + 0.127 | 1.83 | 0.97 | 0.95 | 1.07 | 1.28 | ||||
| P. emblica | 111.59 | 2.18 | 0.99 | 1.24 | 1.08 | 0.94 | ||||
| Cisplatin | 0.839 | 0.91 | 0.95 | 2.32 | 4.08 | 7.19 | ||||
| (D)1 + (D)2 (250:1) | 90.395 + 0.362 | 1.72 | 0.96 | 1.24 | 1.17 | 1.20 | ||||
| T. bellerica | 111.02 | 2.26 | 1.00 | 1.19 | 1.01 | 0.85 | ||||
| Cisplatin | 0.881 | 1.13 | 0.98 | 2.36 | 3.26 | 4.49 | ||||
| (D)1 + (D)2 (250:1) | 93.30 + 0.373 | 1.68 | 0.99 | 1.26 | 1.30 | 1.40 | ||||
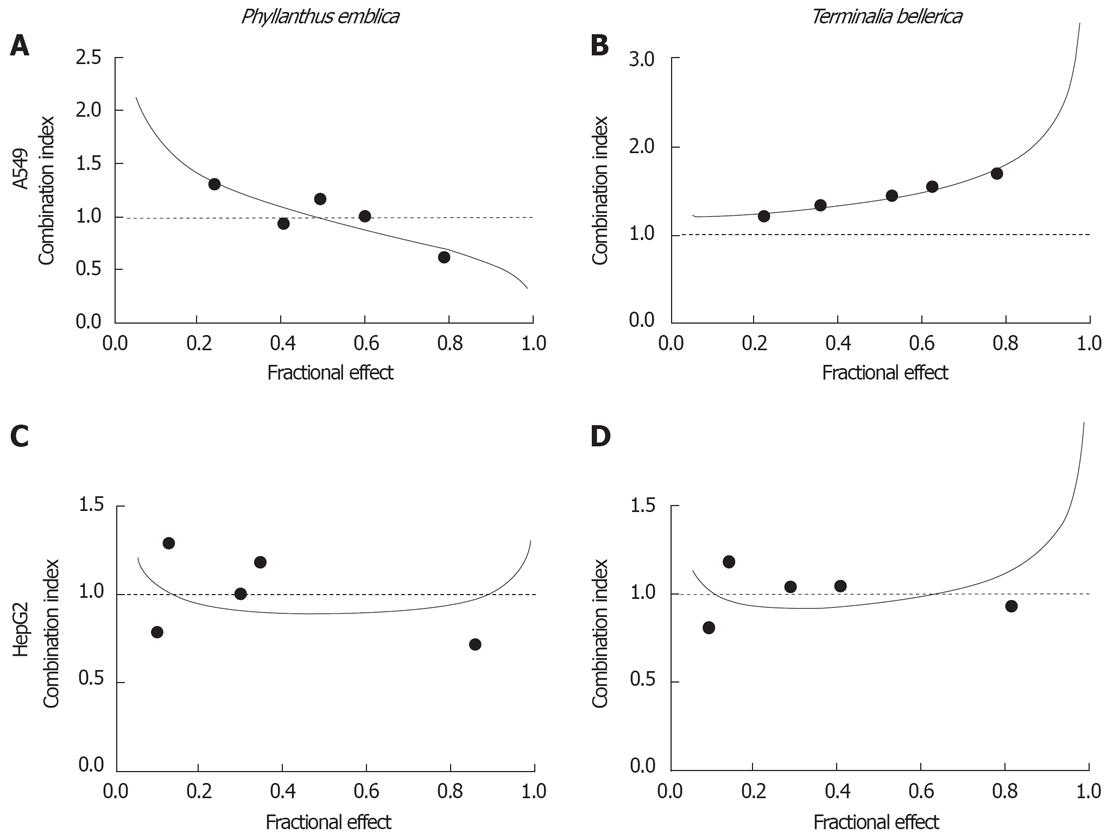
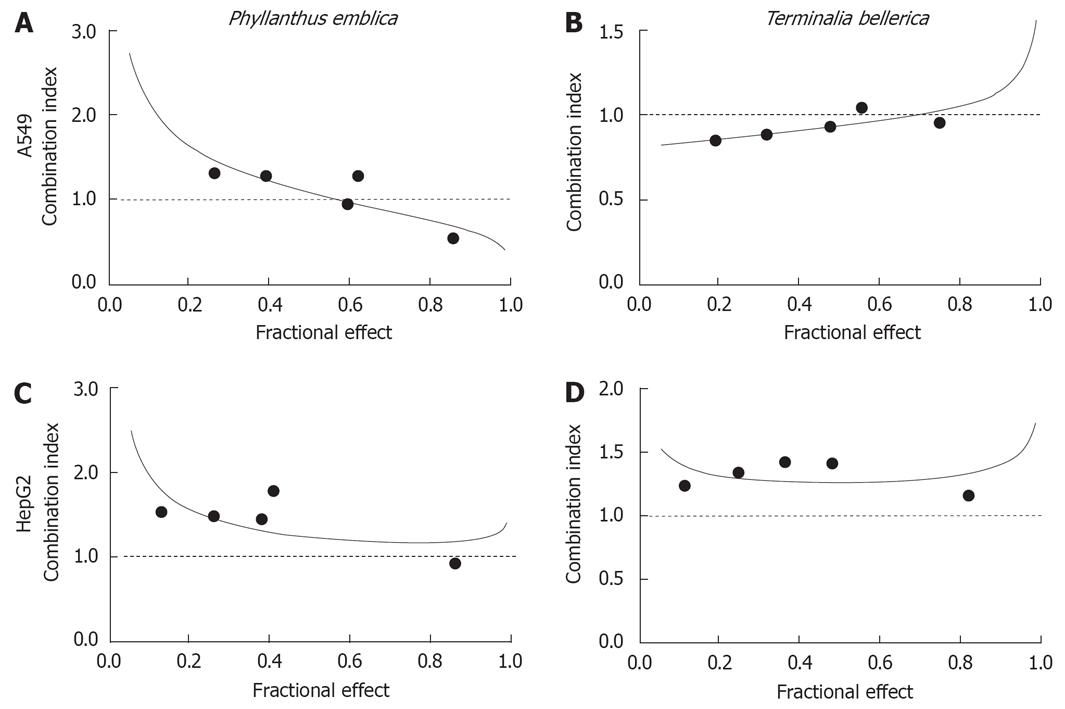
The data were also examined using median effect analysis to determine the type of interactions which occurred, i.e. antagonism (CI > 1), additivity (CI = 1) or synergism (CI < 1) (Figures 3 and 4). With A549 cells, the combinations were synergistic at medium and high dose levels (IC75 and IC90) for the P. emblica/doxorubicin and P. emblica/cisplatin combinations (Table 3, Figures 3 and 4). By contrast, the synergistic and additive effects were recorded at low and medium dose levels (IC50 and IC75) for the T. bellerica/cisplatin combination (Table 3, Figure 4). In HepG2 cells, combinations were synergistic at low and medium dose levels (IC50 and IC75) for the P. emblica/doxorubicin combination or at a low level (IC50) for the T. bellerica/doxorubicin combination (Table 3, Figure 3). All other combinations had antagonistic effects.
The DRI showed a considerable dose reduction for herbal extracts and drugs used as a result of their synergism (Table 3). When using synergistic drug combinations at corresponding dose levels, the DRI indicated that the concentration of doxorubicin necessary to inhibit the growth of 50% of cancer cells (IC50) could be decreased 1.64-fold (A549, P. emblica/doxorubicin) to 4.69-fold (HepG2, P. emblica/doxorubicin), and the IC90 could be reduced 2.59-fold (A549, P. emblica/cisplatin) to 2.60-fold (A549, P. emblica/doxorubicin; Table 3). The dose reduction level was different and specific to each combination and cell line.
In this study, the growth inhibitory activity of the P. emblica and T. bellerica extracts and the chemotherapeutic drugs doxorubicin and cisplatin were investigated in A549, and HepG2 cells. Our results indicate that both plant extracts and chemotherapeutic drugs mediated significant growth inhibitory effects on both cell lines tested in a dose-dependent manner (Tables 1 and 2, Figure 1 and 2).
P. emblica extract combined with doxorubicin or cisplatin resulted in synergistically enhanced growth inhibitory activity at different dose levels in A549 and HepG2 cell lines. The synergistic effects were also demonstrated when T. bellerica extract was combined with doxorubicin or cispaltin in HepG2 and A549 cells. The significance of this finding lies in the fact that doxorubicin and cisplatin are well-known cancer therapeutic agents, but cause high toxicity to normal tissues during cancer therapy[2324].
Several studies have shown that doxorubicin has harmful effects on health (i.e., immunosuppression and secondary cardiomyopathy) and can lead to the development of primary and secondary drug resistance in tumor cells thereby limiting the clinical success of cancer chemotherapy[2526]. Recent reports show that combination (rather than single-agent) chemotherapy is a superior modality and that naturally occurring dietary supplements with known anti-cancer activity could be used in combination chemotherapy to reduce the systemic toxicity of chemotherapeutic agents[2728].
Our study provides corroborative evidence as it showed that P. emblica and T. bellerica extracts were selectively toxic against two cancer cell lines and that, in combination with doxorubicin and cisplatin, produced an increased growth inhibitory effect in both A549 and HepG2 cells. Calculation of the DRI at the IC50 demonstrated possible reductions in doxorubicin concentrations for the drug combinations ranging from 1.64-fold (P. emblica + doxorubicin in A549) to 4.69-fold (P. emblica + doxorubicin in HepG2). This finding supports our hypothesis that combinations of plant extracts and chemotherapeutic agents allow a reduction in the dosage of the latter (i.e., doxorubicin and cisplatin), yet retaining the benefits but minimizing the cytotoxic effects, thus enhancing therapeutic efficacy.
The mechanism of action is unclear and, possibly, multiple compounds in the herbal extracts are involved[29]. Plant derived polyphenols, including tannins and gallic acid, were reported to be the main constituents in P. emblica and T. bellerica[1330]. Marienfeld et al[31] reported that tannic acid (TA) could inhibit the malignant cholangiocyte growth both in vitro and in vivo and also enhance sensitivity of Mz-ChA-1 cholangiocarcinoma cells to camptothecin cytotoxicity which might involve an effect on xenobiotic metabolism. In addition, TA also acts as an inhibitor of the glutathione conjugate export pump. As a result, the sensitivity of tumor cells to anticancer drugs was increased[3233]. This study agreed with the study of Sandhya and Mishra[34] which showed the cytotoxic response of human breast cancer cell lines to Triphala, which contained T. bellerica, P. emblica and T. chebula extracts. It might be possible that T. bellerica and P. emblica extracts could induce ROS in the induction of apoptosis. Apart from the effect of tannic acid, the cytotoxic effect and apoptosis induction of gallic acid in several cancer cell lines have been reported[133435]. It was demonstrated that the effect of gallic acid on cancer cell line particularly lung cancer cells involved caspase activation and oxidative processes[35]. In addition, gallic acid, a major component of T. bellerica[1415] has the capacity to induce apoptosis[35] and increase the efficacy of cisplatin in combined treatment of mice transplanted with LL-2 lung cancer cells[36]. These findings suggest that in the synergistic activity of P. emblica or T. bellerica extracts and doxorubicin it seem to be possible that the extracts could induce cytotoxic to tumor cells by a ROS mediated mechanism in cancer cells but less in normal cell[37] as well as inhibit glutathione conjugate export pump as a result, the extracts would increase the sensitivity of A549 and HepG2 cells to doxorubicin and cisplatin.
In summary, our results demonstrate that combinations of P. emblica or T. bellerica extracts with doxorubicin or cisplatin in A549 and HepG2 cell lines have a better effect than either agent alone. Further studies are needed to assess the underlying mechanism(s), signal transduction pathways, leading to growth inhibition induced by single agents and combinations both in vitro and in vivo. A positive outcome of such studies would be increased efficacy of existing chemotherapies with reduced toxicity to the normal tissues in treatment of human lung carcinoma and hepatocellular carcinoma.
Belleric myrobalan (Terminalia bellerica) and emblic myrobalan (Phyllanthus emblica), are constituents of herbal formulation (Triphala) and is widely used in folk medicine in Southeast Asia. Both extracts have been shown to inhibit cancer cell growth.
Currently, a variety of effective phytochemicals have been tested in cancer treatment, which are used alone, or in combination with chemotherapeutic agents of treatment. The combinations of P. emblica or T. bellerica extracts with doxorubicin or cisplatin have a synergistic growth effect against some cancer cell lines. Depending on the combination ratio, the doses for each drug for a given degree of effect in the combination may be reduced. The mechanism of this synergistic effect is unclear and, possibly, multiple compounds in the herbal extracts are involved.
In this report, we demonstrate that combinations of P. emblica or T. bellerica extracts with doxorubicin or cisplatin in A549 and HepG2 cell lines have a better effect than either agent alone.
P. emblica and T. bellerica extracts appear to be an effective against hepatocellular carcinoma and lung cancer cells and less toxic against normal cells. A combination of an effective P. emblica and T. bellerica extracts with chemotherapeutic agents which combined may be enhancing efficacy while reducing toxicity to normal tissues.
This is a very nice experimental study about the interaction between drugs and plant extracts performed in vitro. In this study, the authors investigated the combination effects of P. emblica and T. bellerica extracts with conventional cytotoxic agents against human cancer cells. The paper shown a synergistic effect of P. emblica and T. bellerica extracts with doxorubicin and cisplatin against human hepatocellular carcinoma and lung cancer cells.
| 1. | Dragsted LO. Natural antioxidants in chemoprevention. Arch Toxicol Suppl. 1998;20:209-226. |
| 2. | Kelloff GJ. Perspectives on cancer chemoprevention research and drug development. Adv Cancer Res. 2000;78:199-334. |
| 3. | Lippman SM, Lee JJ, Sabichi AL. Cancer chemoprevention: progress and promise. J Natl Cancer Inst. 1998;90:1514-1528. |
| 4. | Wargovich MJ. Nutrition and cancer: the herbal revolution. Curr Opin Clin Nutr Metab Care. 1999;2:421-424. |
| 5. | Gowrishanker B, Vivekanandan OS. In vivo studies of a crude extract of Phyllanthus amarus L. in modifying the genotoxicity induced in Vicia faba L. by tannery effluents. Mutat Res. 1994;322:185-192. |
| 6. | Ihantola-Vormisto A, Summanen J, Kankaanranta H, Vuorela H, Asmawi ZM, Moilanen E. Anti-inflammatory activity of extracts from leaves of Phyllanthus emblica. Planta Med. 1997;63:518-524. |
| 7. | Zhang YJ, Nagao T, Tanaka T, Yang CR, Okabe H, Kouno I. Antiproliferative activity of the main constituents from Phyllanthus emblica. Biol Pharm Bull. 2004;27:251-255. |
| 8. | Jose JK, Kuttan G, Kuttan R. Antitumour activity of Emblica officinalis. J Ethnopharmacol. 2001;75:65-69. |
| 9. | Aqil F, Ahmad I. Antibacterial properties of traditionally used Indian medicinal plants. Methods Find Exp Clin Pharmacol. 2007;29:79-92. |
| 10. | Bajpai M, Pande A, Tewari SK, Prakash D. Phenolic contents and antioxidant activity of some food and medicinal plants. Int J Food Sci Nutr. 2005;56:287-291. |
| 11. | Padam SK, Grover IS, Singh M. Antimutagenic effects of polyphenols isolated from Terminalia bellerica myroblan in Salmonella typhimurium. Indian J Exp Biol. 1996;34:98-102. |
| 12. | Valsaraj R, Pushpangadan P, Smitt UW, Adsersen A, Christensen SB, Sittie A, Nyman U, Nielsen C, Olsen CE. New anti-HIV-1, antimalarial, and antifungal compounds from Terminalia bellerica. J Nat Prod. 1997;60:739-742. |
| 13. | Kaur S, Michael H, Arora S, Harkonen PL, Kumar S. The in vitro cytotoxic and apoptotic activity of Triphala--an Indian herbal drug. J Ethnopharmacol. 2005;97:15-20. |
| 14. | Rastogi RP, Mehrotra BN, Central Drug Research Institute (India). Compendium of Indian medicinal plants. Drug research perspectives. Lucknow: Central Drug Research Institute 1990; 388-389. |
| 15. | Satyavati GV, Gupta AK, Tandon N. Medicinal plants of India. New Delhi: Indian Council of Medical Research 1987; 230-239. |
| 16. | Furuya S, Takayama F, Mimaki Y, Sashida Y, Satoh K, Sakagami H. Cytotoxic activity of steroidal saponins against human oral tumor cell lines. Anticancer Res. 2000;20:4189-4194. |
| 17. | Ishihara M, Sakagami H. Application of semiempirical method to estimate the cytotoxic activity of gallic acid and its related compounds. Anticancer Res. 2003;23:2549-2552. |
| 18. | Sakaguchi N, Inoue M, Isuzugawa K, Ogihara Y, Hosaka K. Cell death-inducing activity by gallic acid derivatives. Biol Pharm Bull. 1999;22:471-475. |
| 19. | Saleem A, Husheem M, Harkonen P, Pihlaja K. Inhibition of cancer cell growth by crude extract and the phenolics of Terminalia chebula retz. fruit. J Ethnopharmacol. 2002;81:327-336. |
| 20. | Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107-1112. |
| 21. | Chou TC, Talalay P. Analysis of combined drug effects: a new look at a very old problem. Trends Pharmacol Sci. 1983;4:450-454. |
| 22. | Chou TC, Motzer RJ, Tong Y, Bosl GJ. Computerized quantitation of synergism and antagonism of taxol, topotecan, and cisplatin against human teratocarcinoma cell growth: a rational approach to clinical protocol design. J Natl Cancer Inst. 1994;86:1517-1524. |
| 23. | Chen ST, Pan TL, Tsai YC, Huang CM. Proteomics reveals protein profile changes in doxorubicin--treated MCF-7 human breast cancer cells. Cancer Lett. 2002;181:95-107. |
| 24. | Gandara DR, Perez EA, Weibe V, De Gregorio MW. Cisplatin chemoprotection and rescue: pharmacologic modulation of toxicity. Semin Oncol. 1991;18:49-55. |
| 25. | Raghavan D, Koczwara B, Javle M. Evolving strategies of cytotoxic chemotherapy for advanced prostate cancer. Eur J Cancer. 1997;33:566-574. |
| 26. | Von Hoff DD, Layard MW, Basa P, Davis HL Jr, Von Hoff AL, Rozencweig M, Muggia FM. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91:710-717. |
| 27. | Hortobagyi GN. Progress in systemic chemotherapy of primary breast cancer: an overview. J Natl Cancer Inst Monogr. 2001;91:72-79. |
| 28. | Tyagi AK, Singh RP, Agarwal C, Chan DC, Agarwal R. Silibinin strongly synergizes human prostate carcinoma DU145 cells to doxorubicin-induced growth Inhibition, G2-M arrest, and apoptosis. Clin Cancer Res. 2002;8:3512-3519. |
| 29. | Darzynkiewicz Z, Traganos F, Wu JM, Chen S. Chinese herbal mixture PC SPES in treatment of prostate cancer (review). Int J Oncol. 2000;17:729-736. |
| 30. | Sandhya T, Mishra KP. Cytotoxic response of breast cancer cell lines, MCF 7 and T 47 D to triphala and its modification by antioxidants. Cancer Lett. 2006;238:304-313. |
| 31. | Marienfeld C, Tadlock L, Yamagiwa Y, Patel T. Inhibition of cholangiocarcinoma growth by tannic acid. Hepatology. 2003;37:1097-1104. |
| 32. | Zhang K, Chew M, Yang EB, Wong KP, Mack P. Modulation of cisplatin cytotoxicity and cisplatin-induced DNA cross-links in HepG2 cells by regulation of glutathione-related mechanisms. Mol Pharmacol. 2001;59:837-843. |
| 33. | Suzuki T, Nishio K, Tanabe S. The MRP family and anticancer drug metabolism. Curr Drug Metab. 2001;2:367-377. |
| 34. | Sandhya T, Mishra KP. Cytotoxic response of breast cancer cell lines, MCF 7 and T 47 D to triphala and its modification by antioxidants. Cancer Lett. 2006;238:304-313. |
| 35. | Ohno Y, Fukuda K, Takemura G, Toyota M, Watanabe M, Yasuda N, Xinbin Q, Maruyama R, Akao S, Gotou K. Induction of apoptosis by gallic acid in lung cancer cells. Anticancer Drugs. 1999;10:845-851. |
| 36. | Kawada M, Ohno Y, Ri Y, Ikoma T, Yuugetu H, Asai T, Watanabe M, Yasuda N, Akao S, Takemura G. Anti-tumor effect of gallic acid on LL-2 lung cancer cells transplanted in mice. Anticancer Drugs. 2001;12:847-852. |
| 37. | Sandhya T, Lathika KM, Pandey BN, Mishra KP. Potential of traditional ayurvedic formulation, Triphala, as a novel anticancer drug. Cancer Lett. 2006;231:206-214. |