Published online Jan 7, 2008. doi: 10.3748/wjg.14.132
Revised: October 15, 2007
Published online: January 7, 2008
Oncogenesis of anaplastic carcinoma of the pancreas is a subject of controversy, because it shows sarcomatous nature with extremely poor prognosis. We herein report an unusual case of anaplastic carcinoma occurring with a recurrent mucinous cystic neoplasm in a 38-year-old female. A 10-cm retroperitoneal cystic mass was pointed out in the first pregnancy and a probable diagnosis of mucinous cystic neoplasm was made in October 2000. She refused surgery first and delivered her baby uneventfully. During her second pregnancy in 2002, however, she presented hematemesis and underwent urgent distal pancreatectomy, splenectomy and partial resection of the gastric wall where the tumor perforated. A diagnosis of borderline-type mucinous cystic neoplasm with ovarian-like stroma was made. Nine months later, CT visualized a recurrent cystic tumor near the pancreatic stump, which was subsequently resected. Pathology revealed that the tumor was composed of two different components of borderline-type mucinous cystic neoplasm and anaplastic carcinoma. The latter was intensely positive for vimentin, CD68, p53 and focally for cytokeratin, suggesting both sarcomatous and carcinomatous differentiation. She survived four years after the second surgery without tumor recurrence. Although the origin of anaplastic carcinoma has not been determined yet, it should be remembered that anaplastic carcinoma can occur in association with mucinous cystic neoplasm of more benign histology.
- Citation: Hakamada K, Miura T, Kimura A, Nara M, Toyoki Y, Narumi S, Sasak M. Anaplastic carcinoma associated with a mucinous cystic neoplasm of the pancreas during pregnancy: Report of a case and a review of the literature. World J Gastroenterol 2008; 14(1): 132-135
- URL: https://www.wjgnet.com/1007-9327/full/v14/i1/132.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.132
Anaplastic carcinomas of the pancreas are rare, representing only 0.5%-7% of all non-endocrine malignances in this organ[12]. The origin of the tumor is considered pancreatic ductal cells because they are accompanied with areas of adenocarcinomas in most cases[2]. However, they also contain mesenchymal components such as spindle-shaped cells and osteoclastoid giant cells, reminiscent of having sarcomatous differentiation. The prognosis of pancreatic anaplastic carcinoma is extremely poor. The combination of immature adenocarcinoma and sarcoma components may portend an adverse clinical course.
Mucinous cystic neoplasms of the pancreas, on the contrary, have an indolent clinical course and a much better prognosis than anaplastic carcinoma if respectable[3]. Mucinous cystic neoplasms occur predominantly in women and often have an ovarian-like stroma that contains spindle cells commonly observed in the ovary. The association of anaplastic carcinoma with mucinous cystic neoplasm has rarely been reported to date.
We report an unusual case of pancreatic anaplastic carcinoma coexistent with recurrent mucinous cystic neoplasm in a 38-year-old female, who previously underwent distal pancreatectomy for a borderline-type mucinous cystic neoplasm during her second pregnancy.
A 38-year-old female patient with no previous medical history was introduced to our hospital in October 2000, for further examination of a retroperitoneal cystic tumor that was disclosed incidentally by routine ultrasonography (US) in the first trimester of her first pregnancy. She was completely asymptomatic. Magnetic resonance imaging (MRI) and US showed a large multiloculated cystic tumor with a thick capsule at the tail of the pancreas. It measured 10 cm in diameter and displaced the stomach anteriorly. A probable diagnosis of a mucinous cystic neoplasm was made. We recommended her to receive resection of this potentially malignant tumor soon, but she decided to postpone operation until delivery. She delivered a healthy baby in July 2001. Computed tomography (CT) showed a multiloculated cystic tumor (Figure 1), which seemed similar in size of that at the initial diagnosis. She hesitated to receive surgery and was lost to medical follow-up.
In September 2002, she presented repetitive vomiting and hematemesis, when she noticed her second pregnancy. In November, She presented hematemesis and tarry stool again. MRI and US showed marked enlargement of the cystic tumor. Because of progressive anemia, she underwent urgent operation in the second trimester. At laparotomy, a dense adhesion between the tumor and posterior wall of the stomach was noted, so that an en-block resection was accomplished by distal pancreatectomy, splenectomy and a partial resection of the stomach. The postoperative course was uneventful and she delivered her second baby in April 2003.
In August 2003, a routine medical checkup by US disclosed a 3 cm tumor near the pancreatic stump. The tumor showed both cystic and solid appearances with capsule enhancement. The second operation was performed under the diagnosis of local recurrence of the mucinous cystic neoplasm. At laparotomy, the tumor was located between the pancreatic stump, posterior wall of the stomach and the left adrenal, which was removed en-block by additional partial pancreatectomy, partial gastrectomy, and left adrenalectomy.
The patient was still alive and well four years after the resection. CT revealed no local and distant tumor recurrences at the latest follow-up.
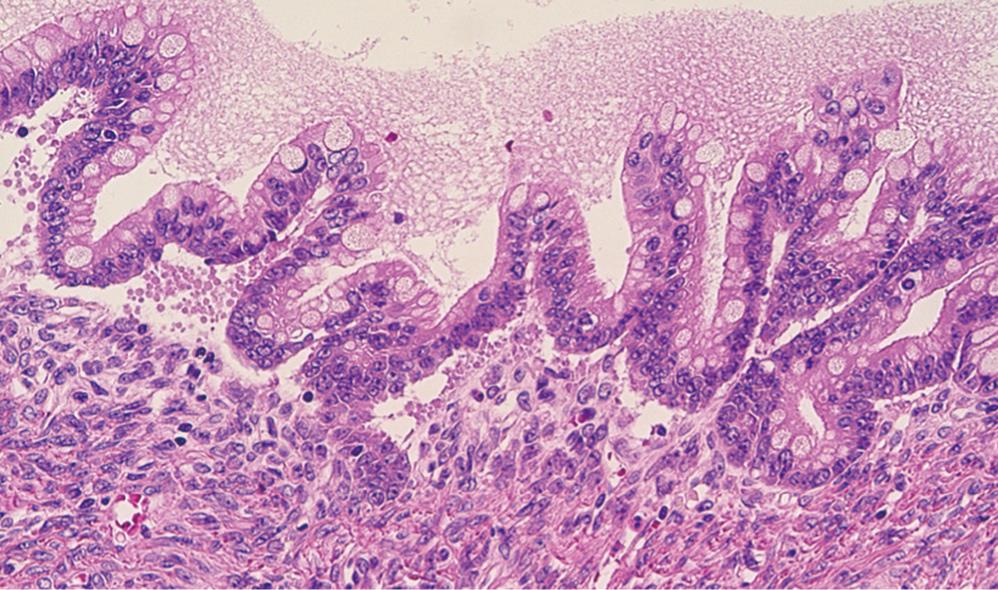
The tail of the pancreas resected at the first operation contained a multiloculated cystic tumor, measuring 14 cm × 14 cm, filled with about one liter of straw-yellow mucinous fluid. The cyst was uniformly lined with smooth inner surface with a thickened capsule of 5-10 mm width. At the cut surface of the maximal dimension, ten blocks were sampled for microscopic examination. The histological diagnosis was a mucinous cystic neoplasm, borderline type (Figure 2). The tumor had an ovarian-like stroma which was immunohistochemically positive for a progesterone receptor. None of the lymph nodes was involved, nor was the pancreatic cut margin. At the site of perforation to the stomach, no neoplastic change was identified in the gastric wall.
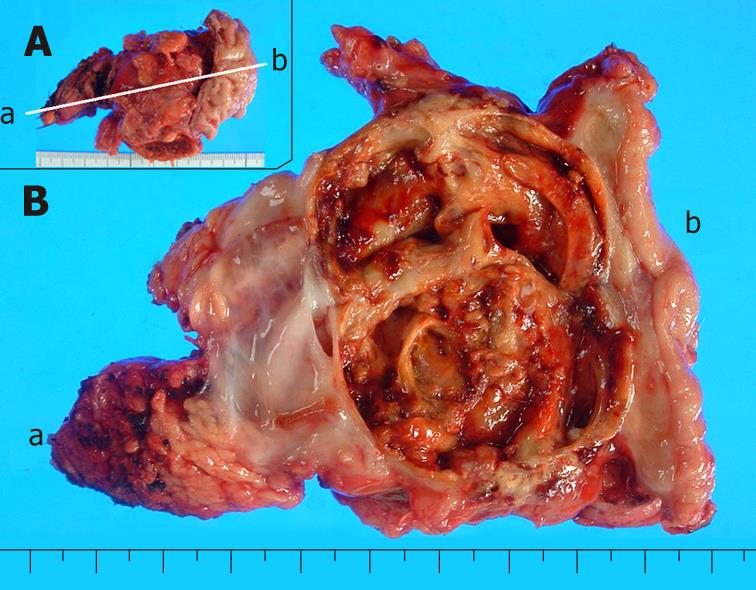
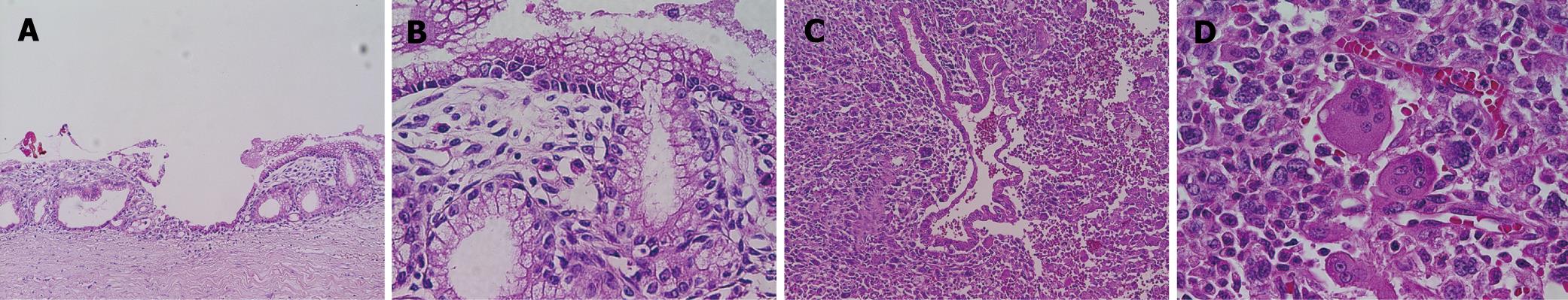
The recurrent tumor was entirely cystic and contained brown, dirty mucus. The tumor was located between the pancreatic stump and posterior wall of the stomach. Macroscopically, it was composed of two different parts. At the pancreatic side, the inner surface was white and smooth, resembling that of the previously resected primary tumor, while at the gastric side, it showed a tan-brown, irregular one. There was no transition area between the two parts (Figure 3). Pathology of the part near the pancreatic stump was a borderline-type mucinous cystic neoplasm. The part at the gastric side, on the other hand, contained an anaplastic carcinoma with osteoclastoid giant cells, atypical spindle-shaped cells and round cells, showing sarcomatous changes (Figure 4). The immunohistochemical the features of anaplastic carcinoma are summarized in Table 1. The recurrent tumor was also positive for progesterone receptor. All components of the anaplastic carcinoma were intensely positive for vimentin. Leukocyte common antigen and CD68 were positive in spindle-shaped cells and giant cells, while cytokeratin was positive in cytoplasm of the obviously epithelial component and focally in some of the spindle-shaped cells. P53 was also positive throughout of anaplastic carcinoma.
| Adenocarcinoma | Spindle cell | Giant cell | |
| Vimentin | + | + | + |
| Leukocyte common antigen | - | + focal | + focal |
| CD68 | - | + | + |
| Cytokeratin | + | + focal | - |
| P53 | + | + | + |
Anaplastic carcinomas associated with mucinous cystic neoplasms are very uncommon. Only several cases have been reported to date (Table 2)[3–12]. Some underwent resection of the cystic tumor at the onset, while others were treated with cystenterostomy first. Because of co-existent adenocarcinomas in the areas of anaplastic cancer, most of the tumors were thought to be of epithelial origin. However, the pathogenesis of these neoplasms, particularly those with epithelial and mesenchymal components, remains a subject of controversy.
| No | Author (reference) | Case (age/sex) | Diagnosis | Anaplastic carcinoma | Outcome (survival time) | |||||||
| Tumorlocation | Size (cm) | Spindle cell | Giant cell | Adeno-carcinoma | Invasion | lymphatic involvement | Distant metastasis | |||||
| 1 | Compagno J[3] | 32/F | Anaplastic carcinoma derived from MCN | Body | ND | ND | ND | + | ND | ND | - | Dead (3 yr) |
| 2 | Gracia Rego JA[4] | 45/F | Mucinous cystadenocarcinoma with pseudosarcomatous mural nodules | Body-tail | 11 | + | ND | + | Capsule | ND | - | Dead (16 mo) |
| 3 | Hartz PH[5] | 56/F | Cystadenocarcinoma with anaplasia | Tail | 6 | + | + | ND | Capsule, peripan-creatic nerve | ND | Liver, peri-toneum | Dead (autopsy) |
| 4 | Lane RB[6] | 25/F | Anaplastic carcinoma with MCN | Tail, liver, lymph node | 15 | + | - | + | ND | + | Liver | ND |
| 5 | Logan SE[7] | 23/F | Pleomorhic adenocarcinoma originated from MCN | Body-tail | 17 | + | ND | + | Stomach | + | Liver | Dead (1 mo) |
| 6 | Marinho A[8] | 70/F | Mucinous cystadenocarcinoma with a mural nodule of anaplastic carcinoma | Body-tail | 4.5 | ND | ND | + | Capsule | ND | - | ND |
| 7 | Sommers SC[9] | 48/M | Cystadenocarcinoma with foci of pleomorphic carcinoma | ND | ND | ND | + | + | ND | + | - | Dead (6 mo) |
| 8 | Tsujimura T[11] | 43/F | Malignant histiocytoma with mucinous cystadenoma | Tail | 16 | + | + | ND | ND | ND | - | ND |
| 9 | 1WenigBM[12] | 67/M | MCN with sarcoma stroma | Tail | 19 | + | ND | + | ND | ND | ND | Dead (15 mo) |
| 10 | 1Wenig BM[12] | 48/F | MCN with sarcoma stroma | Tail | ND | + | ND | + | ND | ND | ND | Alive (12 mo) |
| 11 | 1Wenig BM[12] | 65/F | MCN with sarcoma stroma | Tail, peritoneum | 30 | + | ND | + | Vascular | ND | Omentum, pleura | Dead (9 mo) |
| 12 | Hakamada K | 39/F | Anaplastic carcinoma with recurrent MCN, borderline-type | Tail | 5 | + | + | + | - | - | - | Alive (4 yr) |
In our case, the entire anaplastic carcinoma was intensely immunohistochemical positive for vimentin, an intermediate fragment associated with mesenchymal cells. Areas of spindle-shaped cells and giant cells were also positive for leukocyte common antigen and CD60. These facts suggest the sarcomatous nature of the anaplastic carcinoma[12]. In addition to the positive vimentin, areas of the carcinoma were positive for cytokeratin. The co-expression of cytokeratin and vimentin in the cells with the same hisotological character seemed unusual, but it was reported that vimentin becomes positive when the tumor cells are in a proliferative state[1314]. Immunohistochemical study alone cannot determine the origin of anaplastic carcinoma in the mucinous cystic neoplasms. Van den Berg et al[11] analyzed genetically three cases of anaplastic carcinoma related to mucinous cystic neoplasms, and disclosed that the sarcomatous and carcinomatous components are different phenotypically but genetically uniclone.
It was reported that malignant fibrous histiocytoma coexists with mucinous cystic adenoma[10], suggesting that sarcomas can occur from a relatively benign epithelial histology of mucinous cystic neoplasms. Indeed, the origin of sarcomas and carcinomas in mucinous cystic neoplasm is still unknown, but the theory that both sarcomas and carcinomas are originated from primitive mesenchymal stem cells with totipotential properties in the mucinous cystic neoplasm, might be another acceptable hypothesis for the histogenesis of anaplastic carcinoma.
Another interesting feature of this case is that the tumor experienced pregnancy twice. To the best of our knowledge, there are no reports on the relation of the progression of mucinous cystic neoplasm with pregnancy. Ovarian-like stroma exists exclusively in female patients with mucinous cystic neoplasms, which often are immunohistologically positive for progesterone and/or estrogen receptors. In our case, both the primary and recurrent tumors were positive for progesterone receptors. It is unknown whether physiological changes in blood concentration of these hormones promote the progression of the tumor. In our patient, the tumor size was stable during the first pregnancy, but rapidly enlarged in the second trimeseter of the second pregnancy. Increased mucious production might induce the enlargement of the tumor and subsequent rupture.
Our patient was still alive without any sign of recurrence four years after the second operation. However, close monitoring on tumor recurrence should be required because anaplastic carcinoma reportedly has a dismal prognosis.
| 1. | Matsuno S, Egawa S, Fukuyama S, Motoi F, Sunamura M, Isaji S, Imaizumi T, Okada S, Kato H, Suda K. Pancreatic Cancer Registry in Japan: 20 years of experience. Pancreas. 2004;28:219-230. |
| 2. | Rosai J. Pancreas and Periampullary region. Ackerman's Surgical Pathology. Philadelphia: Mosby-Year Book 1996; 981-982. |
| 3. | Compagno J, Oertel JE. Mucinous cystic neoplasms of the pancreas with overt and latent malignancy (cystadenocarcinoma and cystadenoma). A clinicopathologic study of 41 cases. Am J Clin Pathol. 1978;69:573-580. |
| 4. | Garcia Rego JA, Valbuena Ruvira L, Alvarez Garcia A, Santiago Freijanes MP, Suarez Penaranda JM, Rois Soto JM. Pancreatic mucinous cystadenocarcinoma with pseudosarcomatous mural nodules. A report of a case with immunohistochemical study. Cancer. 1991;67:494-498. |
| 5. | Hartz P, van den Sar A. Cancerous cyst of the tail of the pancreas simulating carcinosarcoma. Am J Clin Pathol. 1946;16:219-224. |
| 6. | Lane RB Jr, Sangueza OP. Anaplastic carcinoma occurring in association with a mucinous cystic neoplasm of the pancreas. Arch Pathol Lab Med. 1997;121:533-535. |
| 7. | Logan SE, Voet RL, Tompkins RK. The malignant potential of mucinous cysts of the pancreas. West J Med. 1982;136:157-162. |
| 8. | Marinho A, Nogueira R, Schmitt F, Sobrinho-Simoes M. Pancreatic mucinous cystadenocarcinoma with a mural nodule of anaplastic carcinoma. Histopathology. 1995;26:284-287. |
| 9. | Sommers SC, Meissner WA. Unusual carcinomas of the pancreas. AMA Arch Pathol. 1954;58:101-111. |
| 10. | Tsujimura T, Kawano K, Taniguchi M, Yoshikawa K, Tsukaguchi I. Malignant fibrous histiocytoma coexistent with mucinous cystadenoma of the pancreas. Cancer. 1992;70:2792-2796. |
| 11. | van den Berg W, Tascilar M, Offerhaus GJ, Albores-Saavedra J, Wenig BM, Hruban RH, Gabrielson E. Pancreatic mucinous cystic neoplasms with sarcomatous stroma: molecular evidence for monoclonal origin with subsequent divergence of the epithelial and sarcomatous components. Mod Pathol. 2000;13:86-91. |
| 12. | Wenig BM, Albores-Saavedra J, Buetow PC, Heffess CS. Pancreatic mucinous cystic neoplasm with sarcomatous stroma: a report of three cases. Am J Surg Pathol. 1997;21:70-80. |
| 13. | Connell ND, Rheinwald JG. Regulation of the cytoskeleton in mesothelial cells: reversible loss of keratin and increase in vimentin during rapid growth in culture. Cell. 1983;34:245-253. |
| 14. | Van Muijen GN, Ruiter DJ, Warnaar SO. Coexpression of intermediate filament polypeptides in human fetal and adult tissues. Lab Invest. 1987;57:359-369. |