Published online Jan 7, 2008. doi: 10.3748/wjg.14.22
Revised: October 19, 2007
Published online: January 7, 2008
Nonalcoholic fatty liver disease (NAFLD) is highly prevalent and can result in nonalcoholic steatohepatitis (NASH) and progressive liver disease including cirrhosis and hepatocellular carcinoma. A growing body of literature implicates the peroxisome proliferators-activated receptors (PPARs) in the pathogenesis and treatment of NAFLD. These nuclear hormone receptors impact on hepatic triglyceride accumulation and insulin resistance. The aim of this review is to describe the data linking PPARα and PPARγ to NAFLD/NASH and to discuss the use of PPAR ligands for the treatment of NASH.
- Citation: Kallwitz ER, McLachlan A, Cotler SJ. Role of peroxisome proliferators-activated receptors in the pathogenesis and treatment of nonalcoholic fatty liver disease. World J Gastroenterol 2008; 14(1): 22-28
- URL: https://www.wjgnet.com/1007-9327/full/v14/i1/22.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.22
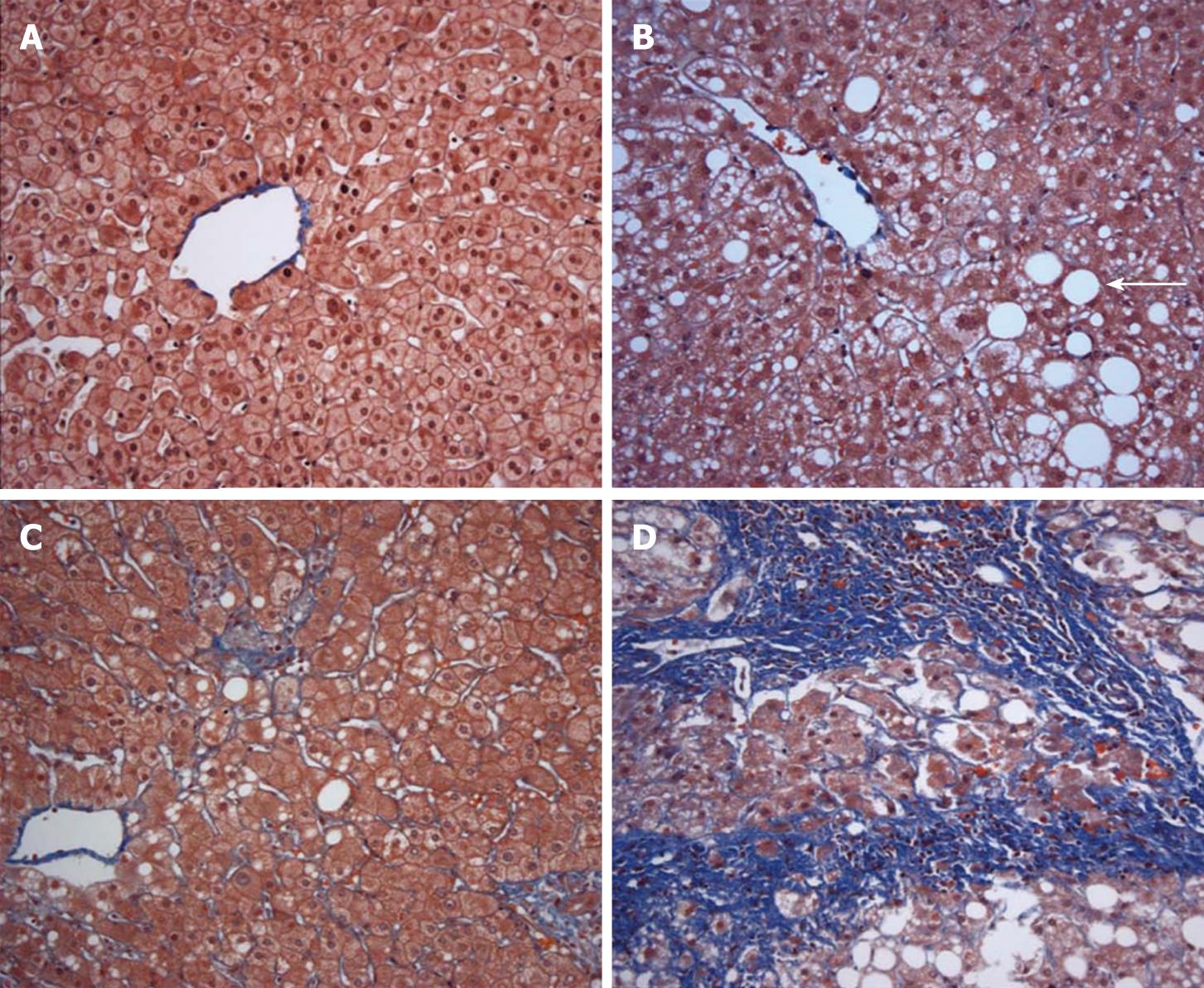
An estimated 30% of adults and 10% of children and adolescents in the United States have nonalcoholic fatty liver disease (NAFLD), defined as liver fat content exceeding 5% (Figure 1)[1–3]. Non-alcoholic fatty liver disease is associated with obesity, non-insulin dependent diabetes, and hypertriglyceridemia and represents the hepatic manifestation of the metabolic syndrome[4]. A subset of persons with NAFLD progresses to nonalcoholic steatohepatitis (NASH), consisting of hepatic steatosis accompanied by inflammation and fibrosis (Figure 1)[5]. Nonalcoholic steatohepatitis affects approximately 3% of the lean population and 19% of obese persons, making it the most prevalent cause of chronic liver disease in the country[6]. Moreover, NASH represents a progressive form of liver disease. Cirrhosis developed in 5% of patients with NASH in a community-based cohort and 20% of NASH patients in a referral population[78]. Nonalcoholic steatohepatitis accounts for up to 75% of cases of cryptogenic cirrhosis and patients with NASH and cirrhosis are at risk for hepatocellular carcinoma[910].
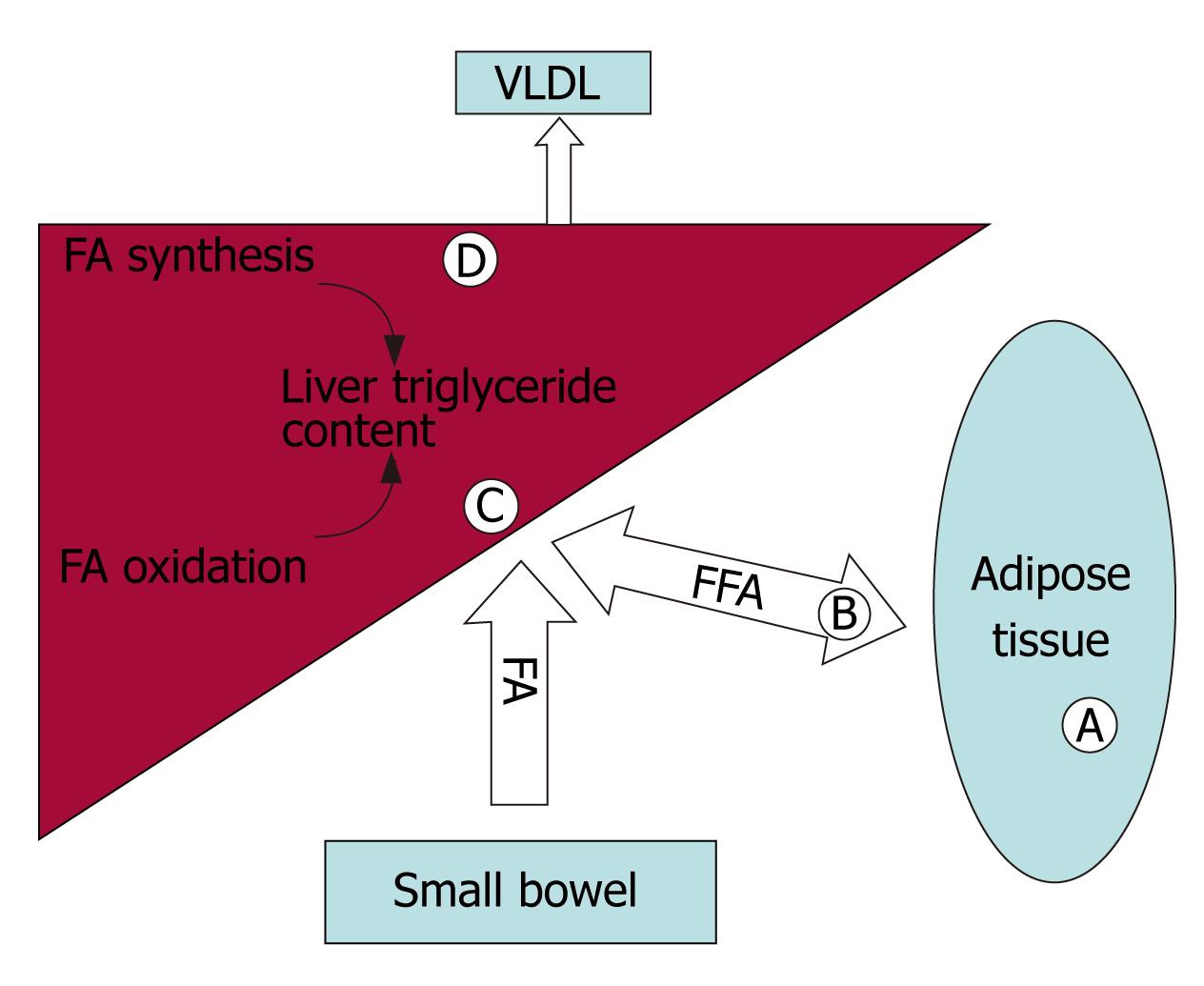
The pathogenesis of NASH is often conceptualized as a two-step process, consisting of hepatic triglyceride accumulation, followed by the development of oxidative stress and cytokine expression leading to steatohepatitis[11]. Multiple metabolic processes can result in hepatocellular triglyceride accumulation including: (1) Excess dietary intake. Dietary triglycerides are delivered to the liver in the form of chylomicrons. In addition, dietary calories stored in adipose tissue as fat represent a source of fatty acids and triglycerides that can be delivered to the liver in the form of lipoprotein particles and free fatty acids. (2) Increased rates of lipogenesis resulting from the de novo synthesis of fatty acids and triglycerides in the liver. (3) Decreased rates of β-oxidation of fatty acids in the liver. (4) Decreased rates of export of cholesterol esters and triglycerides from the liver as very low density lipoprotein (VLDL)[12]. As shown in Figure 2, the PPARs impact on multiple processes involved in lipid trafficking and metabolism.
Insulin resistance and hyperinsulinemia seem to be central to the development of NAFLD. Insulin resistance is associated with increased lipolysis and reduced postprandial uptake and storage of fatty acids in adipose tissue, leading to increased fatty acid flux to the liver[13]. In turn, increased liver fat content contributes to hepatic insulin resistance[14]. Hyperinsulinemia induces sterol regulatory element-binding protein-1c (SREBP-1c) expression and hyperglycemia activates carbohydrate response element binding protein (ChREBP), both of which increase hepatic fatty acid synthesis[15].
PPARs play a key role in modulating hepatic triglyceride accumulation. PPARα regulates fatty acid β-oxidation. PPARγ increases insulin sensitivity as well as regulating triglyceride storage in adipose tissue. Fat labeling studies indicated that the majority of hepatic triglycerides originate from adipose tissue as non-esterified fatty acids[16].
PPARs are part of the nuclear receptor superfamily[17]. There are three isotypes in mammals designated PPARα [NR1C1], PPARδ [NR1C2] and PPARγ [NR1C3][18]. PPARα is activated by ligands termed peroxisome proliferators, which were named for their effects on peroxisomes in rodent livers[1920]. Lipids are natural PPAR ligands, leading to regulation of lipid metabolism and fuel partitioning[17]. PPARs form a heterodimer with the retinoid X receptor (RXR). The PPAR:RXR heterodimer, when bound to a ligand, changes conformation and binds to DNA at PPAR response elements, resulting in gene transcription[2122].
PPARα is expressed in the liver and other metabolically active tissues including striated muscle, kidney and pancreas[2324]. Many of the genes encoding enzymes involved in the mitochondrial and peroxisomal fatty acid β-oxidation pathways are regulated by PPARα. In particular, the acyl-CoA synthetase, the carnitine palmitoyl transferase I, the very long-chain acyl-CoA dehydrogenase and the tri-functional protein genes encoding enzymes in the mitochondrial fatty acid β-oxidation pathway are induced by peroxisome proliferators that activate PPARα[25–28]. Similarly, the acyl-CoA synthetase, the straight-chain acyl-CoA oxidase, the L-bifunctional protein and the 3-ketoacyl-CoA thiolase genes encoding enzymes in the peroxisomal fatty acid β-oxidation pathway are induced by peroxisome proliferators that active PPARα[26272930]. Loss of expression of the PPARα gene in mice results in hepatic steatosis under conditions of increased fatty acid metabolism in the liver such as fasting or a high fat diet[3132]. Administration of a potent PPAR agonist decreases hepatic steatosis in mice receiving a methionine and choline deficient diet[33]. These observations indicate that under conditions of increased hepatic fatty acid influx or decreased hepatic fatty acid efflux, PPARα activation prevents the accumulation of triglycerides by increasing the rate of fatty acid catabolism.
Additional factors appear to interact with PPARα to regulate hepatic triglyceride content. These include adiponectin, which is an adipocyte produced peptide hormone that limits fat accumulation in the liver by a number of mechanisms including activation of PPARαto increase hepatic fatty acid oxidation[34]. In cell culture models, treatment with adiponectin resulted in increased activity of PPARα target genes such as acyl-CoA oxidase, carnitine palmitoyl transferase-I, and fatty acid binding protein[35]. PPARα ligands can increase steaoryl-CoA desaturase-1 (SCD1) activity, which is necessary for VLDL secretion[36]. A PPAR response element was found in the SCD1 promoter[37]. Adiponectin is upregulated by PPARγ, providing a connection between the two isotypes[38].
Most of the data regarding PPARα and hepatic lipid homeostasis comes from mouse models. However, there are important differences in PPARαactivity between rodents and humans. PPARα DNA binding activity and PPARα expression in human hepatocytes is less than 10-fold that observed in mice[3940]. Certain PPAR response elements, such as the acyl CoA oxidase gene, do not respond to PPAR ligands in humans as they do in rodent models[41]. Finally, PPARα activation in rodent models resulted in peroxisomal proliferation, hepatomegaly, and hepatocellular carcinoma[394243], whereas similar changes were not observed in humans[4445]. Further research is needed to determine the relative importance of PPARα in regulating hepatic triglyceride metabolism in humans.
Fibric acid derivatives, which are available for use in humans as lipid lowering agents, serve as PPARα activators[4647]. In a mouse model of fatty liver disease, fenofibrate treatment improved steatosis and increased expression of genes involved in fatty acid metabolism[48]. Trials with fibrates in humans have yielded mixed results. A study involving potential living liver donors with steatosis showed that a combination of diet, exercise, and benzafibrate significantly reduced steatosis and resulted in normalization of alanine aminotransferase levels[49]. However, it was not clear whether the therapeutic benefit was related to benzafibrate or to a 1000 kilocalorie/day diet and a 600 kilocalorie/day exercise regimen. In addition to being a PPARα ligand, benzofibrate activates PPARγ and improves insulin sensitivity in animal models[4647], an effect not seen with fenofibrate[50]. Another study demonstrated that 42% of 62 patients with NAFLD had biochemical and ultrasound improvement on fenofibrate, but histologic data were not collected[51]. A small controlled study of gemfibrozil versus placebo for four weeks found improved aminotransferase levels with the use of gemfibrozil in patients with NAFLD[52]. These studies are in contrast to a another small series, which demonstrated no change in aminotransferases and no histologic improvement after one year of clofibrate therapy for NAFLD[53].
Omega-3 polyunsaturated fatty acids (PUFA) present in fish oil, and their metabolites, provide another source of PPARα ligands. Omega-3 PUFA also inhibit lipogenesis by antagonizing activation of LXR[5455], thus reducing expression of SREBP-1c[56], which results in the down regulation of key enzymes involved in hepatic lipid biosynthesis. In mouse models, omega-3 PUFA supplementation was associated with improvement in hepatic steatosis and insulin sensitivity, as well as lower fasting free fatty acid concentrations and lower serum triglyceride levels[5758]. Two human studies reported a decline in serum aminotransferase levels and improvement in ultrasound features of fatty liver with omega-3 PUFA supplementation[5960]. However, no histologic data were provided. Omega-3 PUFA supplementation also reduces serum triglyceride levels in the fasting and postprandial state[61–63], but was not found to improve insulin sensitivity in humans[626465].
PPARγ is expressed in high levels in adipose tissue[66] and plays a role in increasing insulin sensitivity as well as in promoting fatty acid uptake into adipocytes and adipocyte differentiation. The net effect of these processes is to increase triglyceride storage in adipocytes, reducing delivery of fatty acids to the liver. Patients with dominant negative mutations in PPARγ have NAFLD and the metabolic syndrome while lacking adipose tissue suggesting increased triglyceride delivery to the liver[67]. PPARγ is present in the liver to a lesser degree than in adipose tissue. Liver-specific PPARγ deficient mice are protected against the development of steatosis suggesting a role for hepatic PPARγ in liver triglyceride accumulation[6869].
Insulin resistance is integral to the development of NAFLD, leading to increased fatty acid flux to the liver and increased hepatic fatty acid synthesis[1315]. PPARγ increases insulin sensitivity by upregulating GLUT4, an insulin dependent glucose transporter in adipose tissue and striated muscle[70], and inducing expression of the c-Cbl associated protein, which is involved in insulin signaling[71]. Additionally, in mouse models of insulin resistance, PPARγ activation attenuated induction of supressor of cytokine signaling 3 (SOCS3), which is involved in the development of insulin resistance[72].
PPARγ also promotes adipocyte differentiation and expression of proteins in adipocytes involved in fatty acid uptake[1773], fatty acid transport[7475] and fatty acid synthesis[76]. Differentiation of preadipocytes to adipocytes requires transcription factors including the CCAT-enhancer-binding proteins (C/EBPs) and the adipocyte differentiation and determination factor (ADD)-1/SREBP-1[77–80]. C/EBP plays an important role in inducing and maintaining PPARγ expression in adipogenesis[8182]. ADD-1/SREBP-1 is strongly adipogenic, is enhanced by PPARγ, and results in the expression of lipogenic genes including fatty acid synthase[80]. These transcription factors guide the cell through proliferation, clonal expansion, growth arrest, and eventually adipocyte specific genes are activated resulting in lipid accumulation[82]. PPARγ also increases expression of lipoprotein lipase, an enzyme that serves to partition fat to adipocytes, limiting fatty acid flux to the liver. Similar to PPARγ, PPARγ ligands upregulate SCD1 activity, which promotes VLDL secretion. Thiazolidinediones (TZDs), ligands for PPARγ have also been shown to increase arachidonic acid content in triglycerides through SCD1, which has been associated with increased insulin sensitivity[83]. Other effects of PPARγ include induction of uncoupling protein-2, which might decrease hepatic triglyceride accumulation by increasing energy expenditure[84]. PPARγ expression also might reduce hepatic inflammation by decreasing expression of proinflammatory cytokines, such as TNFα[85].
TZDs are PPARγ agonists, which improve glycemic control in patients with type 2 diabetes mellitus by increasing insulin sensitivity[86]. The TZD-mediated increase in insulin sensitivity was demonstrated in adipose tissue, the liver, and skeletal muscle[8788]. TZD therapy increases adiponectin levels, which are associated with improved insulin sensitivity[89]. Furthermore, adiponectin impacts on hepatic fat accumulation by enhancing fatty acid oxidation in muscle, and by activating PPARα to increase fatty acid oxidation in the liver[34].
Thiazolidinediones also increase expression of AMP-activated protein kinase[8890]. This protein kinase increases fatty acid oxidation as well as decreasing lipogenesis[9192]. The reduction in lipogenesis is mediated through phosphorylation and inhibition of acetyl-CoA carboxylase, which decreases malonyl CoA formation and down regulates SREBP and the carbohydrate response element binding protein (ChREBP)[93]. Finally, TZDs have anti-inflammatory and anti-fibrotic properties that might be beneficial in NASH. Serum high-sensitivity CRP, IL-6 and IL-18 levels were significantly reduced in patients on TZD therapy[9495] and the TZD pioglitazone reduced activation of hepatic stellate cells in an animal model[96]. Increased adiponectin may also contribute to the anti-inflammatory effects of TZD therapy. Adiponectin was shown to block TNFα activation of inflammatory genes in endothelial cells[97], decrease macrophage growth and function[98–100], and increase release of the anti-inflammatory cytokines IL-10 and IL-1RA with a concomitant decrease in interferon-γ production[100].
Studies of the TZDs rosiglitazone and pioglitazone demonstrated reduction in aminotransferase levels and improvement in liver histology in patients with NASH[87101–106]. One study that compared pioglitazone plus vitamin E to vitamin E alone for the treatment of NASH found significant improvement in steatosis, hepatocellular ballooning, and pericellular fibrosis in the combination therapy arm, but not in patients treated with vitamin E alone[103]. In a study of pioglitazone plus diet versus placebo plus diet in patients with biopsy proven NASH and insulin resistance, pioglitazone therapy was associated with a significant reduction in mean serum aminotransferase levels and improved glycemic control[107]. There were significant improvements in hepatic insulin resistance as well as histologic parameters including hepatic steatosis, ballooning, and inflammation, although not fibrosis with six months of treatment. Further evaluation of the efficacy and the cardiovascular risk of TZD therapy[108] is needed before this class of medications is routinely prescribed for the treatment of NASH.
The nuclear hormone receptors PPARα and PPARγ appear to play an important role in modulating hepatic triglyceride accumulation, the primary process in the development of NAFLD. PPARα activity reduces liver fat by increasing β-oxidation of fatty acids and PPARγ increases insulin sensitivity as well as reducing fatty acid flux to the liver. PPAR ligands show promise in the treatment of NAFLD, although further human studies are needed to define the therapeutic role of these agents.
| 1. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. |
| 2. | Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387-1395. |
| 3. | Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388-1393. |
| 4. | Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917-923. |
| 5. | Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202-1219. |
| 6. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. |
| 7. | Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113-121. |
| 8. | Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413-1419. |
| 9. | Caldwell SH, Oelsner DH, Iezzoni JC, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology. 1999;29:664-669. |
| 10. | Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36:1349-1354. |
| 11. | Day CP, James OF. Steatohepatitis: a tale of two "hits"? Gastroenterology. 1998;114:842-845. |
| 12. | Reddy JK, Rao MS. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am J Physiol Gastrointest Liver Physiol. 2006;290:G852-G858. |
| 13. | Yu YH, Ginsberg HN. Adipocyte signaling and lipid homeostasis: sequelae of insulin-resistant adipose tissue. Circ Res. 2005;96:1042-1052. |
| 14. | Kim JK, Fillmore JJ, Chen Y, Yu C, Moore IK, Pypaert M, Lutz EP, Kako Y, Velez-Carrasco W, Goldberg IJ. Tissue-specific overexpression of lipoprotein lipase causes tissue-specific insulin resistance. Proc Natl Acad Sci USA. 2001;98:7522-7527. |
| 15. | Yamashita H, Takenoshita M, Sakurai M, Bruick RK, Henzel WJ, Shillinglaw W, Arnot D, Uyeda K. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc Natl Acad Sci USA. 2001;98:9116-9121. |
| 16. | Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343-1351. |
| 17. | Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649-688. |
| 19. | Hess R, Staubli W, Riess W. Nature of the hepatomegalic effect produced by ethyl-chlorophenoxy-isobutyrate in the rat. Nature. 1965;208:856-858. |
| 20. | Svoboda DJ, Azarnoff DL. Response of hepatic microbodies to a hypolipidemic agent, ethyl chlorophenoxyisobutyrate (CPIB). J Cell Biol. 1966;30:442-450. |
| 21. | Bardot O, Aldridge TC, Latruffe N, Green S. PPAR-RXR heterodimer activates a peroxisome proliferator response element upstream of the bifunctional enzyme gene. Biochem Biophys Res Commun. 1993;192:37-45. |
| 22. | Gearing KL, Gottlicher M, Teboul M, Widmark E, Gustafsson JA. Interaction of the peroxisome-proliferator-activated receptor and retinoid X receptor. Proc Natl Acad Sci USA. 1993;90:1440-1444. |
| 23. | Mukherjee R, Jow L, Noonan D, McDonnell DP. Human and rat peroxisome proliferator activated receptors (PPARs) demonstrate similar tissue distribution but different responsiveness to PPAR activators. J Steroid Biochem Mol Biol. 1994;51:157-166. |
| 24. | Auboeuf D, Rieusset J, Fajas L, Vallier P, Frering V, Riou JP, Staels B, Auwerx J, Laville M, Vidal H. Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator-activated receptors and liver X receptor-alpha in humans: no alteration in adipose tissue of obese and NIDDM patients. Diabetes. 1997;46:1319-1327. |
| 25. | Louet JF, Chatelain F, Decaux JF, Park EA, Kohl C, Pineau T, Girard J, Pegorier JP. Long-chain fatty acids regulate liver carnitine palmitoyl transferase I gene (L-CPT I) expression through a peroxisome-proliferator-activated receptor alpha (PPARalpha)-independent pathway. Biochem J. 2001;354:189-197. |
| 26. | Schoonjans K, Watanabe M, Suzuki H, Mahfoudi A, Krey G, Wahli W, Grimaldi P, Staels B, Yamamoto T, Auwerx J. Induction of the acyl-coenzyme A synthetase gene by fibrates and fatty acids is mediated by a peroxisome proliferator response element in the C promoter. J Biol Chem. 1995;270:19269-19276. |
| 27. | Aoyama T, Peters JM, Iritani N, Nakajima T, Furihata K, Hashimoto T, Gonzalez FJ. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor alpha (PPARalpha). J Biol Chem. 1998;273:5678-5684. |
| 28. | Reddy JK, Hashimoto T. Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: an adaptive metabolic system. Annu Rev Nutr. 2001;21:193-230. |
| 29. | Tugwood JD, Issemann I, Anderson RG, Bundell KR, McPheat WL, Green S. The mouse peroxisome proliferator activated receptor recognizes a response element in the 5' flanking sequence of the rat acyl CoA oxidase gene. EMBO J. 1992;11:433-439. |
| 30. | Hansmannel F, Clemencet MC, Le Jossic-Corcos C, Osumi T, Latruffe N, Nicolas-Frances V. Functional characterization of a peroxisome proliferator response-element located in the intron 3 of rat peroxisomal thiolase B gene. Biochem Biophys Res Commun. 2003;311:149-155. |
| 31. | Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, Fernandez-Salguero PM, Westphal H, Gonzalez FJ. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol. 1995;15:3012-3022. |
| 32. | Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J Clin Invest. 1999;103:1489-1498. |
| 33. | Nagasawa T, Inada Y, Nakano S, Tamura T, Takahashi T, Maruyama K, Yamazaki Y, Kuroda J, Shibata N. Effects of bezafibrate, PPAR pan-agonist, and GW501516, PPARdelta agonist, on development of steatohepatitis in mice fed a methionine- and choline-deficient diet. Eur J Pharmacol. 2006;536:182-191. |
| 34. | Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762-769. |
| 35. | Yoon MJ, Lee GY, Chung JJ, Ahn YH, Hong SH, Kim JB. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes. 2006;55:2562-2570. |
| 36. | Attie AD, Krauss RM, Gray-Keller MP, Brownlie A, Miyazaki M, Kastelein JJ, Lusis AJ, Stalenhoef AF, Stoehr JP, Hayden MR. Relationship between stearoyl-CoA desaturase activity and plasma triglycerides in human and mouse hypertriglyceridemia. J Lipid Res. 2002;43:1899-1907. |
| 37. | Miller CW, Ntambi JM. Peroxisome proliferators induce mouse liver stearoyl-CoA desaturase 1 gene expression. Proc Natl Acad Sci USA. 1996;93:9443-9448. |
| 38. | Neschen S, Morino K, Rossbacher JC, Pongratz RL, Cline GW, Sono S, Gillum M, Shulman GI. Fish oil regulates adiponectin secretion by a peroxisome proliferator-activated receptor-gamma-dependent mechanism in mice. Diabetes. 2006;55:924-928. |
| 39. | Holden PR, Tugwood JD. Peroxisome proliferator-activated receptor alpha: role in rodent liver cancer and species differences. J Mol Endocrinol. 1999;22:1-8. |
| 40. | Palmer CN, Hsu MH, Griffin KJ, Raucy JL, Johnson EF. Peroxisome proliferator activated receptor-alpha expression in human liver. Mol Pharmacol. 1998;53:14-22. |
| 41. | Lambe KG, Woodyatt NJ, Macdonald N, Chevalier S, Roberts RA. Species differences in sequence and activity of the peroxisome proliferator response element (PPRE) within the acyl CoA oxidase gene promoter. Toxicol Lett. 1999;110:119-127. |
| 42. | Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645-650. |
| 43. | Reddy JK, Azarnoff DL, Hignite CE. Hypolipidaemic hepatic peroxisome proliferators form a novel class of chemical carcinogens. Nature. 1980;283:397-398. |
| 44. | Blumcke S, Schwartzkopff W, Lobeck H, Edmondson NA, Prentice DE, Blane GF. Influence of fenofibrate on cellular and subcellular liver structure in hyperlipidemic patients. Atherosclerosis. 1983;46:105-116. |
| 45. | Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, Huttunen JK, Kaitaniemi P, Koskinen P, Manninen V. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317:1237-1245. |
| 46. | Fruchart JC, Staels B, Duriez P. The role of fibric acids in atherosclerosis. Curr Atheroscler Rep. 2001;3:83-92. |
| 47. | Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: from orphan receptors to drug discovery. J Med Chem. 2000;43:527-550. |
| 48. | Harano Y, Yasui K, Toyama T, Nakajima T, Mitsuyoshi H, Mimani M, Hirasawa T, Itoh Y, Okanoue T. Fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, reduces hepatic steatosis and lipid peroxidation in fatty liver Shionogi mice with hereditary fatty liver. Liver Int. 2006;26:613-620. |
| 49. | Nakamuta M, Morizono S, Soejima Y, Yoshizumi T, Aishima S, Takasugi S, Yoshimitsu K, Enjoji M, Kotoh K, Taketomi A. Short-term intensive treatment for donors with hepatic steatosis in living-donor liver transplantation. Transplantation. 2005;80:608-612. |
| 50. | Nakano S, Inada Y, Masuzaki H, Tanaka T, Yasue S, Ishii T, Arai N, Ebihara K, Hosoda K, Maruyama K. Bezafibrate regulates the expression and enzyme activity of 11beta-hydroxysteroid dehydrogenase type 1 in murine adipose tissue and 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab. 2007;292:E1213-E1222. |
| 51. | Athyros VG, Mikhailidis DP, Didangelos TP, Giouleme OI, Liberopoulos EN, Karagiannis A, Kakafika AI, Tziomalos K, Burroughs AK, Elisaf MS. Effect of multifactorial treatment on non-alcoholic fatty liver disease in metabolic syndrome: a randomised study. Curr Med Res Opin. 2006;22:873-883. |
| 52. | Basaranoglu M, Acbay O, Sonsuz A. A controlled trial of gemfibrozil in the treatment of patients with nonalcoholic steatohepatitis. J Hepatol. 1999;31:384. |
| 53. | Laurin J, Lindor KD, Crippin JS, Gossard A, Gores GJ, Ludwig J, Rakela J, McGill DB. Ursodeoxycholic acid or clofibrate in the treatment of non-alcohol-induced steatohepatitis: a pilot study. Hepatology. 1996;23:1464-1467. |
| 54. | Sampath H, Miyazaki M, Dobrzyn A, Ntambi JM. Stearoyl-CoA desaturase-1 mediates the pro-lipogenic effects of dietary saturated fat. J Biol Chem. 2007;282:2483-2493. |
| 55. | Nakatani T, Katsumata A, Miura S, Kamei Y, Ezaki O. Effects of fish oil feeding and fasting on LXRalpha/RXRalpha binding to LXRE in the SREBP-1c promoter in mouse liver. Biochim Biophys Acta. 2005;1736:77-86. |
| 56. | Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, Shan B, Brown MS, Goldstein JL, Mangelsdorf DJ. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14:2819-2830. |
| 57. | Alwayn IP, Andersson C, Zauscher B, Gura K, Nose V, Puder M. Omega-3 fatty acids improve hepatic steatosis in a murine model: potential implications for the marginal steatotic liver donor. Transplantation. 2005;79:606-608. |
| 58. | D'Alessandro ME, Lombardo YB, Chicco A. Effect of dietary fish oil on insulin sensitivity and metabolic fate of glucose in the skeletal muscle of normal rats. Ann Nutr Metab. 2002;46:114-120. |
| 59. | Hatzitolios A, Savopoulos C, Lazaraki G, Sidiropoulos I, Haritanti P, Lefkopoulos A, Karagiannopoulou G, Tzioufa V, Dimitrios K. Efficacy of omega-3 fatty acids, atorvastatin and orlistat in non-alcoholic fatty liver disease with dyslipidemia. Indian J Gastroenterol. 2004;23:131-134. |
| 60. | Capanni M, Calella F, Biagini MR, Genise S, Raimondi L, Bedogni G, Svegliati-Baroni G, Sofi F, Milani S, Abbate R. Prolonged n-3 polyunsaturated fatty acid supplementation ameliorates hepatic steatosis in patients with non-alcoholic fatty liver disease: a pilot study. Aliment Pharmacol Ther. 2006;23:1143-1151. |
| 61. | Harris WS, Connor WE, Alam N, Illingworth DR. Reduction of postprandial triglyceridemia in humans by dietary n-3 fatty acids. J Lipid Res. 1988;29:1451-1460. |
| 62. | Rivellese AA, Maffettone A, Iovine C, Di Marino L, Annuzzi G, Mancini M, Riccardi G. Long-term effects of fish oil on insulin resistance and plasma lipoproteins in NIDDM patients with hypertriglyceridemia. Diabetes Care. 1996;19:1207-1213. |
| 63. | Sanders TA, Oakley FR, Miller GJ, Mitropoulos KA, Crook D, Oliver MF. Influence of n-6 versus n-3 polyunsaturated fatty acids in diets low in saturated fatty acids on plasma lipoproteins and hemostatic factors. Arterioscler Thromb Vasc Biol. 1997;17:3449-3460. |
| 64. | Sirtori CR, Paoletti R, Mancini M, Crepaldi G, Manzato E, Rivellese A, Pamparana F, Stragliotto E. N-3 fatty acids do not lead to an increased diabetic risk in patients with hyperlipidemia and abnormal glucose tolerance. Italian Fish Oil Multicenter Study. Am J Clin Nutr. 1997;65:1874-1881. |
| 65. | Mori TA, Bao DQ, Burke V, Puddey IB, Watts GF, Beilin LJ. Dietary fish as a major component of a weight-loss diet: effect on serum lipids, glucose, and insulin metabolism in overweight hypertensive subjects. Am J Clin Nutr. 1999;70:817-825. |
| 66. | Dreyer C, Krey G, Keller H, Givel F, Helftenbein G, Wahli W. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell. 1992;68:879-887. |
| 67. | Savage DB, Tan GD, Acerini CL, Jebb SA, Agostini M, Gurnell M, Williams RL, Umpleby AM, Thomas EL, Bell JD. Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-gamma. Diabetes. 2003;52:910-917. |
| 68. | Matsusue K, Haluzik M, Lambert G, Yim SH, Gavrilova O, Ward JM, Brewer B Jr, Reitman ML, Gonzalez FJ. Liver-specific disruption of PPARgamma in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J Clin Invest. 2003;111:737-747. |
| 69. | Gavrilova O, Haluzik M, Matsusue K, Cutson JJ, Johnson L, Dietz KR, Nicol CJ, Vinson C, Gonzalez FJ, Reitman ML. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem. 2003;278:34268-34276. |
| 70. | Wu Z, Xie Y, Morrison RF, Bucher NL, Farmer SR. PPARgamma induces the insulin-dependent glucose transporter GLUT4 in the absence of C/EBPalpha during the conversion of 3T3 fibroblasts into adipocytes. J Clin Invest. 1998;101:22-32. |
| 71. | Ribon V, Johnson JH, Camp HS, Saltiel AR. Thiazolidinediones and insulin resistance: peroxisome proliferatoractivated receptor gamma activation stimulates expression of the CAP gene. Proc Natl Acad Sci USA. 1998;95:14751-14756. |
| 72. | Shi H, Cave B, Inouye K, Bjorbaek C, Flier JS. Overexpression of suppressor of cytokine signaling 3 in adipose tissue causes local but not systemic insulin resistance. Diabetes. 2006;55:699-707. |
| 73. | Schoonjans K, Peinado-Onsurbe J, Lefebvre AM, Heyman RA, Briggs M, Deeb S, Staels B, Auwerx J. PPARalpha and PPARgamma activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J. 1996;15:5336-5348. |
| 74. | Motojima K, Passilly P, Peters JM, Gonzalez FJ, Latruffe N. Expression of putative fatty acid transporter genes are regulated by peroxisome proliferator-activated receptor alpha and gamma activators in a tissue- and inducer-specific manner. J Biol Chem. 1998;273:16710-16714. |
| 75. | Frohnert BI, Hui TY, Bernlohr DA. Identification of a functional peroxisome proliferator-responsive element in the murine fatty acid transport protein gene. J Biol Chem. 1999;274:3970-3977. |
| 76. | Castelein H, Gulick T, Declercq PE, Mannaerts GP, Moore DD, Baes MI. The peroxisome proliferator activated receptor regulates malic enzyme gene expression. J Biol Chem. 1994;269:26754-26758. |
| 77. | Christy RJ, Yang VW, Ntambi JM, Geiman DE, Landschulz WH, Friedman AD, Nakabeppu Y, Kelly TJ, Lane MD. Differentiation-induced gene expression in 3T3-L1 preadipocytes: CCAAT/enhancer binding protein interacts with and activates the promoters of two adipocyte-specific genes. Genes Dev. 1989;3:1323-1335. |
| 78. | Freytag SO, Paielli DL, Gilbert JD. Ectopic expression of the CCAAT/enhancer-binding protein alpha promotes the adipogenic program in a variety of mouse fibroblastic cells. Genes Dev. 1994;8:1654-1663. |
| 79. | Tontonoz P, Kim JB, Graves RA, Spiegelman BM. ADD1: a novel helix-loop-helix transcription factor associated with adipocyte determination and differentiation. Mol Cell Biol. 1993;13:4753-4759. |
| 80. | Kim JB, Spiegelman BM. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996;10:1096-1107. |
| 81. | Wu Z, Bucher NL, Farmer SR. Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol Cell Biol. 1996;16:4128-4136. |
| 82. | Fajas L, Fruchart JC, Auwerx J. Transcriptional control of adipogenesis. Curr Opin Cell Biol. 1998;10:165-173. |
| 83. | Riserus U, Tan GD, Fielding BA, Neville MJ, Currie J, Savage DB, Chatterjee VK, Frayn KN, O'Rahilly S, Karpe F. Rosiglitazone increases indexes of stearoyl-CoA desaturase activity in humans: link to insulin sensitization and the role of dominant-negative mutation in peroxisome proliferator-activated receptor-gamma. Diabetes. 2005;54:1379-1384. |
| 84. | Camirand A, Marie V, Rabelo R, Silva JE. Thiazolidinediones stimulate uncoupling protein-2 expression in cell lines representing white and brown adipose tissues and skeletal muscle. Endocrinology. 1998;139:428-431. |
| 85. | Hofmann C, Lorenz K, Braithwaite SS, Colca JR, Palazuk BJ, Hotamisligil GS, Spiegelman BM. Altered gene expression for tumor necrosis factor-alpha and its receptors during drug and dietary modulation of insulin resistance. Endocrinology. 1994;134:264-270. |
| 86. | Miyazaki Y, Mahankali A, Matsuda M, Mahankali S, Hardies J, Cusi K, Mandarino LJ, DeFronzo RA. Effect of pioglitazone on abdominal fat distribution and insulin sensitivity in type 2 diabetic patients. J Clin Endocrinol Metab. 2002;87:2784-2791. |
| 87. | Promrat K, Lutchman G, Uwaifo GI, Freedman RJ, Soza A, Heller T, Doo E, Ghany M, Premkumar A, Park Y. A pilot study of pioglitazone treatment for nonalcoholic steatohepatitis. Hepatology. 2004;39:188-196. |
| 88. | Saha AK, Avilucea PR, Ye JM, Assifi MM, Kraegen EW, Ruderman NB. Pioglitazone treatment activates AMP-activated protein kinase in rat liver and adipose tissue in vivo. Biochem Biophys Res Commun. 2004;314:580-585. |
| 89. | Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094-2099. |
| 90. | Fryer LG, Parbu-Patel A, Carling D. The Anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J Biol Chem. 2002;277:25226-25232. |
| 91. | Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339-343. |
| 92. | Hardie DG. Minireview: the AMP-activated protein kinase cascade: the key sensor of cellular energy status. Endocrinology. 2003;144:5179-5183. |
| 93. | Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147-152. |
| 94. | Esposito K, Ciotola M, Carleo D, Schisano B, Saccomanno F, Sasso FC, Cozzolino D, Assaloni R, Merante D, Ceriello A. Effect of rosiglitazone on endothelial function and inflammatory markers in patients with the metabolic syndrome. Diabetes Care. 2006;29:1071-1076. |
| 95. | Haffner SM, Greenberg AS, Weston WM, Chen H, Williams K, Freed MI. Effect of rosiglitazone treatment on nontraditional markers of cardiovascular disease in patients with type 2 diabetes mellitus. Circulation. 2002;106:679-684. |
| 96. | Kawaguchi K, Sakaida I, Tsuchiya M, Omori K, Takami T, Okita K. Pioglitazone prevents hepatic steatosis, fibrosis, and enzyme-altered lesions in rat liver cirrhosis induced by a choline-deficient L-amino acid-defined diet. Biochem Biophys Res Commun. 2004;315:187-195. |
| 97. | Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296-1301. |
| 98. | Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, Kihara S, Funahashi T, Tenner AJ, Tomiyama Y. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood. 2000;96:1723-1732. |
| 99. | Tsatsanis C, Zacharioudaki V, Androulidaki A, Dermitzaki E, Charalampopoulos I, Minas V, Gravanis A, Margioris AN. Adiponectin induces TNF-alpha and IL-6 in macrophages and promotes tolerance to itself and other pro-inflammatory stimuli. Biochem Biophys Res Commun. 2005;335:1254-1263. |
| 100. | Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630-635. |
| 101. | Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Oliver D, Bacon BR. Improved nonalcoholic steatohepatitis after 48 weeks of treatment with the PPAR-gamma ligand rosiglitazone. Hepatology. 2003;38:1008-1017. |
| 102. | Neuschwander-Tetri BA, Brunt EM, Wehmeier KR, Sponseller CA, Hampton K, Bacon BR. Interim results of a pilot study demonstrating the early effects of the PPAR-gamma ligand rosiglitazone on insulin sensitivity, aminotransferases, hepatic steatosis and body weight in patients with non-alcoholic steatohepatitis. J Hepatol. 2003;38:434-440. |
| 103. | Sanyal AJ, Mofrad PS, Contos MJ, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, Shiffman ML, Clore J, Mills AS. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2004;2:1107-1115. |
| 104. | Carey DG, Cowin GJ, Galloway GJ, Jones NP, Richards JC, Biswas N, Doddrell DM. Effect of rosiglitazone on insulin sensitivity and body composition in type 2 diabetic patients (corrected). Obes Res. 2002;10:1008-1015. |
| 105. | Bajaj M, Suraamornkul S, Pratipanawatr T, Hardies LJ, Pratipanawatr W, Glass L, Cersosimo E, Miyazaki Y, DeFronzo RA. Pioglitazone reduces hepatic fat content and augments splanchnic glucose uptake in patients with type 2 diabetes. Diabetes. 2003;52:1364-1370. |
| 106. | Mayerson AB, Hundal RS, Dufour S, Lebon V, Befroy D, Cline GW, Enocksson S, Inzucchi SE, Shulman GI, Petersen KF. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes. 2002;51:797-802. |
| 107. | Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297-2307. |
| 108. | Home PD, Pocock SJ, Beck-Nielsen H, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJ. Rosiglitazone evaluated for cardiovascular outcomes-an interim analysis. N Engl J Med. 2007;357:28-38. |