Published online Jan 7, 2008. doi: 10.3748/wjg.14.114
Revised: September 19, 2007
Published online: January 7, 2008
AIM: To investigate the therapeutic effects of the combined use of rosiglitazone and aminosalicylate on mild or moderately active ulcerative colitis (UC).
METHODS: According to the national guideline for diagnosis and treatment of inflammatory bowel disease (IBD) in China, patients with mild or moderately active UC in our hospital were selected from July to November, 2004. Patients with infectious colitis, amoebiasis, or cardiac, renal or hepatic failure and those who had received corticosteroid or immunosuppressant treatment within the last month were excluded. Following a quasi-randomization principle, patients were allocated alternatively into the treatment group (TG) with rosiglitazone 4 mg/d plus 5-ASA 2 g/d daily or the control group (CG) with 5-ASA 2 g/d alone, respectively, for 4 wk. Clinical changes were evaluated by Mayo scoring system and histological changes by Truelove-Richards’ grading system at initial and final point of treatment.
RESULTS: Forty-two patients completed the trial, 21 each in TG and CG. The Mayo scores in TG at initial and final points were 5.87 (range: 4.29-7.43) and 1.86 (range: 1.03-2.69) and those in CG were 6.05 (range: 4.97-7.13) and 2.57 (range: 1.92-3.22) respectively. The decrements of Mayo scores were 4.01 in TG and 3.48 in CG, with a remission rate of 71.4% in TG and 57.1% in CG, respectively. Along with the improvement of disease activity index (DAI), the histological grade improvement was more significant in TG than in CG (P < 0.05).
CONCLUSION: Combined treatment with rosiglitazone and 5-ASA achieved better therapeutic effect than 5-ASA alone without any side effects. Rosiglitazone can alleviate colonic inflammation which hopefully becomes a novel agent for UC treatment.
- Citation: Liang HL, Ouyang Q. A clinical trial of combined use of rosiglitazone and 5-aminosalicylate for ulcerative colitis. World J Gastroenterol 2008; 14(1): 114-119
- URL: https://www.wjgnet.com/1007-9327/full/v14/i1/114.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.114
Ulcerative colitis (UC) is chronic intestinal inflammation with uneven relapsing course. Most patients need to take daily medications lifelong. The conventional therapies for UC include 5-aminosalicylate (5-ASA), glucocorticoid, and immunomodulator. 5-ASA and its derivatives were widely used to induce and maintain remission in patients with UC. But the general response rate was only 70%-80% and the relapse rate varied depending on the maintenance of the medications and other relapsing factors. It is necessary to search for special target of inflammatory cascades in UC so as to improve the therapeutic effects. With deeper understanding of its pathogenesis, more and more therapeutic targets have been found, and more and more novel clinical trials are directed to these targets with encouraging results. Peroxisome proliferators-activated receptor γ (PPAR-γ), highly expressed in the colon, is a subgroup of ligand-activated nuclear receptors responsible for the regulation of cellular events ranging from lipid homeostasis to cell differentiation and apoptosis. Recent studies showed its ligands can suppress the inflammatory response by inhibiting the activity of macrophages, cytokines production and NF-κB transcriptional activities. Thiazolidinediones (TZDs), such as rosiglitazon, are PPAR-γ synthetic ligands which have been used in type 2 diabetes mellitus for a long time. James et al[1] reported that refractory UC patients achieved clinical and endoscopic remissions using rosiglitazone, and concluded that it might represent a novel approach of UC therapy. However, it is an open-label trial with a small sample size and needs to be validated by expanded trials.
In this study we observed the therapeutic effects of combined use of rosiglitazone and 5-ASA in UC clinically and improvement pathologically.
Patients with mild or moderately active UC, who met with the criteria of suggested guidelines for the diagnosis and treatment of inflammatory bowel disease (IBD), were enrolled[2]. Patients with infectious colitis, acute or chronic cardiac, renal and hepatic failure and abnormal liver-associated chemistries were excluded. Patients who took glucocorticoid or immunomodulators, such as AZA, 6-MP, within the last month were also excluded. Included patients all provided informed consent. During the trial, the patients were not allowed to take traditional Chinese medicines (TCM), glucocorticoid and immunomodulator.
The patients were assigned into treatment group (TG) or control group (CG), with even number allocated into TG and odd number into CG. Both groups took 5-ASA 2 g/d (500 mg granule, Beaufour Ipsen Pharmaceutical Co. Ltd), but rosiglitazone 4 mg/d (4 mg/tablet, GlaxoSmithKline Pharmaceutical Co. Ltd.) was added to the treatment group for 4 wk.
The patients were assessed from 0 to the 4th wk and interviewed by phone every week and returned for a visit at the 2nd wk, and received the colonoscopic examination and colonic mucosal sampling at initial and final points. The evaluation of disease activity was performed at 0 and the 4th wk according to the Mayo indices[3] (also called Sutherland index, Table 1).
| Stool frequency | |
| 0 | Normal number of stools for this patient |
| 1 | 1 to 2 more stools than normal |
| 2 | 3 to 4 more stools than normal |
| 3 | 5 or more stools than normal |
| Rectal bleeding | |
| 0 | No blood seen |
| 1 | Streaks of blood with stool less than half the time |
| 2 | Obvious blood with stool most of the time |
| 3 | Blood alone passed |
| Findings of endoscope | |
| 0 | Normal or inactive disease |
| 1 | Mild disease (erythema, decreased vascular pattern, mild friability) |
| 2 | Moderate disease (marked erythema, absent vascular pattern, friability, erosions) |
| 3 | Severe disease (spontaneous bleeding, ulceration) |
| Physician’s global assessment | |
| 0 | Normal |
| 1 | Mild disease |
| 2 | Moderate disease |
| 3 | Severe disease |
Patients with a final DAI score of ≤ 2 were defined to achieve clinical remission and patients with a final score ≥ 3 but reduction in the DAI of ≥ 2 were defined to achieve partial remission.
All patients were included in the safety analysis. The adverse events and concomitant medication were carefully documented. Safety evaluations included vital signs, patients’ symptoms, physical examination, hematology, serum biochemistry, fecal routine, and urinalysis. All the patients received these tests at the initial and the final points of the study.
At least 2 colon samples were taken from each patient at initial and final points of the trial through colonoscopy. Tissues were fixed in 10% buffered neutral formalin and embedded in paraffin. Four &mgr;m sections were stained with the hematoxylin and eosin (HE stain) for histological evaluation. Tissue slides were blindly assessed by experienced pathologists based on the Truelove-Richards histological grading system[4] as follows: 0 = no polymorphs, 1 = small number of polymorphs in the lamina propria with minimal infiltration of crypts, 2 = prominent polymorphs in the lamina propria with infiltration of > 50% of crypts, 3 = florid polymorph infiltrate with crypt abscesses, and 4 = florid acute inflammation with ulceration.
Labeled streptavidin-biotin (LsAB) methods were used for PPAR-γ and NF-κB p65 detection. Paraffin-embedded colonic tissue samples were dewaxed in xylene for 5 min twice, rehydrated in a series of ethanol (100%-70%) for 3 min each followed by rehydration in PBS for 30 min. After rehydration, the endogenous peroxidase was blocked with 0.3% hydrogen peroxide followed by antigen retrieval by autoclaving sections in citrate buffer pH 6.0 (10 mmol/L Na citrate). After antigen retrieval, the sections were stained using the above-mentioned kit according to manufacturer’s recommendations, but with the following modifications. Sections were incubated with the primary antibody at 37°C for 2 h. The following antibodies were used at the indicated dilutions: PPAR-γ (E-8: sc-7273, Santa Cruz, Santa Cruz, CA USA) 1:100 and NF-κB p65 (Boshide Bio Corp, Wuhan, China) 1:200. Each section had its own control using the secondary antibody only. Preimmune serum was initially used to ensure specificity of the signal with each of the antibodies. The lipoma resection sample was used as the positive control and the substitute monoclonal antibody of PPAR-γ with PBS as the blank control.
Statistical analysis was performed with the SPSS 10.0 software. The t test of the sample mean value of the designed group was used to examine the Mayo scores and positive cell numbers in the immunohistochemistry. The rank sum test was adopted for the histological grading. A P value < 0.05 was considered statistically significant.
We enrolled 42 patients with mild and moderately active UC into our trial and 21 patients each in TG and CG. The pre-study characteristics of patients are summarized in Table 2. The age ranged from 16 to 57 years (average, 38.8 ± 10.4 years) in TG and from 14 to 60 years (average, 37.1 ± 8.01 years) in CG, respectively. The extent of disease in TG was 8 proctitis (38.1%), 12 proctosigmoiditis (57.1%), and 1 left-side colitis (4.8%) and 9 (42.9%), 11 (52.4%), 1 (4.7%) in CG respectively. All patients received 5-ASA agents before enrolled into this study. The statistical analysis on the age, sex, location, type, and disease activity suggested similar baseline characteristics in the two groups (P > 0.05).
| TG (n = 21) | CG (n = 21) | ||
| Age (yr) | 38.8 ± 10.4 | 37.1 ± 8.01 | |
| Sex | Male (%) | 16 (76.2) | 15 (71.4) |
| Female (%) | 5 (23.8) | 6 (28.6) | |
| Location | Rectum (%) | 8 (38.1) | 9 (42.9) |
| Rectosigmoid (%) | 12 (57.1) | 11 (52.4) | |
| Left colon (%) | 1 (4.8) | 1 (4.7) | |
| Type | Chronic relapse (%) | 13 (61.9) | 12 (57.1) |
| Chronic persistence (%) | 8 (38.1) | 9 (42.9) | |
| Severity | Mild (%) | 5 (23.8) | 6 (28.6) |
| Moderate (%) | 16 (76.2) | 15 (71.4) |
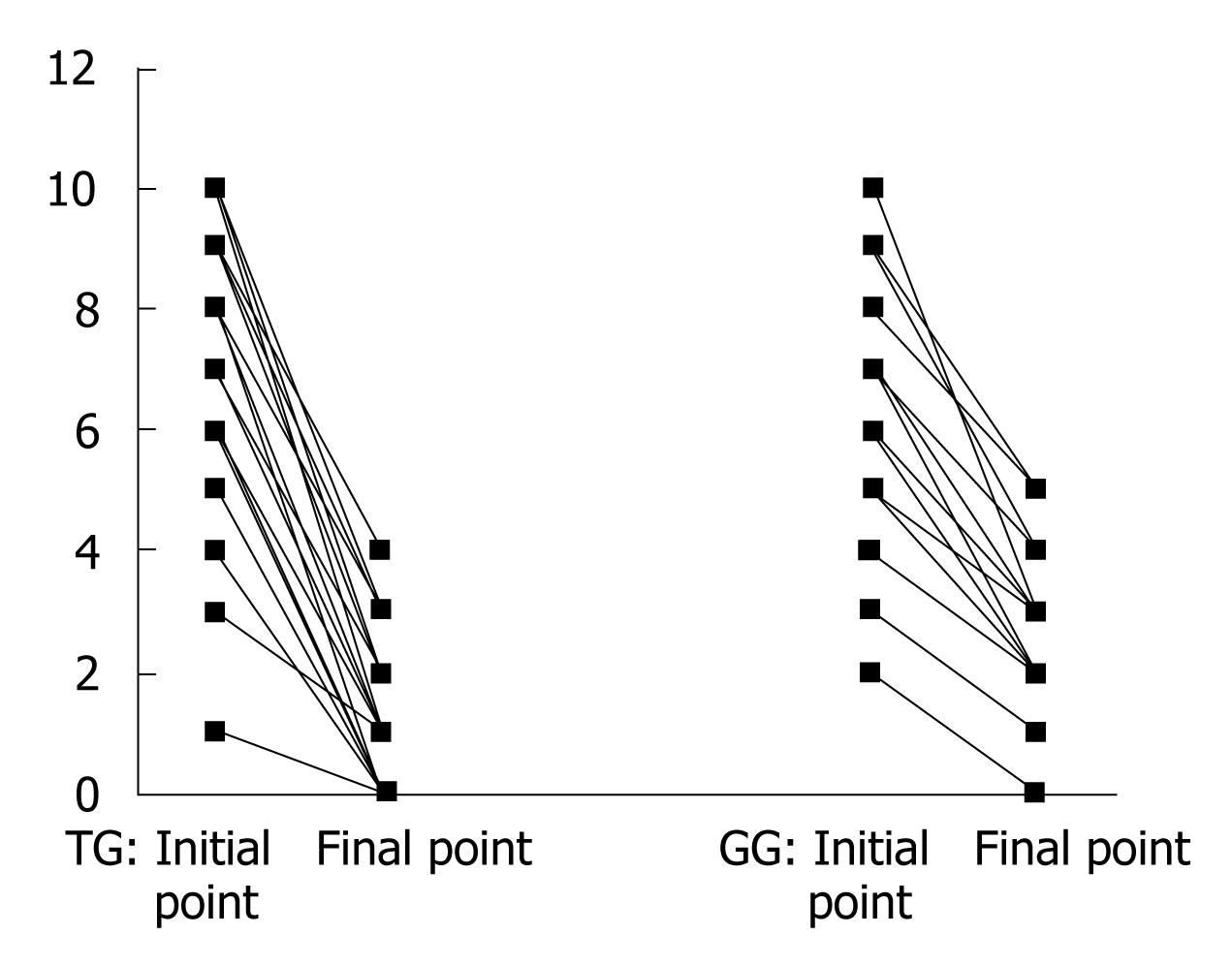
The Mayo score changes in each patient from 0 to the end of 4 wk were figured as below (Figure 1): the DAI in TG at initial and final points were 5.87 (range, 4.29-7.43) and 1.86 (range, 1.03-2.69), respectively, and those in CG were 6.05 (range, 4.97-7.13) and 2.57 (range, 1.92-3.22) respectively, (Table 3). There was a significant difference between the two groups and the therapeutic effect of TG was better than CG (P < 0.05).
| Mayo scores | Initial point | Final point |
| TG (n = 21)a | 5.87 (4.29-7.43) | 1.86 (1.03-2.69) |
| CG (n = 21) | 6.05 (4.97-7.13) | 2.57 (1.92-3.22) |
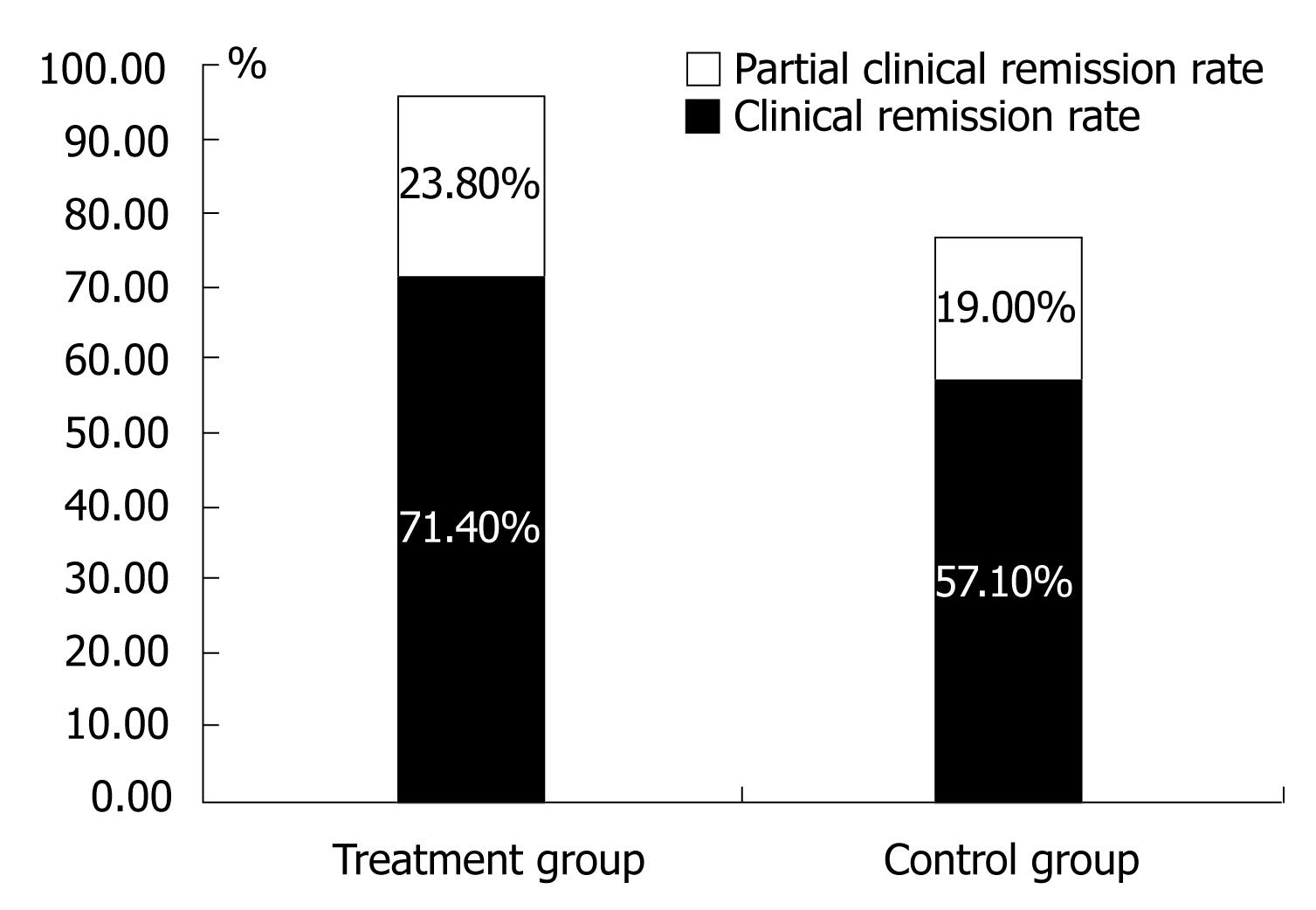
According to the definition of outcome from the guideline, the clinical remission was achieved in 15 patients in TG (remission rate, 71.4%) and 12 patients in CG (remission rate, 57.1%), the partial remission was achieved in 5 patients (23.8%) and 4 patients (19.0%) in TG and CG respectively (Figure 2).
According to Truelove and Richards’s histological grading system, the histological sections by H&E staining were graded in Table 4. The grade II and III of histology was found at the initial point (80.95%) and grade I (57.14%) at the final points in TG, respectively, as compared with 33% grade I at the final point in CG.
The positive percentage of PPAR-γ at initial and final points were 26.20%, 47.76% in TG and 24.90%, 39.24% in CG, respectively. The positive percentage of NF-κB were 58.09%, 21.19% and 58.00%, 27.00%, accordingly (Table 5). The relation between histological grade and immunohistochemistrical positive percentages was showed on Table 6.
| Expression positivity (100%) | |||||
| I | II | III | IV | ||
| Initial points | n | 4 | 10 | 7 | 0 |
| NF-κB | 46.09-51.91 | 52.11-56.89 | 61.35-70.65 | 0 | |
| PPAR-γ | 31.57-39.93 | 24.54-29.06 | 16.30-20.55 | 0 | |
| Final points | n | 12 | 6 | 3 | 0 |
| NF-κB | 21.55-26.62 | 18.93-20.07 | 8.42-20.92 | 0 | |
| PPAR-γ | 37.20-44.97 | 49.82-56.51 | 55.50-69.84 | 0 | |
During the observation period, no adverse events and abnormalities in the blood biochemical test were found in the patients. No jaundice or edema was found either.
As it is known, except for open-label trial by James et al[1], there are few clinical trials about rosiglitazone as adjunctive agent for UC conventional therapy. In this trail, treatment group showed a greater decrease in DAI and higher clinical remission rate than control group. Our results showed similar effects as those of James and his colleagues and the adjunctive effect of rosiglitazone on the UC therapy. However, we investigated the effects of rosiglitazone in mild and moderately active UC, but James did in refractory UC.
The PPAR-γ, highly expressed on adipo-tissue and colons, can regulate gene transcription of lipoprotein, cell differentiation, inflammation and immune response. PPAR-γ plays a key role not only in adipocyte differentiation, insulin sensitivity and nonalcoholic fatty liver[6–10], but also in innate and adaptive immunity[11–13]. Activation of PPAR-γ can promote macrophage desensitization, thus attenuating the oxidative burst[14]. Arnold and Konig[15] observed that PPAR γ ligands inhibited dose-dependently the release of TNF-α, GM-CSF, IL-1α, IL-6, IL-8 and CCL5 from RSV-infected A549 cells while diminishing the cellular amount of mRNA of IL-6, IL-8 and CCL5 and binding activity of the transcription factors NF-κB and AP-1, respectively. Belvisi et al[16] also outlined the anti-inflammatory effects of PPAR-γ ligands. Pan et al[17] revealed this anti-inflammation effect on human gallbladder epithelial cells as well. PPAR-γ ligands can prevent intestinal inflammation by blocking the activation of NF-κB, down-regulate the production of ICAM-1 and TNF-α in intestinal epitheliums, suppress expressions of TNF-α and IL-1β, etc.
Dubuquoy et al[5] found that expression of PPAR-γ in colon mucosa was decreased in active UC and was negatively related with UC severity. PPAR-γ was regarded as a new therapeutic target in IBD, especially in UC[18]. Christel et al[19] even found that the intestinal anti-inflammatory effect of 5-ASA depends on the PPAR-γ in chemically induced colitis in mice heterozygous at the PPAR-γ locus. These results strongly suggested a reasonable combination of these two agents for the therapeutic purpose on UC.
There are two kinds of PPAR-γ ligands, one is natural ligands such as prostaglandins J2, the other is synthetic ligands including TZDs. TZDs (e.g. rosiglitazone and pioglitazone) can reduce the productions of several pro-inflammatory cytokines and ameliorate the intestinal inflammation. Adachi M et al[20] reported that PPAR-γ in colonic epithelial cells plays an anti-inflammatory role and protects against experimental IBD. Marina Sánchez-Hidalgo et al[21] draws a similar conclusion. Sasaki et al[22] reported that troglitazone significantly reduced the TNF-α mediated induction of endothelial MAdCAM-1 in a dose-dependent manner; it also lowered the VCAM-1, ICAM-1 and E-selectin expression and significantly reduced α4β7-integrin dependent lymphocyte adhesion. Bassaganya et al[23] found that activation of PPAR-γ can inhibit the activation of NF-κB and ameliorate experimental colitis. NF-κB controls the transcription of a large cohort of genes, its dysregulated activation is linked to various biological disorders including inflammatory, and immune disorders[24]. In IBD, NF-κB plays an important role and is a target of various anti-inflammatory drugs. The ability of TZDs to ameliorate the experimental colitis is closely related to inhibition of NF-κB activation[19]. Another unpublished study of ours also investigated colonic mucosal expressions of PPAR-γ and NF-κB in the oxalzolone-induced experimental colitis, and found that the PPAR-γ ligand could increase the expression of PPAR-γ and inhibit the activation of NF-κB in colonic epithelium, ameliorating the colon inflammation in experimental colitis. Lawrence et al[25] showed that PPAR-γ ligands could provide anti-inflammatory protection by maintaining the cytokine balance and shifting transcriptional regulation of T cells away from Th1 and towards Th2 predominance in acute DSS colitis. Lytle et al[26] found that rosiglitazone could slow down the onset of spontaneous IBD in IL-10 (-/-) mice. Sánchez-Hidalgo et al[27] also reported that rosiglitazone for TNBS colitis can correct mucosal lesions, and significantly lower the ulceration index, myeloperoxidase (MPO), and the levels of TNF-α. Meanwhile, it increased prostaglandin (PG) E2 production and returned PG D2 to basal levels and reduced COX-2 and NF-κB proteins expression. Pedersen et al[28] reported that PPAR-γ mRNA and adipophilin expressions (a marker of PPAR activation) were markedly lower in colonic epithelium cells (CEC) from the patients with active UC and were clearly associated with the inhibition of PPAR-γ function and the stimulation with rosiglitazone fully restored PPAR-γ activation in CEC. Zhang YQ et al[29] reported that rosiglitazone enhances apoptosis of HT-29 cells by activating PPAR-γ. Li et al[30] reported troglitazone can not inhibit cell proliferation, and induce apoptosis in HepG2 cells, but down-regulate the expression of COX-2 mRNA and protein.
Our study showed the therapeutic effects of rosiglitazone on human UC, but it is required to demonstrate whether the therapeutic effects of rosiglitazone or other PPAR-γ ligands depend on PPAR-γ NF-κB signal pathway or on other inflammatory pathway, such as P38 MAPK, or toll-like receptor. A larger sample size clinical trail is also needed to confirm the effects of rosiglitazone on human UC.
Rosiglitazone has few adverse effects except for an ALT increase and exacerbation of cardiac failure induced by the body fluid retention. In our study, we excluded the patients with hepatic, cardiac failure and closely observed related clinical and chemical changes. No adverse events were found in our study, including 4 patients who took rosiglitazone for 12 wk. Based on the above observation, we conclude that TZDs, ligands of PPAR-γ can be used in the UC as an adjunctive agent efficiently and safely.
The peroxisome proliferators activated receptor γ (PPAR-γ) is highly expressed in the colon and play a crucial role in intestinal inflammation. Regulation of colon inflammation by PPAR-γ has been well demonstrated in experimental colitis. Recently, rosiglitazone achieved quite well therapeutic effects in refractory ulcerative colitis (UC) patients in the study of James D. This result suggested that PPAR-γ is a hopefully novel target for UC treatment in the future.
The hotspots in this field of studies include mechanism of PPAR-γ in intestinal inflammation and finding its high-affinity ligands.
An open-label trial of rosiglitazone for UC by James D et al was a first research of clinical application of thiazolidinediones (TZDs); however, their study merely focused on refractory UC. We mainly studied the patients with active UC and explored the mechanisms of PPAR-γ ligands.
PPAR-γ has multiple functions in the immune system and in some major inflammatory diseases such as atherosclerosis, inflammatory bowel disease and rheumatoid arthritis. If the ligands of PPAR-γ can be utilized for UC treatment, it will provide another therapeutic target and improve the effects of UC treatment.
PPAR-γ is a member of nuclear receptors family, proved to be a key transcription factor of adipocyte differentiation, lipid and glucose homeostasis and an important target in type 2 diabetes and metabolic syndrome. Besides its role in metabolic tissues, it appears to be expressed in several other cells, including immune cells such as macrophages, dendritic cells, eosinophils, T cells and B cells. It was pointed to a role in the immune system and its new aspect was revealed and developed in parallel: its potential anti-inflammatory activity. DAI is an abbreviation of disease activity index and widely used to evaluate the conditions and therapeutic effect for UC. There are several DAI systems; however, Mayo system is widely accepted.
The authors aim to investigate the therapeutic effects of rosiglitazone on mild or moderately active ulcerative colitis and explore the relation between PPAR-γ and intestinal inflammation and NF-κB. Their results revealed the ligand of PPAR-γ can alleviate the inflammation of UC and this effect may be related with depression of NF-κB by PPAR-γ activation. Rosiglitazone can alleviate colonic inflammation and hopefully become a novel agent for the treatment of UC. PPAR-γ may be another therapeutic target of UC in the future.
| 1. | Lewis JD, Lichtenstein GR, Stein RB, Deren JJ, Judge TA, Fogt F, Furth EE, Demissie EJ, Hurd LB, Su CG. An open-label trial of the PPAR-gamma ligand rosiglitazone for active ulcerative colitis. Am J Gastroenterol. 2001;96:3323-3328. |
| 2. | Ou-Yang Q, Pan GZ, Wen ZH, Wan XH, Hu RW, Lin SR, Hu PJ. Suggested guideline for the diagnosis and treatment of inflammatory bowel disease. Chin J Dig Dis. 2001;21:236-239. |
| 3. | Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625-1629. |
| 5. | Dubuquoy L, Jansson EA, Deeb S, Rakotobe S, Karoui M, Colombel JF, Auwerx J, Pettersson S, Desreumaux P. Impaired expression of peroxisome proliferator-activated receptor gamma in ulcerative colitis. Gastroenterology. 2003;124:1265-1276. |
| 6. | Spiegelman BM, Flier JS. Adipogenesis and obesity: rounding out the big picture. Cell. 1996;87:377-389. |
| 7. | Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597-609. |
| 8. | Vamecq J, Latruffe N. Medical significance of peroxisome proliferator-activated receptors. Lancet. 1999;354:141-148. |
| 10. | Dong H, Lu FE, Gao ZQ, Xu LJ, Wang KF, Zou X. Effects of emodin on treating murine nonalcoholic fatty liver induced by high caloric laboratory chaw. World J Gastroenterol. 2005;11:1339-1344. |
| 11. | Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002;2:748-759. |
| 12. | Meier CA, Chicheportiche R, Juge-Aubry CE, Dreyer MG, Dayer JM. Regulation of the interleukin-1 receptor antagonist in THP-1 cells by ligands of the peroxisome proliferator-activated receptor gamma. Cytokine. 2002;18:320-328. |
| 13. | Gosset P, Charbonnier AS, Delerive P, Fontaine J, Staels B, Pestel J, Tonnel AB, Trottein F. Peroxisome proliferator-activated receptor gamma activators affect the maturation of human monocyte-derived dendritic cells. Eur J Immunol. 2001;31:2857-2865. |
| 14. | Von Knethen AA, Brune B. Delayed activation of PPARgamma by LPS and IFN-gamma attenuates the oxidative burst in macrophages. FASEB J. 2001;15:535-544. |
| 15. | Arnold R, Konig W. Peroxisome-proliferator-activated receptor-gamma agonists inhibit the release of proinflammatory cytokines from RSV-infected epithelial cells. Virology. 2006;346:427-439. |
| 16. | Belvisi MG, Hele DJ, Birrell MA. Peroxisome proliferator-activated receptor gamma agonists as therapy for chronic airway inflammation. Eur J Pharmacol. 2006;533:101-109. |
| 17. | Pan GD, Wu H, Liu JW, Cheng NS, Xiong XZ, Li SF, Zhang GF, Yan LN. Effect of peroxisome proliferator-activated receptor-gamma ligand on inflammation of human gallbladder epithelial cells. World J Gastroenterol. 2005;11:6061-6065. |
| 18. | Dubuquoy L, Rousseaux C, Thuru X, Peyrin-Biroulet L, Romano O, Chavatte P, Chamaillard M, Desreumaux P. PPARgamma as a new therapeutic target in inflammatory bowel diseases. Gut. 2006;55:1341-1349. |
| 19. | Rousseaux C, Lefebvre B, Dubuquoy L, Lefebvre P, Romano O, Auwerx J, Metzger D, Wahli W, Desvergne B, Naccari GC. Intestinal antiinflammatory effect of 5-aminosalicylic acid is dependent on peroxisome proliferator-activated receptor-gamma. J Exp Med. 2005;201:1205-1215. |
| 20. | Adachi M, Kurotani R, Morimura K, Shah Y, Sanford M, Madison BB, Gumucio DL, Marin HE, Peters JM, Young HA. Peroxisome proliferator activated receptor gamma in colonic epithelial cells protects against experimental inflammatory bowel disease. Gut. 2006;55:1104-1113. |
| 21. | Sanchez-Hidalgo M, Martin AR, Villegas I, de la Lastra CA. Rosiglitazone, a PPARgamma ligand, modulates signal transduction pathways during the development of acute TNBS-induced colitis in rats. Eur J Pharmacol. 2007;562:247-258. |
| 22. | Sasaki M, Jordan P, Welbourne T, Minagar A, Joh T, Itoh M, Elrod JW, Alexander JS. Troglitazone, a PPAR-gamma activator prevents endothelial cell adhesion molecule expression and lymphocyte adhesion mediated by TNF-alpha. BMC Physiol. 2005;5:3. |
| 23. | Bassaganya-Riera J, Reynolds K, Martino-Catt S, Cui Y, Hennighausen L, Gonzalez F, Rohrer J, Benninghoff AU, Hontecillas R. Activation of PPAR gamma and delta by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology. 2004;127:777-791. |
| 24. | Burke JR. Targeting I kappa B kinase for the treatment of inflammatory and other disorders. Curr Opin Drug Discov Devel. 2003;6:720-728. |
| 25. | Saubermann LJ, Nakajima A, Wada K, Zhao S, Terauchi Y, Kadowaki T, Aburatani H, Matsuhashi N, Nagai R, Blumberg RS. Peroxisome proliferator-activated receptor gamma agonist ligands stimulate a Th2 cytokine response and prevent acute colitis. Inflamm Bowel Dis. 2002;8:330-339. |
| 26. | Lytle C, Tod TJ, Vo KT, Lee JW, Atkinson RD, Straus DS. The peroxisome proliferator-activated receptor gamma ligand rosiglitazone delays the onset of inflammatory bowel disease in mice with interleukin 10 deficiency. Inflamm Bowel Dis. 2005;11:231-243. |
| 27. | Sanchez-Hidalgo M, Martin AR, Villegas I, Alarcon De La Lastra C. Rosiglitazone, an agonist of peroxisome proliferator-activated receptor gamma, reduces chronic colonic inflammation in rats. Biochem Pharmacol. 2005;69:1733-1744. |
| 28. | Pedersen G, Matthiessen MW, Brynskov J. Selective downregulation of peroxisome proliferators-activated receptor expression and function in colonic epithelium of patients with ulcerative colitis. Gut. 2005;54 A:160. |
| 29. | Zhang YQ, Tang XQ, Sun L, Dong L, Qin Y, Liu HQ, Xia H, Cao JG. Rosiglitazone enhances fluorouracil-induced apoptosis of HT-29 cells by activating peroxisome proliferator-activated receptor gamma. World J Gastroenterol. 2007;13:1534-1540. |
| 30. | Li MY, Deng H, Zhao JM, Dai D, Tan XY. PPARgamma pathway activation results in apoptosis and COX-2 inhibition in HepG2 cells. World J Gastroenterol. 2003;9:1220-1226. |