CASE REPORT
A 31-year-old woman was admitted to our hospital with perimenstrual lower and mid-abdominal pain irradiating to the back and lower abdominal fullness for 3 years, at first monthly, but later continuous, and gradually increasing in severity. She had a history of low-grade fever with chills, which occured monthly during her menstrual periods. The abdominal pain was crampy and associated with borborygmi, anorexia, nausea and vomiting, without hematemesis or coffee-ground emesis. The patient had lost 10 kg in weight over the previous 6 mo, and was having two to three semiformed stools per day. She also gave a history of moderate dysmenorrhea and menorrhagia, but no dyspareunia. Her only medication was an oral contraceptive. She denied any history of smoking, alcohol consumption, or illicit drug use. The patient had no history of sexually transmitted disease. She had delivered a healthy baby. There was no family history of inflammatory bowel disease or endometriosis.
Despite no pathological evidence, her gynecologist at a women’s health clinic had diagnosed her with small bowel endometriosis, based on interviews and her clinical course. Since only oral contraceptive therapy was started, the symptoms due to mechanical subileus had gradually improved. Although he had no pathological evidence, lack of response to oral contraceptive therapy had encouraged him to perform an exploratory laparotomy 1 mo before she was admitted to our institution for further evaluation and management. At Pfannenstiel transverse laparotomy, extensive adhesion formation and inflammation around the cecum, terminal ileum, sigmoid colon, uterus and the right adnexa were found. The gynecologist was only able to perform an incisional biopsy from the highly inflamed areas. Biopsy results showed only chronic inflammatory infiltrate and granulation tissue. The patient was then referred to us to identify the underlying pathology.
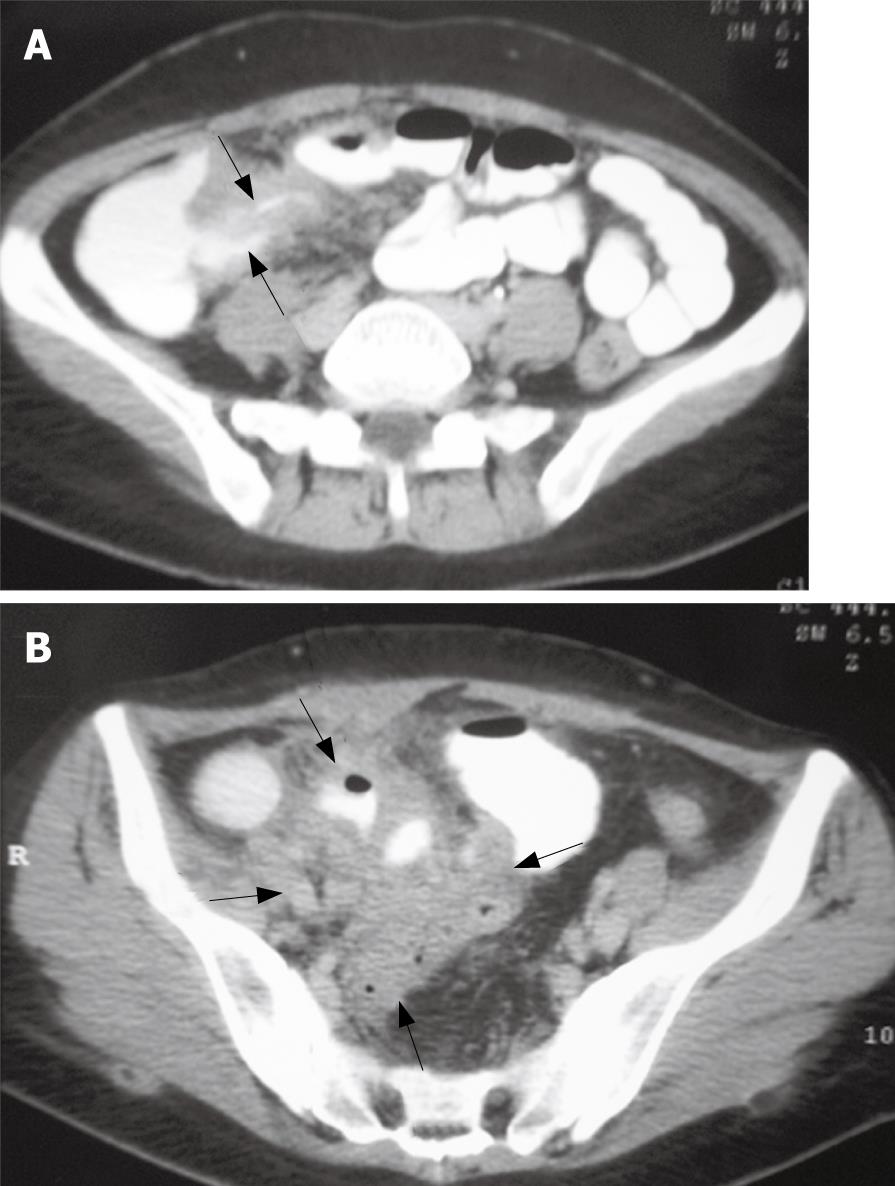
A physical examination on admission revealed a thin and wasted woman weighing 48 kg. The patient was hypotensive with a blood pressure of 95/65 mm Hg and a heart rate of 108 beats/min. The patient’s temperature was 37.5°C. The abdomen was soft and distended with right-lower-quadrant tenderness to palpation and fullness. A Pfannenstiel scar was present, but there was no scar tenderness. There was no hepatosplenomegaly or lymphoadenopathy. She demonstrated no arthralgia. Skin rash and erythema nodosum were not found. Rectal examination was unremarkable. There was no perianal fistula. A pelvic examination revealed cervical motion tenderness and right adnexal tenderness. Results of laboratory studies on admission showed: white blood cell count, 28 000/mm3 with 85.7% neutrophils; hemoglobin, 8.3 g/dL; hematocrit, 24.3%; erytrocyte sedimentation rate, 72 mm/h; C-reactive protein, 26.2 mg/dL; albumin, 3.2 mg/dL; β-human chorionic gonadotropin (β-hCG), 0.1 mIU/mL; carcinoembryonic antigen (CEA), 0.58 ng/mL; and cancer antigen (CA)125, 13.71 U/mL. Urinalysis was in the normal range. Stool studies for ova, parasites, cultures including Yersinia enterocolitica and acid-fast bacilli and Clostridium difficile toxin were negative. A plain abdominal roentgenogram was consistent with partial small bowel obstruction. Abdominal ultrasonography demonstrated the presence of an inflammatory mass surrounding the terminal ileum and/or appendix, wall thickening and absence of peristalsis in the small intestine, with a small amount of ascites. Abdominopelvic CT scanning was performed with oral and intravenous contrast enhancement. There was a narrowing of the terminal ileum and bowel-wall thickening (Figure 1A). Adjacent to the terminal ileum in the right lower quadrant, a complex predominantly inflammatory mass of large size (6.4 cm × 6.1 cm) was present (Figure 1B). The mass was surrounded by fat stranding, with obliteration of the adjacent psoas muscle fat planes. The appendix was not identified separately from the mass. Colonoscopy with random biopsies from the colon and rectum was unremarkable.
Figure 1 Contrast-enhanced scan showing the mural thickening of terminal ileum (arrows) (A), and a complex, predominantly inflammatory mass of large size (6.
4 cm × 6.1 cm) (arrows) (B).
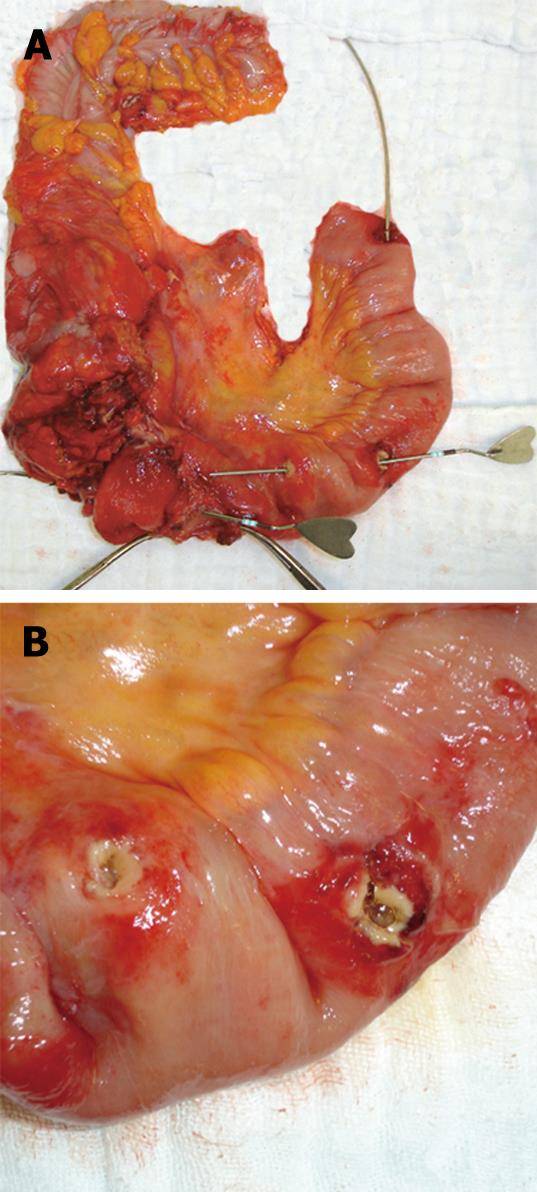
A presumptive diagnosis of mechanical small bowel obstruction was made. The patient was initially treated conservatively with nasogastric suction, intravenous fluids and medication, and responded to this treatment. However, after ingesting a small amount of food she again complained of abdominal pain, and plain radiography once more showed mechanical small bowel obstruction. After improvement with conservative measures and obtaining adequate informed consent, an exploratory laparotomy was performed. At surgery, the proximal small bowel was dilated and the colon was collapsed. There was a small amount of free fluid in the abdomen. No macroscopic evidence of endometriosis was noted at laparatomy. Multiple stenotic ileal loops, adhering firmly to each other, were wrapped as a mass lesion around the cecum, ascending colon, sigmoid colon, right adnexa and uterus. The adhesions were released, and ileal loops were freed from adjacent organs by meticulous sharp dissection. The serosa overlying these areas was congested and hyperemic. The right ureter was completely exposed. Four strictures were noted in the distal 40 cm of the terminal ileum, and three internal fistulas were detected between the terminal ileum and the cecum, between the terminal ileum and the adjacent loop of small bowel, and between the two loops of ileum (Figure 2A and B). The macroscopic appearance was thought to indicate Crohn’s disease, but in view of the close relationship of the ovaries, tubes and uterus, an immediate gynecological opinion was obtained. The on-call gynecology registrar did not consider the appearance to be due to primary gynecological pathology, and therefore, a right hemicolectomy and partial distal ileum resection was performed with an end-to-end ileocolonic anastomosis.
Figure 2 Gross appearance of the resected specimen showing Crohn’s ileitis with multiple fistulas probed with instruments (A), and ileal segment with two adjacent openings of an internal enteric fistula after separation of adhesions (B).
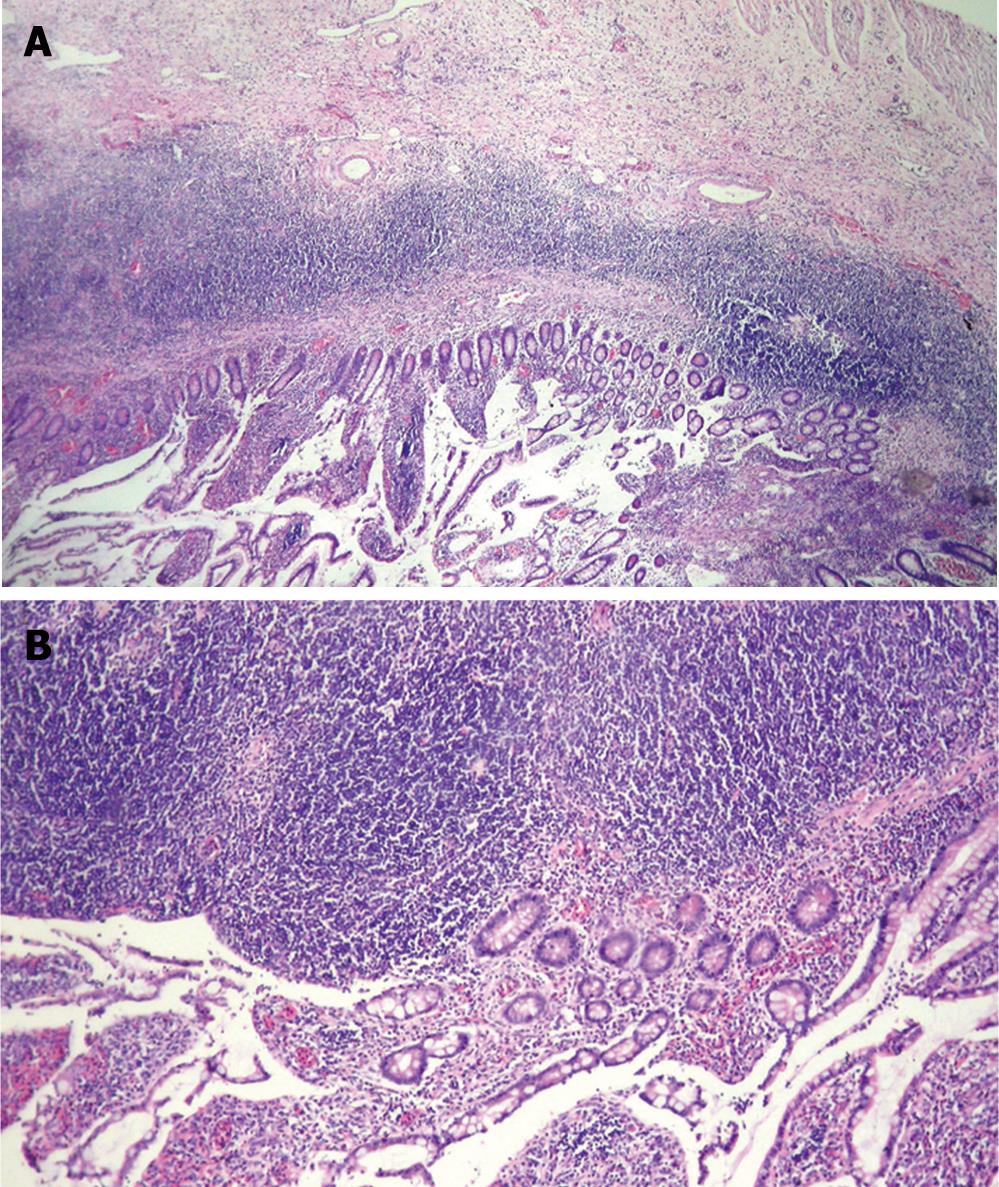
On opening the specimen, there was severe narrowing of the terminal ileum and bowel-wall thickening chara-cteristic of Crohn’s disease. Ulceration measuring 3 cm in diameter, with an irregular margin, was detected at the level of ileocecal valve. Multiple ulcerations, which ranged in sized from 0.5 cm to 2.5 cm in diameter, were also observed on the oral side of the terminal ileum and adjacent loops of small intestine. The mucosa was edematous, pale and marked with deep linear ulcers, and the bowel wall was thickened. Histological examination of the resected terminal ileum revealed marked ulcers and fissures, with transmural inflammatory cell infiltration (Figure 3A), lymphoid aggregates (Figure 3B), and fibrosis. No endometrial tissue was noted in any segment of the resected specimen. The patient made an uneventful recovery from this procedure and was discharged home 10 d post-operatively. Ileocolic resection led to rapid resolution of the symptoms. She has been asymptomatic for over 1 year after her surgery.
Figure 3 Microscopic examination of the resected specimen revealed transmural inflammatory cell infiltration with crypt distortion (A), and transmural lymphoid aggregates (B) (HE, A × 10, B × 20).
DISCUSSION
Endometrosis is a common condition that occurs most frequently in the pelvis, in association with the ovaries, tubes and pelvic peritoneum, with related gynecological symptoms. The incidence of intestinal endometriosis varies between 3% and 37% of all cases of endometriosis, affecting the rectosigmoid colon in 50%-90%, rectovaginal septum in 10%-20%, cecum in 2%-5%, appendix in 3%-18% and small intestine in 2%-16%[13–17]. Small intestinal involvement is nearly always confined to the terminal ileum, which can be explained by its proximity to the tubes and ovaries. However, the correct diagnosis is often delayed because intestinal endometriosis may masquerade clinically as regional enteritis, ulcerative colitis, appendicitis, ischemic enteritis or colitis, diverticulitis, acute self-limited colitis, irritable bowel syndrome, ileocolonic intussusception, or a neoplasm such as colonic or small bowel cancer[18]. Therefore, many cases of bowel endometriosis have been clinically diagnosed based on interviews and response to hormone therapy.
This case demonstrated multiple strictures and internal fistulas that are frequently observed in complicated Crohn’s disease by intestinal contrast, abdominal CT, or at surgery, but we could not make an accurate preoperative diagnosis. There were probably several major reasons for this misdiagnosis. First, our patient presented symptoms of mechanical subileus including intermittent abdominal pain, nausea, vomiting and weight loss, which occured monthly during her menstrual periods. Second, she lacked extraintestinal manifestations associated with Crohn’s disease, including stomatitis, aphthous ulcer, colitic arthritis, pericholangitis, erythema nodosum, pyoderma gangrenosum, episcleritis, uveitis, nephrolithiasis, and hydronephrosis. Third, a histopathological diagnosis of the endoscopic biopsy specimen was reported to be negative for inflammatory bowel disease.
Endometriosis of the intestinal tract is a common disorder that, when symptomatic, may be difficult to diagnose accurately. Intestinal endometriosis has a predilection for the terminal ileum, leads to obstruction, and affects women of reproductive age. Crohn’s ileitis must be considered in the differential diagnosis of enteric endometriosis, as with our patient. Other conditions that may cause difficulty in young women with apparent terminal ileal disease include nodular terminal hyperplasia, Yersinia enterocolitis, tuberculous enteritis, lymphogranuloma, small bowel lymphoma, carcinoid tumors of the terminal ileum, primary adenocarcinoma of the small intestine, and Behcet’s disease[910]. Many of these conditions will be diagnosed only with laparotomy.
The distinction of ileal endometriosis from Crohn’s disease may be difficult pre-operatively. Indeed, there is a similarity between the two entities in terms of clinical presentation, symptomatology, radiological appearances, surgical and pathological findings. The nature of presentation is mainly dependent on the region of bowel affected for both ileal endometriosis and Crohn’s disease. The ileum is the third most affected area, after the rectosigmoid colon and appendix, which is found to be involved in up to 7% of patients with intestinal endometriosis[19]. It usually involves the distal ileum and is limited to the serosa. More extensive and deeper involvement may produce intestinal obstruction by stenosis, kinking or adhesions. Fibrosis may cause puckering and kinking of the mucosa, which results in intestinal bleeding[20]. The presentation of ileal involvement may be an incidental mass found on barium enema or CT, or as a lead point for ileal intussusception. However, Crohn’s disease almost invariably affects the gastrointestinal tract. Crohn’s ileitis, affecting the ileum only, accounts for 30% of cases. There are three categories of disease presentation in Crohn’s disease: stricturing, penetrating, and inflammatory (non-stricturing, non-penetrating disease)[21]. The clinical presentation of our patient with evidence of small bowel obstruction, multiple ileal strictures and internal fistulas at laparotomy suggested Crohn’s ileitis, probably due to a combination of inflammatory mass in the right lower quadrant, proximal bowel distention, narrowing of the terminal ileum and bowel-wall thickening.
Endometriosis may present with a wide variety of symptoms that are more commonly associated with other diseases. Gastrointestinal endometriosis is suggested by dysmenorrhea, menorrhagia or perimenstrual symptoms. Intestinal involvement is usually only serosal, not associated with any intestinal symptoms, and only coincidentally noted at the time of open surgery or laparoscopy for gynecological symptoms. Frank intestinal symptoms are usually associated with intestinal obstruction[22]. While intestinal symptoms may occur during or be exacerbated by the menses, this association may not always be present. The symptoms coincide with menstruation in only 18%-40% of the cases in some series[23]. A recurring crampy lower or mid-abdominal pain is the most common presenting symptom for both enteric endometriosis and Crohn’s disease. Moreover, right-sided or right-lower-quadrant abdominal pain often precipitates laparotomy or laparoscopy, possibly for the suspicion of appendicitis. Other symptoms that may occur in ileal endometriosis as well as Crohn’s disease include diarrhea, constipation, nausea, vomiting, fever, anorexia, and weight loss. We initially considered ileal involvement by gastrointestinal endometriosis because of a history of the relationship between menstruation and presenting symptoms, and also previous surgery for misdiagnosis of enteric endometriosis in our patient.
Small bowel endometriosis is capable of producing both acute and chronic symptoms that can present a diagnostic and therapeutic challenge to the surgeon. Imaging studies may not provide an accurate diagnosis. The radiologic appearances of ileal endometriosis may be so similar to ileal Crohn’s disease that a differential diagnosis is not possible. Plain abdominal X-ray or barium enema may be helpful. Contrast studies may demonstrate extrinsic bowel compression, abdominal mass, and bowel stenosis or angulation[24]. Typically, it appears as a long filling defect with tapering margins. Non-invasive radiological diagnostic aids are provided by CT or ultrasonography. Abdominal ultrasonography demonstrates the size, shape and location of lesions, but unequivocal diagnosis is rarely possible. In our patient, ultrasonography demonstrated the presence of an inflammatory mass in the right lower quadrant, and also wall thickening and the absence of peristalsis in the small intestine, with a small amount of ascites. CT is useful for evaluating the small bowel with enteroclysis protocols. The findings of enteric endometriosis on CT include one or more focal masses on the bowel wall or a lesion compressing the colon. Abdominal CT is especially useful when looking for intra-abdominal complications of Crohn’s disease, such as abscesses, small bowel obstruction, or fistulas[25]. When thin-section abdominopelvic images with multidetector CT were obtained from our patient, there was a narrowing of the terminal ileum and bowel-wall thickening. It was not certain whether this was due to extrinsic compression or a primary intestinal lesion. Adjacent to the terminal ileum in the right lower quadrant, a complex, predominantly inflammatory mass was also detected on CT. Nevertheless, multiple stenotic ileal segments and small intestinal fistulas associated with Crohn’s disease were not seen on CT slices, possibly due to extensive adhesion formation and inflammation. Therefore, we were not able to exclude enteric endometriosis according to the presented radiological findings pre-operatively.
Crohn’s disease usually has a distinctive appearance operatively which is not to be confused with the findings in endometriosis, even if multiple site ileal endometriotic deposits are present[26]. Furthermore, in both diseases the surgical intention is limited resection of obstructing lesions, so there is no conflict of interest. An even more difficult, but more important operative distinction is malignant disease, and clearly this is relevant for cecal and ileal lesions, such as colonic or small bowel cancer. At operation, intestinal endometriosis appears as tumors having a glistening gray color on cross section[19]. In our patient, multiple stenotic ileal loops, which adhered firmly to each other, were wrapped as an inflammatory mass around the adjacent organs. The serosa overlying these areas was congested and hyperemic. Multiple ileal strictures and internal enteric fistulas were detected at laparotomy. Intraoperative findings were thought to indicate Crohn’s disease, hence a right hemicolectomy and partial distal ileum resection was the treatment of choice for obstructive Crohn’s ileitis.
Both small bowel endometriosis and Crohn’s disease are characterized grossly by patchy involvement of the small intestine with intervening, uninvolved skip areas of intestine. Moreover, both conditions may be transmural processes with marked chronic inflammatory changes resulting in the formation of strictures, adhesions, mucosal thickening, mural fibrosis, bowel angulation, stenosis, fibrosis and obstruction. Although transmural involvement by Crohn’s disease is the result of chronic insult, strictures and masses in endometriosis result largely from profound smooth muscle hypertrophy around endometriotic foci present in the muscularis propria[27]. Other manifestations of Crohn’s disease, such as perianal abscesses or fistulas, and inflammatory pseudotumors involving the cecum, terminal ileum and appendix, have been described in intestinal endometriosis[15]. In rare cases, endometriosis may cause deep fissures and even fistulous tracts, further mimicking regional enteritis. In the presented case, severe mural thickening of terminal ileum, with variable luminal stenosis or stricture formation and entero-enteric fistulas, were gross abnormalities. An approximately 40-cm segment of distal ileum, ileocecal valve and cecum had four strictures and three internal enteric fistulas, with associated serosal adhesions.
Endometriosis may cause many of the histological changes that are associated with Crohn’s disease. The endometriotic deposists consist of well-defined endometrial glands embedded in a dense cytogenic stroma[29]. Previous studies have observed chronic mucosal changes ranging from focally branching surface crypts, mildly hyperplastic surface epithelium overlying endometriotic foci, hemorrhagic mottling, ulceration, ulceration with inflammation, occasional neutrophils and eosinophils, or a non-specific inflammatory cell infiltrate, to focal glandular architectural abnormality with edema and siderophages[30]. Chronic inflammatory infiltrates composed of lymphocytes and plasma cells surround the deep crypts, and transmural lymphoid aggregates are also seen. Superimposed acute changes with crypt abscesses and surface ulceration may be noted. The changes are focal in most cases and are flanked by normal, unremarkable mucosa[27]. However, histopathological features used to reach the diagnosis of Crohn’s disease in the present case included both gross and microscopic features such as a discontinuous, asymmetric distribution of the lesions, patchy inflammation, ulceration, fissuring, transmural distribution of the inflammatory infiltrate and lymphoid aggregates.
To the best of our knowledge, there have been no other case reports of Crohn’s disease complicated by multiple ileal strictures and internal enteric fistulas clinically mimicking small bowel endometriosis. Involvement of the intestinal tract with endometriosis may mimic clinically and pathologically a wide spectrum of diseases including infectious etiology, ischemic enteritis/colitis, inflammatory bowel disease, and neoplasms. The differential diagnosis of Crohn’s disease with intestinal endometriosis may be difficult pre-operatively. The two entities share many overlapping clinical, radiological, and pathological features. Nevertheless, when it is difficult to identify the cause of intestinal obstruction in a woman of child-bearing age with cyclical symptoms suggestive of small bowel endometriosis, Crohn’s disease should be included in the differential diagnosis.