Published online Feb 28, 2007. doi: 10.3748/wjg.v13.i8.1286
Revised: December 26, 2006
Accepted: January 29, 2007
Published online: February 28, 2007
We report here a case of clinically significant liver toxicity after a brief course of rosuvastatin, which is the first statin approved by the regulatory authorities since the withdrawal of cerivastatin. Whether rosuvastatin has a greater potential compared with other statins to damage the liver is unclear and the involved mechanisms are also unknown. However, rosuvastatin is taken up by hepatocytes more selectively and more efficiently than other statins, and this may reasonably represent an important variable to explain the hepatotoxic potential of rosuvastatin. Our report supports the view that a clinically significant risk of liver toxicity should be considered even when rosuvastatin is given at the range of doses used in common clinical practice.
- Citation: Famularo G, Miele L, Minisola G, Grieco A. Liver toxicity of rosuvastatin therapy. World J Gastroenterol 2007; 13(8): 1286-1288
- URL: https://www.wjgnet.com/1007-9327/full/v13/i8/1286.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i8.1286
Liver toxicity is a well recognized adverse effect of treatment with statins[1]. However pre-marketing studies have suggested that rosuvastatin may have a lesser potential to cause liver toxicity as compared with other statins[2]. We report here a case of clinically significant liver toxicity after a brief course of rosuvastatin.
A 64-year-old man presented with a seven-days history of malaise, anorexia, upper abdominal discomfort, and jaundice. Four months earlier the patient had an acute myocardial infarction that was treated with angioplasty and stenting of a culprit lesion in the right coronary artery; liver function tests were normal and he was discharged on clopidogrel, aspirin, metoprolol, ramipril, and atorvastatin (40 mg daily). One week later, rosuvastatin (10 mg daily) was prescribed instead of atorvastatin as the patient reported an itching skin rash that developed soon after he took the second tablet of atorvastatin; at that time serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were 55 U/L and 45 U/L (normal range 10-36 U/L for both), respectively, with bilirubin, γ-glutamyltransferase (γ-GT), and alkaline phosphatase (AP) within the normal limits.
At the present admission, he was fully alert and oriented, apyrexial, with mild cutaneous and scleral jaundice, and no flapping tremor or stigmata of chronic liver disease; the remaining physical examination was normal. A laboratory work-up revealed AST 880 U/L, ALT 775 U/L, total bilirubin 2.6 mg/dL (normal range 0.2-1) with conjugated bilirubin 0.8 mg/dL; γ-GT, AP, ammonia, α-fetoprotein, electrolytes, hematologic and coagulation parameters, and renal function tests were normal. Serological screening for viral hepatitis (hepatitis A, B, C, E and G virus; cytomegalovirus; herpes simplex; and Epstein–Barr virus) was negative and HBV-DNA and HCV-RNA were not detected in the peripheral blood. Search for autoimmune liver disorders (antinuclear antibodies, anti-smooth-muscle antibodies, and antimitochondrial antibodies) was also negative as were results of iron, copper, ceruloplasmin metabolism and α1-antitrypsin concentrations.
Ultrasonography and contrast-enhanced computed tomography (CT) showed a normal liver and no expanded bile ducts or gallbladder abnormalities; there was no caval or portal thrombosis and no peri-hepatic or perisplenic intraperitoneal fluid. Echocardiography was also normal with no evidence of valvular disease, pericardial effusion, pulmonary hypertension, or left ventricular systolic or diastolic dysfunction.
The patient had an otherwise unremarkable medical record with no previous history of acute or chronic liver disease. He also denied toxic and alcoholic habits or using any other medications, including over-the-counter medications, or herbal remedies.
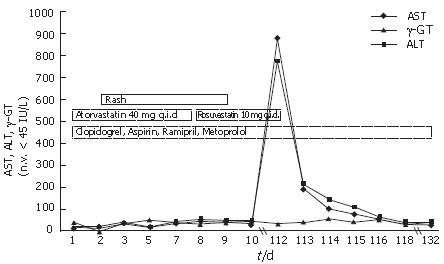
Rosuvastatin was withdrawn and AST and ALT levels fell to 216 U/L and 198 U/L, respectively, and bilirubin to 1.8 mg/dL on the 3rd day; ammonia and coagulation parameters remained within the normal range. Over the subsequent course symptoms gradually resolved, which was paralleled by declining levels of liver enzymes and bilirubin; at no time were flapping tremor or other signs or symptoms of liver failure or encephalopathy observed and the patient was discharged on the 6th day with instructions for close follow-up as an outpatient. At that time, AST and ALT were 40 U/L and 32 U/L, respectively; bilirubin, ammonia, and coagulation parameters were normal. The patient refused a liver biopsy and a re-challenge test with rosuvastatin was not done for ethical reasons. At a follow-up visit two weeks later, he was doing well with normal liver function tests and a normal coagulation profile. The clinical and biochemical course of this patient is summarized in Figure 1.
Until today the incidence of elevated serum liver enzymes among patients on treatment with statins generally ranges from 2% to 3%[1] while pre-marketing studies have shown a 20 to 30-fold lower incidence if rosuvastatin is used[2]. We report here a case of clinically significant liver toxicity after a brief course of rosuvastatin, which is the first statin approved by the regulatory authorities since the withdrawal of cerivastatin. According to the Naranjo probability scale our patient had a highly probable rosuvastatin-related adverse event[3]; furthermore, liver function tests were normal before statin treatment was started and we did not find any other plausible alternative cause to explain the onset of such a severe hepatitis in this case. As a matter of fact, we identified a clear temporal relationship between initiation of rosuvastatin therapy and the elevation of liver enzymes and the patient rapidly achieved a complete clinical and biochemical recovery after rosuvastatin was interrupted.
The episode of urticaria accompanied by a modest increase of liver enzymes after the patient was given atorvastatin, which was his first exposure to a statin, raises the view of an idiosyncratic or hypersensitivity cross-reaction between atorvastatin and rosuvastatin as an underlying mechanism of the liver injury that occurred during treatment with rosuvastatin. Even though the true correlation remains to be understood, we might reasonably conceive that allergic cross-reactions between the two statins might have contributed, at least to a certain extent, to the onset of severe liver damage in this case. Our patient had normal eosinophil count and remained apyrexial throughout the entire course. However all these features along with the absence of lymphadenopathy and the negative search for antinuclear and anti-smooth-muscle antibodies do not substantially argue against an allergic or immune-mediate mechanism of liver injury.
The patient was also receiving other medications in combination with rosuvastatin, but this is not a true confounding factor in our opinion. Our Medline search yielded only a few anecdotal reports of hepatitis associated with the use of clopidogrel or ramipril[4-7] and none of liver toxicity linked with metoprolol. In addition, aspirin-related hepatitis has been described, but this is a dose-related phenomenon caused by the intrinsic salicylate hepatotoxicity which generally does occur only when aspirin is given in full anti-inflammatory doses and not with the 75-300 mg used for anti-platelet indications[8]. One important point is that rosuvastatin is neither an inhibitor nor an inducer of cytochrome P450 isoenzymes and rosuvastatin itself is a poor substrate for these isoenzymes. It suggests that fewer drug interactions resulting from cytochrome P450-mediated metabolism should be expected with rosuvastatin compared with other statins[9,10]. There is no available evidence of any clinically significant pharmacokinetic interactions in subjects taking concomitant rosuvastatin and clopidogrel, ramipril, metoprolol, or aspirin.
Whether rosuvastatin has a greater potential compared with other statins to damage the liver is unclear and the involved mechanisms are also unknown. Results from a recent meta-analysis demonstrate that treatment with statins as a class is not associated with a significant risk of liver dysfunction and hepatitis, but studies with rosuvastatin were not included in this meta-analysis[1]. In contrast, a post-marketing analysis has shown that use of rosuvastatin, at least over its first year of marketing, was significantly more likely than atorvastatin, pravastatin, and simvastatin to be associated with a composite end point of adverse events including rhabdomyolysis, proteinuria, nephropathy, and renal failure, and there was also a significant trend in favor of an increased liver toxicity with rosuvastatin compared with the other statins even though this was not targeted as the primary end point of the study[11]. Furthermore, rosuvastatin is taken up by hepatocytes more selectively and more efficiently than other statins, and this may reasonably represent an important variable associated with the hepatotoxic potential of rosuvastatin[12].
Our report supports the view that a clinically significant risk of liver toxicity should be considered even when rosuvastatin is given at the range of doses used in common clinical practice.
S- Editor Liu Y L- Editor Zhu LH E- Editor Lu W
| 1. | de Denus S, Spinler SA, Miller K, Peterson AM. Statins and liver toxicity: a meta-analysis. Pharmacotherapy. 2004;24:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 151] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 2. | Rosenson RS. Rosuvastatin: a new inhibitor of HMG-coA reductase for the treatment of dyslipidemia. Expert Rev Cardiovasc Ther. 2003;1:495-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7061] [Cited by in RCA: 8430] [Article Influence: 187.3] [Reference Citation Analysis (4)] |
| 4. | Ramos Ramos JC, Sanz Moreno J, Calvo Carrasco L, García Díaz Jde D. Clopidogrel-induced hepatotoxicity. Med Clin (Barc). 2003;120:156-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Durán Quintana JA, Jiménez Sáenz M, Montero AR, Gutiérrez MH. Clopidogrel probably induced hepatic toxicity. Med Clin (Barc). 2002;119:37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Willens HJ. Clopidogrel-induced mixed hepatocellular and cholestatic liver injury. Am J Ther. 2000;7:317-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Yeung E, Wong FS, Wanless IR, Shiota K, Guindi M, Joshi S, Gardiner G. Ramipril-associated hepatotoxicity. Arch Pathol Lab Med. 2003;127:1493-1497. [PubMed] |
| 8. | Fry SW, Seeff LB. Hepatotoxicity of analgesics and anti-inflammatory agents. Gastroenterol Clin North Am. 1995;24:875-905. [PubMed] |
| 9. | White CM. A review of the pharmacologic and pharmacokinetic aspects of rosuvastatin. J Clin Pharmacol. 2002;42:963-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 99] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 10. | Tornio A, Pasanen MK, Laitila J, Neuvonen PJ, Backman JT. Comparison of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) as inhibitors of cytochrome P450 2C8. Basic Clin Pharmacol Toxicol. 2005;97:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Alsheikh-Ali AA, Ambrose MS, Kuvin JT, Karas RH. The safety of rosuvastatin as used in common clinical practice: a postmarketing analysis. Circulation. 2005;111:3051-3057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 114] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 12. | Nezasa K, Higaki K, Matsumura T, Inazawa K, Hasegawa H, Nakano M, Koike M. Liver-specific distribution of rosuvastatin in rats: comparison with pravastatin and simvastatin. Drug Metab Dispos. 2002;30:1158-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 89] [Article Influence: 3.7] [Reference Citation Analysis (0)] |