Published online Jan 7, 2008. doi: 10.3748/wjg.14.108
Revised: September 26, 2007
Published online: January 7, 2008
AIM: To explore the expression of phosphatase and tensin homolog deleted on chromosome ten (PTEN) in liver of athymic mice with hepatocellular carcinoma (HCC) and the effect of Fuzheng Jiedu Decoction (FJD).
METHODS: Forty eight male BALB/c athymic mice models were built by Bel-7402 with an indirect method. After 24 h of postoperation, the 48 athymic mice were distributed randomly into 4 groups: A, B, C, D, each group had 12 athymic mice. Group A were treated by intragastric administration with FT207 (Tegafur) for 4 wk. Group B, C and D were treated by intragastric administration with FJD (complex prescription of Chinese crude drug) that had been delegated into 3 kinds of density as the low, middle, and high for 4 wk. At last, athymic mice were put to death, live time, volume of tumors, exponent of tumors and the tumor metastasis in livers were observed; and PTEN was detected in hepatic tissue, latero-cancer tissue and cancer tissue by immunohistochemistry.
RESULTS: Four weeks later, the total survival rate in treatment group (A + B + C) was 50% and higher than the control group (0%) treated by FT207, (P < 0.01). The survival rate in group A, B, C was higher than in group D, and except group A with D, there was significant differentces (Fisher’s Exact Test P = 0.05 or 0.01). And no differences were observed between the treatment groups and the control group in volume of tumors and exponent of tumors (P > 0.05). Tumor metastasis in livers of the treatment group was less than the controls (Fisher’s Exact Test, P = 0.021). The result of immunohistochemistry showed that the intensity of PTEN in latero-cancer tissue was the highest, and then the hepatic tissue, the lowest was cancer tissue (Kruskal-Wallis test, χ2 = 60.67, P = 0.000). It also showed that the intensity of PTEN in treatment groups (A, B, C) was higher than the control group (D) (F = 5.90, P = 0.002 in hepatic tissue and F = 15.99, P = 0.000 in latero-cancer tissue and χ2 = 26.08, P = 0.000 in cancer tissue), and group B is the highest in the treatment groups (P < 0.05, r = 0.01. respectively). However, there was no significant statistic difference between group A and group C (P > 0.05).
CONCLUSION: FJD can prolong the survival time and decrease tumor metastasis in livers of these experimental mice. Mechanisms of FJD healing HCC may partially be explained by enhancing the expression of PTEN in liver.
- Citation: Yin LR, Chen ZX, Zhang SJ, Sun BG, Liu YD, Huang HZ. Expression of phosphatase and tensin homolog deleted on chromosome ten in liver of athymic mice with hepatocellular carcinoma and the effect of Fuzheng Jiedu Decoction. World J Gastroenterol 2008; 14(1): 108-113
- URL: https://www.wjgnet.com/1007-9327/full/v14/i1/108.htm
- DOI: https://dx.doi.org/10.3748/wjg.14.108
Hepatocellular carcinoma (HCC) is one of the major cancer killers. Although surgical resection, liver transplantation and percutaneous ablation are considered as effective treatment for HCC[1–3], Traditional Chinese Medicine (TCM) has been widely used as combined therapies in treating the disease in China. According to the theory of TCM, the main mechanism of HCC is deficiency of vital qi and exuberant pathogens, thus strengthening body resistance and disintoxication is the major method of treating HCC[4]. The previous study demonstrated that reduced expression levels of PTEN are involved in the pathogenesis of HCC. Moreover, decreased phosphatase and tensin homolog deleted on chromosome ten (PTEN) expression was correlated with tumor progression and poor prognosis in patients with HCC[5–7], whether TCM can down-regulate the expression of PTEN in HCC is still unknown. The aim of our study is to explore the effect of Fuzheng Jiedu Decoction (FJD), complex prescription of Chinese crude drug in treating BALB/c athymic mice with HCC, as well as the expression of PTEN. We proclaim that the animal study was acknowledged by the Ethical Committee of The First Affiliated Hospital of Sun Yat-Sen University committee in the materials and methods section. The results are reported as following.
Forty eight BALB/c athymic male mice, 4-6 wk, were purchased from The Experimental Animal Center of Traditional Chinese Medicine university of Guangzhou. The animals were housed individually in cages and kept in a room maintained at a temperature of 23 with a relative humidity (RT) of 55 with a 12-h/12-h light/dark cycle. Solid rodent chow and tap water were given ad libitum.
FJD (Application: 200710026976.6) consists of the following indegredients: Ezhu (Rhizoma Curcumae) 15 g, Banzhilian (Herba Scutellariae Darbatae ) 30 g, etc. They were decocted routinely and then made into a final concentration of 2 g/mL which extracted and prepared by college of pharmacy of Traditional Chinese Medicine University of Guangzhou. The prepared herbal pieces were purchased from Guangzhou City Pharmacy Company and consistent with the requirement of Pharmacopoeia of the People's Republic of China[8]; FT207 parenteral solution (Tegafur parenteral solution), 5 mL/0.2 g per ramus, Shandongqilu production (production batch number 06020032); rabbit anti-human PTEN polyclonal antibody (production batch number 60182150), Power VisionTM Two-Step Histostaining Reagent (production batch number 125135), were bought from Beijing Zhongshan Jinqiao biotechnology limited company.
The athymic mice with indirect orthotopic transplantation tumor models were established in accordance with the method by Dr. Zheng Jianhua[9]. The animals were inoculated Bel-7402 hepatoma carcinoma cell with concentration of 1 million cells/mL on their waist and back, until subcutaneous transplantation tumor grew to diameter 1 cm, then cut down the tumor. Remove necrotic tissue in the tumor and cut into pieces about 1 mm3 in Hanks liquid. Anesthetize the athymic mice in abdominal cavity with Pentobarbital 45 mg/kg weight, transverse incise the left upper quadrant, expose liver, take 1 piece of tumor tissue, use bodkin pinhead (20° angle of slope, deep 3 mm) imbed the tumor tissue in deep part of hepatic lobes parenchyma of athymic mouse in ex vivo 40 min, compresse the incision to stop bleeding then close abdomen.
Athymic mice were raised in SPF condition in divided cages. After 24 h of postoperation, the 48 athymic mice were distributed randomly into 4 groups, every group has 12 mice. Low concentration group (A): drench the Ganaifang (0.25 g Chinese crude drug/mL, 0.2 mL/10 g weight) with the dose of 10 times human unit kilogram weight. Middle concentration group (B): drench the Ganaifang (0.5 g Chinese crude drug/mL, 0.2 mL/10 g weight) with the dose of 20 times human unit kilogram weight. High concentration group (C): drench the Ganaifang (1.0 g Chinese crude drug/mL, 0.2 mL/10 g weight) with the dose of 40 times human unit kilogram body weight. Chemotherapy group (D): drench the FT207 parenteral solution (Tegafur parenteral solution) (8 mg/mL, 0.2 mL/10 g weight) with the dose of 5 times human per day.
Record live time of each athymic mouse in the process of observation. When the treatment was ended, get blood from athymic mice eyeball, and put them to death, then dissect them, meanwhile record the tumor volume, tumor index number (the weight of the tumor/the weight of the mouse), fix the normal hepatic tissue, latero-cancer tissue and cancer tissue in 4% neutral formalin, sent to pathology laboratory to make paraffin imbedding microtome sections (4 mm thick serial sections) fixed in silicification glass.
Histologic detection: dying with the routine hematoxilin -eosine (HE).
Immunohistochemistry detection: 4 &mgr;m thick paraffin sections were baked at 65°C until get deparaffinage and hydration; incubate in 3% Hydrogen Dioxide about 5-10 min to inactivate the endogenous peroxidase; microwave repairs the antigen; sealed by 10% normal goat serum; PTFN multiclone rabbit antibody (1:100) was incubated overnight at 4°C, washed by PBS 2 min for 3 times; dropwise goat anti-rabbit IgG antibody-HRP multimer, incubated about 30 min at 37°C, washed by PBS, 2 min × 3 times; DAB coloration; washed thoroughly by distilled water. In the same time, PBS was used as first-antibody and second-antibody in negative control.
Evalution of coloration result: using Bresalier[10] semiquantitative formula to judge coloration result. Selecting randomly 10 fields of vision from every section when enlarged 200 times, then we classified the cell coloration intensity into four categories: Negative, cell hasn’t colored (0); Cell has colored buff (1); claybank (2); chocolatebrown (3). Count the number of field of vision of each intensity, and according to the formula calculate the average coloration intensity. IS (intensity score) = ∑[(0 × F0) + (1 × F1) + (2 × F2) + (3 × F3)], F = n/10 (n = 0, 1, 2, 3 the number of field of vision of different score). Two people read the section two times with double blind method, calculate IS for each time, and get the two times average as the result.
Using SPSS 14.0 statistic software, variance analysis and rank-sum test were used for measurement data; chi square test and rank-sum test were used for numeration data.
The dissection witness that achievement ratio of making model is 100%; at the end of the experiment, get 48 utility pathologic samples. Most tumors were enormous, some were accompanied multilesions in the liver and/or lung.It was demonstrated that the model was stable and facility, consistent with dissection characteristic of human liver cancer.
After 3 wk of drug intervention, the total survival rate in treatment group (A + B + C) was 94.4% and much higher than the chemotherapy group (58.3%) (P = 0.007, Fisher’s Exact Test). Though the survival rate in group A was higher than that of group D, no differences were observed between them (P > 0.05). The survival rate in group Band C is higher than in group D, there was a significant difference (P = 0.014). 4 wk later, the total survival rate in treatment group (A + B + C) was 50% and much higher than the chemotherapy group (0%) (P = 0.02). Though the survival rate in group A was higher than that of group D, no difference were observed between them (P > 0.05). The survival rate in group B and C was higher than in group D, there was significant difference, (P < 0.05 or 0.01) (Table 1).
No differences was observed between the group A, group B, group C and the group D in volume and index of tumors (P > 0.05) (Table 2).
| Group | n | Volume (mm3) | Index |
| A | 12 | 466.43 ± 645.66 | 0.0037 ± 0.0403 |
| B | 12 | 215.91 ± 275.23 | 0.0305 ± 0.0403 |
| C | 12 | 325.23 ± 464.30 | 0.0313 ± 0.0436 |
| D | 12 | 309.7 ± 309.72 | 0.0462 ± 0.0296 |
After the transplantation tumors in liver of each mouse was taken out, then the remain liver were observe by microscope, if it existed hepatoma carcinoma cell, We diagnosed the liver had the metastases. Tumor metastasis in livers of group A and group C were both 58.3% (7/12) and much less than the group D 100% (12/12) P = 0.037. Tumor metastasis in livers of group A, group B and group C was 63.9% (23/36) and much less than the group D (P = 0.021), but there was no difference among group A, group B and group C (Table 3).
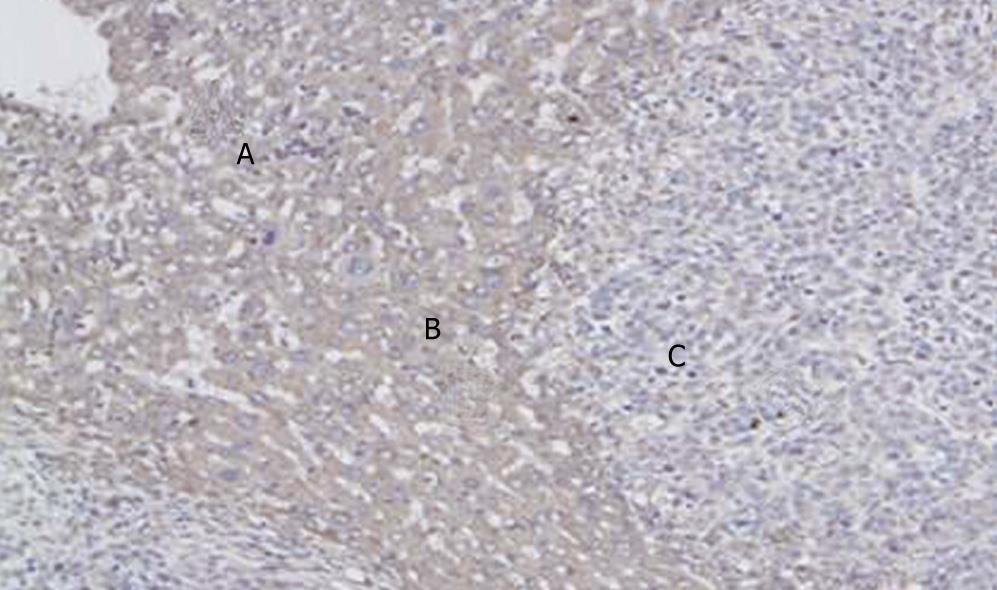
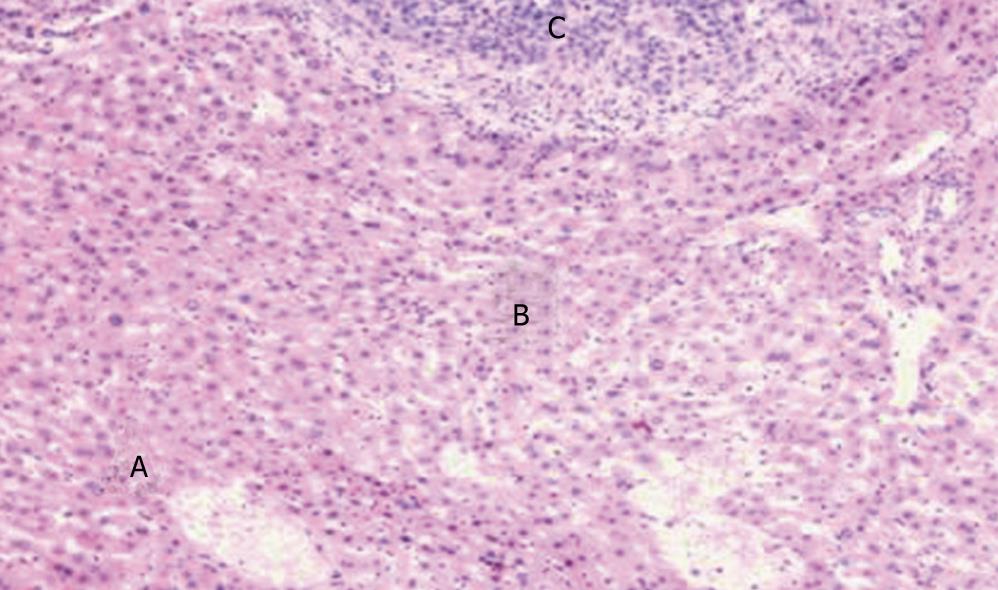
Three groups were compared with each other applied Kruskal-Wallis test, χ2 = 60.67, P = 0.000. The result showed that the intensity of PTEN in latero-cancer tissue was the highest, and then the normal hepatic tissue, the lowest was cancer tissue (Table 4, Figures 1 and 2).
| Kind of tissue | n | PTEN intensity median |
| Normal hepatic tissue | 48 | 1.00 |
| Latero-cancer tissue | 48 | 1.31 |
| Cancer tissue | 48 | 0.23 |
Tested by One-way ANOVA, LSD, Tamhane Test was used in normal hepatic tissue and latero-cancer tissue, and Mann-Whitney U in cancer tissue. in normal hepatic tissue (Test of Homogeneity of Variances, Levene Statistic = 2.18, P = 0.104; and One-way ANOVA Test, F = 5.90, P = 0.002), A > D, P = 0.001; B > D, P = 0.031; C > D, P = 0.001. It represented that the intensity of PTEN in Chinese crude drug group was higher than the Tegafur group. However, there was no significant statistic difference among Chinese crude drug group in difference concentrations (P > 0.05). In latero-cancer tissue, (Test of Homogeneity of Variances, Levene Statistic = 3.09, P = 0.037; and One-way ANOVA Test, F = 15.99, P = 0.000), A < B, P = 0.170; A > C, P = 0.091; B > C, P = 0.017; C > D, P = 0.028, it showed that expression intensity of PTEN under the intervention of Chinese crude drug was higher than that of Tegafur, the expression intensity of PTEN in medium dose group was the highest (P = 0.05). In cancer tissue, A < B, U = 30.5, P = 0.016; A > C, U = 44, P = 0.104; A > D, U = 24, P = 0.005; C > D, U = 18, P = 0.002; B < C, U = 9.5, P = 0.000; B > D, U = 4, P = 0.000. It represents that the intensity of PTEN in medium dose group was highest among the 3 kinds of tissue. In cancer tissue, A < B, P = 0.014; A > C, P = 0.114; A > D, P = 0.005; C > D, P = 0.001; B > D, P = 0.000, It also represented that the intensity of PTEN in medium dose group was highest (P < 0.001). However, there was no significant statistic difference between medium dose group and low group, low dose group and high dose group (P > 0.05) (Table 5).
| Group | n | Normal hepatic tissue | Latero-cancer tissue | Cancer tissue(median) |
| A | 12 | 1.05 ± 0.34 | 1.42 ± 0.24 | 0.36 |
| B | 12 | 0.90 ± 0.31 | 1.75 ± 0.42 | 0.86 |
| C | 12 | 1.06 ± 0.18 | 1.27 ± 0.21 | 0.20 |
| D | 12 | 0.59 ± 0.48 | 0.64 ± 0.61 | 0.05 |
PTEN/MMAC1 (mutated in multiple advanced cancer 1) or TEP1 (TGF-regulated and epithelial cell-riched phosphatase1), located in human chromosome band 10q23[11], was recently identified together as tumor suppressor gene by three America research teams. As the first discovered dual-specific phosphatase, it may suppress tumor cell growth, regulate tumor cell invasion and metastasis through inhibiting many signal pathways of cell proliferation[12–15].
FT-207/tegafur, which belongs to the Second-generation fluoropyrimidine drugs, is metabolized to 5-fluorouracil through certain hepatic metabolizing enzymes and the cytochrome P450 (CYP450) system. Its anticancer mechanism is the same with that of 5-FU[16]. The advantage of Tegafur is highly fat-soluble, rapid gastrointestinal absorption, much longer half-life, as well as suitable for oral administration, its side effects is only one seventh that of 5-FU, while the efficacy index is twice higher. As the representative of the Second-generation 5-FU, Tegafur is used to treat several types tumors of alimentary canal as a routine chemotherapy drugs[17–19].
TCM includes primary hepatic carcinoma in the category of diseases such as: liver mass, abdominal mass, ZhengJia, tympanites, jaundice, etc. The etiological factors are concerned with yin-yang disbalance caused by the reception humid heat, long-term eating and drinking without temperance, being addicted to drink, internal injury caused by excess of seven emotions. When the vital qi gets deficient, evil factor easily invades the body and are stagnated in the liver cause the depression of liver-QI, activities of qi is stagnated and the blood circulation is blocked; Phlegm knobbing stagnant blood leads to the formation of abdominal mass, finally resulting in the formation of liver cancer[2021]. The primary pathogenesis of liver cancer lies in the qi-stagnancy and blood stasis, the stagnation of humid heat, the discord of the spleen and the liver, as well as the weakness of vital qi[22]. At the early stage, this disease manifests itself in the unimpaired vital-qi, the type is most likely be sthenia syndrome or syndrome of blood stasis; at the middle stage, vital vital-qi is impaired, and asthenia and sthenia complicated with each other; at the advanced stage, the main syndrome is asthenia syndrome. Thus, the main treatment of TCM for liver cancer lies in strengthening body resistance and eliminating pathogen; the former is the mian way, the later is the assistant way[2324]. Correlated investigation confirmed that the therapy of invigorating the spleen and regulating the qi could inhibit or delay the tumorous growth and metastasis, strengthen body’s immunity, prolong the life span, and was more effective than the therapy of promoting blood flow and dissolving the stagnated blood and of[2526], what’s more, different therapeutic methods of TCM can in different degree regulate the transcription of some important oncogenes which play an important role in the process of occurrence and development of the liver cancer[27], thereby have some anti-tumorous effect. Therefore, TCM becomes one of important combined therapies for cancer in China. In dealing with tumor, Chinese crude drugs have such advantages as following: guidance of wholism, strengthening the internal anticancer mechanism, mild toxicity, without pain, easily be accepted, it can relieve the symptoms, improve patient’s function, lighten the toxicity and side effect meanwhile enhance effectiveness of the radiotherapy or chemotherapy, and accelerate recovery from operation, further more, can inhibit tumor growth, control or delay its recurrence, improve life quality, prolong survival time. During to the traditionary chemotherapy with the characteristic of being exist without tumor impacts the patient's exist quality, while people pay close attention to TCM with the characteristic of being exist with tumor.
FJD (Appl.: 200710026976.6) has been widely used to treat the hepatocellular carcinoma for years in our department. According to the special pathogenesis of middle and advanced hepatocellular carcinoma, the decoction can smooth the liver and strengthen the spleen, meanwhile can strengthen the body resistance, remove toxin and treat Biaol and Ben. So the decoction has the effect of strengthening the spleen and replenishing qi, diminishing stagnation by detoxification, relieving the depressed liver qi and regulating the blood. Previous research showed that this decoction had a certain anticancer effect on liver cancer cell lines in vitro[28] and in clinic[29]. In this study, we found that each TCM groups had distinct superiority in prolonging the live time and raising the survival rate of athymic mouse bearing cancer compared with the chemotherapy group. At the same time, middle and high concentration groups were more effective in prolonging the live time than that in low concentration group, which indicated TCM groups in prolonging the live time had dose-effect relationship. However, the TCM groups and the chemotherapy group has the similar effect in shortening tumor. Moreover, each group of the medicinal herbs were better than chemotherapy group chemotherapy group in inhibiting tumor diffusion and reducing tumor metastasis in liver. Compared with chemotherapy group, every TCM groups could reduce the number of tumor metastasis in liver, there was no difference between TCM groups and chemotherapy group in tumor metastasis rate in liver, which might relate with the insufficient the number of the sample. As mentioned previously, FJD could inhibit tumor metastasis and prolong the live time, the anti-tumorous effect of FJD might through certain way to inhibiting tumor diffusion and metastasis other than shorten tumor, thus slowed down pathogenetic condition progressing, and prolonged the live time[30].
What on earth is the mechanism of the anticancer treatment by the Chinese herbs that strengthen the body resistance and remove toxic substances? Our research showed that the low, middle and high concentrations of the Chinese herbs could obviously increase expression of PTEN on the athymic mice bearing tumor respectively, compared with chemotherapy by ft207, which indicated the antitumous mechanism of medicinal herbs in this study. Interestingly, we found the expression of the PTEN in adjacent cancerous tissues of athymic mice bearing tumor was higher than that of the normal tissues, we inferred that this phenomenon might be a protection of the body itself to prevent cancer cell from further developing by high expression of PTEN in adjacent cancerous tissues under stressful condition. Therefore, the expression density of PTEN closely relates with the occurrence and development of liver cancer. Our study showed that FJD can provocate the expression of PTEN, and high expression of the PTEN in adjacent cancerous tissues seem to explain the reason why the intrahepatic metastasis rate in TCM groups is lower than that of chemotherapy group. The comparison among the TCM groups indicated that middle concentration achieved the best curative effect, low and high groups ranked secondly, this result illustrates that it is necessary for us to pay more attention to the dosage of medicinal herbs in clinic practice, because only the moderate dosage may acquire the best therapeutic effect. However, the best concentration of the FJD in treating HCC needs to be studied furtherly.
However, a control group of mice which were inoculated HCCs in the liver and were not given FZDJT and tegafur had been allocated. It's a pity that due to the too much cellular necrosis in pathological section we cannot get the result of immunohistochemistry. Next time we should do better.
In conclusion, our study showed that FJD can prolong the survival time and decrease tumor metastasis in livers of these experimental mice. Mechanisms of FJD healing HCC may partially be explained by enhancing the expression of PTEN in liver.
Traditional Chinese Medicine (TCM) which is useful to improve life quality, prolong survival time has been widely used as combined therapies in treating hepatocellular carcinoma (HCC). The primary pathogenesis of liver cancer lies in the qi-stagnancy and blood stasis, the stagnation of humid heat, the discord of the spleen and the liver, as well as the weakness of vital qi. Fuzheng Jiedu Decoction (FJD) (Appl.: 200710026976.6) has been widely used to treat the hepatocellular carcinoma for years. Previous research showed that this decoction had a certain antitumous effect on liver cancer cell lines in vitro and in clinic.
TCM has been widely used as combined therapies in treating HCC in China. The novelty and innovation of the research consist in researching the mechanism of treatment of FJD to the hepatocellular carcinoma with mole-biological method. Mechanisms of FJD healing HCC may partially be explained by enhancing the expression of PTEN in liver.
TCM has been widely used as combined therapies in treating HCC, whether it can down-regulate the expression of PTEN in HCC is still unknown. The aim of our study is to explore the effect of FJD, complex prescription of Chinese crude drug in treating BALB/c athymic mice with HCC, as well as the expression of PTEN.
The mechanism of treatment of FJD to the hepatocellular carcinoma may partially be explained by enhancing the expression of PTEN tumor suppressor gene. We also found that different concentration of FJD had different effect in prolonging survival time and decreasing metastatic tumour. This study was indicated that FJD can be used as one of combined therapies in treating HCC and which was the best concentration of FJD.
PTEN: Phosphatase and tensin homolog deleted on chromosome ten. FJD: Fuzheng Jiedu Decoction. FT207: Tegafur. TCM :Traditional Chinese Medicine.
This is a good study in which the main objective is to explore the expression of PTEN in liver of athymic mice with HCC and the effect of FJD. PTEN was recently identified together as tumor suppressor gene. FJD can prolong the survival time and decrease Tumor metastasis in livers of these experimental mice. Mechanisms of FJD healing HCC may partially be explained by enhancing the expression of PTEN in liver.
| 1. | Chen XP, Qiu FZ, Wu ZD, Zhang ZW, Huang ZY, Chen YF. Long-term outcome of resection of large hepatocellular carcinoma. British Journal of Surgery. 2006;93:600-606. |
| 2. | Taura K, Ikai I, Hatano E, Fujii H, Uyama N, Shimahara Y. Implication of frequent local ablation therapy for intrahepatic recurrence in prolonged survival of patients with hepatocellular carcinoma undergoing hepatic resection: an analysis of 610 patients over 16 years old. Annals of Surgery. 2006;244:265-273. |
| 3. | Earle SA, Perez EA, Gutierrez JC, Sleeman D, Livingstone AS. Franceschi D. Levi JU. Robbins C. Koniaris LG. Hepatectomy enables prolonged survival in select patients with isolated noncolorectal liver metastasis. JACS. 2006;203:436-446. |
| 4. | Lin JJ, Jin CN, Zheng ML, Ouyang XN, Zeng JX, Dai XH. Clinical study on treatment of primary hepatocellular carcinoma by Shenqi mixture combined with microwave coagulation. Chin J Integr Med. 2005;11:104-110. |
| 5. | Hu TH, Huang CC, Lin PR, Chang HW, Ger LP, Lin YW, Changchien CS, Lee CM, Tai MH. Expression and prognostic role of tumor suppressor gene PTEN/MMAC1/TEP1 in hepatocellular carcinoma. Cancer. 2003;97:1929-1940. |
| 6. | Wan XW, Wang HY, Jiang M, He YQ, Liu SQ, Cao HF, Qiu XH, Tang L, Wu MC. PTEN expression and its significance in human primary hepatocellular carcinoma. Zhonghua Gan zangbing Zazhi. 2003;11:490-492. |
| 7. | D DL, Xi RZ, Xiang RC. Expression and signifi cance of new tumor suppressor gene PTEN in primary liver cancer. J Cell Mol Med. 2003;7:67-71. |
| 8. | China pharmacopoeia committee. Pharmacopoeia of the People's Republic of China. 2005ed. Beijing: Chemical industry publishing house 2005; 77-199. |
| 9. | Sun FX, Tang ZY, Liu KD, Xue Q, Gao DM, Yu YQ, Zhou XD, Ma ZC. Metastatic models of human liver cancer in nude mice orthotopically constructed by using histologically intact patient specimens. J Cancer Res Clin Oncol. 1996;122:397-402. |
| 10. | Bresalier RS, Ho SB, Schoeppner HL, Kim YS, Sleisenger MH, Brodt P, Byrd JC. Enhanced sialylation of mucin-associated carbohydrate structures in human colon cancer metastasis. Gastroenterology. 1996;110:1354-1367. |
| 12. | Yamada KM, Araki M. Tumor suppressor PTEN: modulator of cell signaling, growth, migration and apoptosis. J Cell Sci. 2001;114:2375-2382. |
| 13. | Kawamura N, Nagai H, Bando K, Koyama M, Matsumoto S, Tajiri T, Onda M, Fujimoto J, Ueki T, Konishi N. PTEN/MMAC1 mutations in hepatocellular carcinomas: somatic inactivation of both alleles in tumors. Jpn J Cancer Res. 1999;90:413-418. |
| 14. | Guo SP, Wang WL, Wang WY, Li QL. Inhibitory effect of tumor suppressor gene PTEN on hepatocellular carcinoma cell line HHCC proliferation and its mechanisms of action. Zhonghua Zhongliu Zazhi. 2005;27:591-594. |
| 15. | Guo SP, Wang L, Wang WL, Li QL, Wang WY, Zhang J. Mutations of tumor suppressor gene PTEN mutations in hepatocellular carcinoma and its implications in tumor proliferation and apoptosis. Zhonghua Binglixue Zazhi. 2006;35:467-472. |
| 16. | Kawata S, Noda S, Imai Y, Tamura S, Saitoh R, Miyoshi S, Minami Y, Tarui S. Hepatic conversion of 1-(tetrahydro-2-furanyl)-5-fluorouracil into 5-fluorouracil in patients with hepatocellular carcinoma. Gastroenterol Jpn. 1987;22:55-62. |
| 17. | Nakanishi T, Kawakami H. Maintenance chemotherapy by UFT after trans-arterial embolization for hepatocellular carcinoma. Gan To Kagaku Ryoho. 1986;13:1589-1595. |
| 18. | Hasegawa K, Takayama T, Ijichi M, Matsuyama Y, Imamura H, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Uracil-tegafur as an adjuvant for hepatocellular carcinoma: a randomized trial. Hepatology. 2006;44:891-895. |
| 19. | Itsubo M. The present status of chemotherapy for hepatocellular carcinoma. Gan To Kagaku Ryoho. 1993;20:889-895. |
| 20. | Pan MQ, Pan B, Jiang YL, Zeng PH, Li YH. Epidemiological Investigation and Analysis of Symptoms, Signs, Disease Position and Using Medicine of Liver cancer in Traditional Chinese Medicine. Shijie Zhongxiyi Jiehe Zazhi. 2006;1:69-71. |
| 21. | Liu Q, Zhang YB, Ma CH, Yue XQ, Ling CQ. Analysis of literature on therapeutic methods and medicines of traditional Chinese medicine for primary liver cancer. Zhongxiyi Jiehe Xuebao. 2005;3:260-262. |
| 22. | Liu LM, Yu EX. Treatment of pain according to syndrome differentiation in 169 cases of liver cancer. Zhongyi Zazhi. 1989;9:277-280. |
| 23. | Wang J, Gu LG, Wang QG, Peng GY, Li RS, Wang XQ. Research on the impact of liver-stagnation and spleen-deficiency on experimental hepatocarcinoma induced by DEN in Rats. Zhongguo Zhongyiyao Xinxi Zazhi. 2003;10:18-20. |
| 24. | Dong XL, Li CH, Li W. Clinical study on treatment of primary hepatocarcinoma based on deficiency of spleen. Shandong Zhongyi Zazhi. 2001;20:459-461. |
| 25. | Guan DY, Fang ZQ. Research Progress of Spleen-Nourishing and Qi-Regulating Therapy for Liver Cancer. Shanghai Zhongyiyao Daxue Xuebao. 2005;19:60-63. |
| 26. | Li YJ, Fang ZQ, Tang CL, Ma J, Guan DY, Chen DS. Clinical epidemiological investigation and research of syndrome distribution law in Chinese medicine of 2060 cases of primary liver carcinoma. Zhongguo Yiyao Xuebao. 2003;3:144-146. |
| 27. | Guan D, Fang Z, Lu H, Li H. Preliminary investigation on regulating effects of different TCM treatments on transcription of the correlated genes of liver cancer in rats. J Tradit Chin Med. 2003;23:62-66. |
| 28. | Chen ZX, Chen W, Zhang SJ. The study of the influence of Decoction on apoptosis and proliferation in tumor cell. Zhongyi Zazhi. 2000;23:477-478. |
| 29. | Chen ZX, Zhang SJ, Hu HT, Sun BG, Yin LR. Clinical study of method of strengthening body resistance and disintoxication disintoxication in patients with HCC of post-TACE. Zhongguo Zhongyao Zazhi. 2007;32:1211-1213. |
| 30. | Lin LZ. The quality of life and oncology of traditional chinese medicine (TCM). Xiandai Kangfu. 2000;4:1300-1306. |