Published online Feb 21, 2007. doi: 10.3748/wjg.v13.i7.1060
Revised: December 5, 2006
Accepted: December 25, 2006
Published online: February 21, 2007
AIM: To study the induction of T cellular immune responses in BALB/c mice immunized with uric acid and dendritic cells (DCs) pulsed with hepatitis B virus surface antigen (HBsAg).
METHODS: DCs were generated from bone-marrow cells of BABL/c mice, and then pulsed or unpulsed with HBsAg protein (HBsAg-pulsed-DCs or unpulsed-DCs) in vitro. BABL/c mice were immunized with HBsAg-pulsed-DCs (1 × 106) and uric acid, injected through the tail vein of each mouse. The mice in control groups were immunized with HBsAg-pulsed-DCs alone, unpulsed-DCs alone or 200 μg uric acid alone or PBS alone. The immunization was repeated 7 d later. Cytotoxic T lymphocytes (CTLs) in vivo were determined by the CFSE labeled spleen lysis assay. Spleen cells or spleen T cells were isolated, and re-stimulated in vitro with HBsAg for 120 h or 72 h. Production of IFN-γ and IL-4 secreted by spleen cells were determined by ELISA method; proliferation of spleen T cells were detected by flow cytometry.
RESULTS: The cytotoxicities of HBsAg-specific-CTLs, generated after immunization of HBsAg-pulsed-DCs and uric acid, were 68.63% ± 11.32% and significantly stronger than that in the control groups (P < 0.01). Compared with control groups, in mice treated with uric acid and HBsAg-pulsed-DCs, the spleen T cell proliferation to HBsAg re-stimulation was stronger (1.34 ± 0.093 vs 1.081 ± 0.028, P < 0.01), the level of IFN-γ secreted by splenocytes was higher (266.575 ± 51.323 vs 135.223 ± 32.563, P < 0.01) , and IL-4 level was lower (22.385 ± 2.252 vs 40.598 ± 4.218, P < 0.01).
CONCLUSION: Uric acid can strongly enhance T cell immune responses induced by HBsAg-pulsed-DCs vaccine. Uric acid may serve as an effective adjuvant of DC vaccine against HBV infection.
- Citation: Ma XJ, Tian DY, Xu D, Yang DF, Zhu HF, Liang ZH, Zhang ZG. Uric acid enhances T cell immune responses to hepatitis B surface antigen-pulsed-dendritic cells in mice. World J Gastroenterol 2007; 13(7): 1060-1066
- URL: https://www.wjgnet.com/1007-9327/full/v13/i7/1060.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i7.1060
It is generally accepted that dendritic cells (DCs) are the most efficient and powerful antigen presenting cells and play a center role in exciting T cell immune reactions. T-cell mediated immune responses, especially Hepatitis B virus (HBV) specific- cytotoxic T lymphocyte (CTL) response, may play an important role in resolving HBV infection[1,2]. hepatitis B surface antigen (HBsAg) pulsed DCs can activate lymphocytes to become HBsAg-specific CTLs or specific CD4+ T cells in vivo[3]. Shimizu et al[4] immunized HBV transgenic mice with DCs loading HBsAg, and found that DC vaccine could break tolerance to HBV and induce an effective anti-viral immune response. Chen et al[5] reported that HBsAg-pulsed DCs from the peripheral blood could effectively suppress HBV replication in chronic hepatitis B patients. However, anti-HBV immune effects of DC vaccine are varied and instable. This may be due to the diversity of DC vaccine preparations; however, the main cause may be the insufficiency of DC vaccines. For example, some researchers carried out similar immune therapy in volunteers, but no evident immune response was shown[3,6-8]. Therefore, the basic research of DC vaccine as well as how to improve the immune response to DC vaccine is still a significant challenge.
Recently, it was reported that uric acid (UA) could stimulate DCs to mature, promote DCs to present foreign antigens and stimulate T lymphocytes[9,10]. These findings demonstrate the adjuvant effects of uric acid and encourage the potential application of uric acid in vaccination.
This study aimed to observe the T cell immune response after immunization with HBsAg pulsed DCs (HBsAg-pulsed-DCs) and uric acid in mice. The results demonstrated that administration of uric acid could enhance the T cell immune response to HBsAg-pulsed-DCs.
Male or female BALB/c (H-2d) mice aged 8 to 10 wk were obtained from the Department of Experimental Animals, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China. All animal experiments followed the guidelines for the care and use of animals established by Tongji Medical College, Huazhong University of Science and Technology, and were approved by the Ethics Committee of Tongji Medical College.
Uric acid (UA, Sigma-aldrich) was dissolved at a concentration of 5 mg/mL in 0.1 mol/L sodium borate buffer (pH 8.5) for more than 72 h[9,11]. The HBsAg protein was synthesized by Shanghai SanGon Company, China. The purity (> 99%) of the protein was confirmed by high performance liquid chromatography (HPLC) and mass spectrometry. Carboxy-fluorescein diacetate, succinimidyl ester (CFSE, Molecular Probes, USA) was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 10 mmol/L.
DCs were prepared as described previously[12]. Briefly, bone marrow cells were collected from the femur and tibiae of BALB/c mice, and DCs were grown from precursors at a starting concentration of 2 × 106 cells per ml in complete RPMI 1640 (RPMI 1640 supplemented with 10% inactivated fetal calf serum, 2 mmol/L L-glutamine, 100 U/mL penicillin G and 100 μg/mL streptomycin), and cultured in six-well flat bottom plates (Falcon) at 37°C, 5% CO2 for 3 h and then non-adherent cells were washed out. rmGM-CSF at 10 ng/mL (PeproTech, Rocky Hill, NJ) and rmIL-4 at 10 ng/mL (PeproTech, Rocky Hill, NJ) were added to the culture. On days 3, 5 and 7, half of the medium was replaced with a fresh medium. On day 7, cells were incubated with uric acid (100, 200 and 400 μg/mL) or 1 μg/mL lipopolysaccharide (LPS, Sigma), respectively. Serum-free RPMI 1640 was used as control. On day 9, cells and culture supernatants were collected for further experiments and analysis.
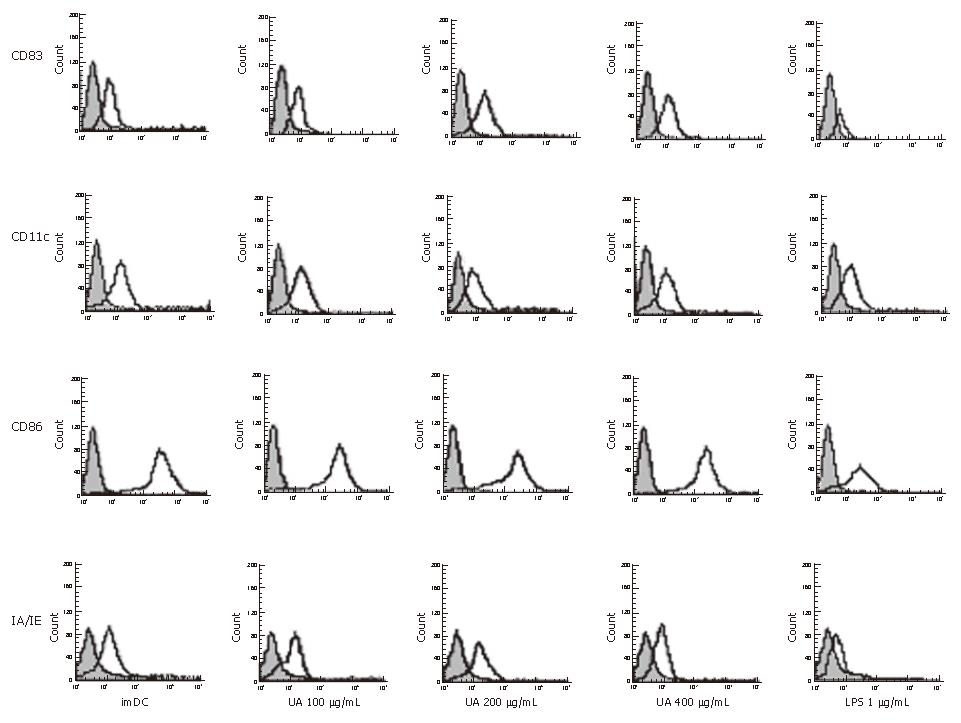
Expression of DC cell surface molecules (CD11c, CD83, IA/IE, CD86) were determined by flow cytometric analysis. Cells were washed twice with an ice cold FACScan buffer (PBS containing 2% FCS and 0.1% sodium azide). The same buffer was used for the incubation with antibodies as well as for all washes. Twenty percent of mixed sera of mice and rats were used to prevent nonspecific antibody binding. FITC-conjugated anti-mouse CD1lc (Clone: N418, eBioscience) and IA/IE(Clone:M5/114.15.2, eBioscience) or PE-conjugated anti-mouse CD83 (Clone:Michel-17, eBioscience) and CD86 (Clone: RMMP-2, Caltag Laboratories) were added, respectively, to the cells and the samples were left on ice for 45 min in the dark. Fluorescence profiles were generated on an FACScan flow cytometer (Becton Dickinson). Histogram was produced with the CellQuest software package.
The concentration of IL-12P70 in DC culture supernatants was determined by using an enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems) according to the manufacturer’s instructions.
Mature DCs stimulated by 1 μg/mL LPS were collected and used to pulse with HBsAg. The procedure for pulsing DCs was the same as previously described[13]. Briefly, 1 × 106 mature DCs were incubated with 10 μg/mL HBsAg for 6 h. DCs were washed three times and resuspended in PBS.
Totally 1 × 106 HBsAg-pulsed-DCs (total volume 200 μL) were injected through the tail vein of each mouse, together with 200 μg uric acid. The mice immunized with HBsAg-pulsed-DCs (1 × 106) or unpulsed-DCs (1 × 106) alone were used as controls. The mice treated with 200 μg uric acid or 200 μL PBS alone were used as controls as well. Ten mice were in each group. The immunization was repeated 7 d later.
Seven days after immunization with DCs for the second and last time, each mouse received spleen cells labeled with CFSE. To prepare target cells to detect in vivo cytotoxic activity[14,15], erythrocytes were removed from naive BALB/c spleen cell suspensions by lysis in ammonium chloride solution. The cells were then washed and split into two populations. One population was pulsed with 10 μg/mL HBsAg, incubated at 37°C for 4 h, and labeled with a high concentration of CFSE (5.0 μmol/L) (CFSEhigh cells). Another population as control target cells was left without HBsAg and was labeled with a low concentration of CFSE (0.5 μmol/L) (CFSElow cells)[16]. An equal number of cells from each population were mixed together, and each mouse received a total of 2 × 107 mixed cells in 400 μL of PBS. Cells were intravenously injected into all mice as above. And 10 h later, the mice were sacrificed and their spleen cells were obtained. Cell suspensions were analyzed by flow cytometry, and each population was detected for their differential CFSE fluorescence intensities. Up to 1 × 104 CFSE-positive cells were collected for analysis. To calculate specific lysis, the following formula was used: Ratio = (percentage CFSElow/percentage CFSEhigh). Percentage specific lysis = [1- (ratio unprimed/ratio primed) × 100][14,15].
Spleen cells from the immunized mice were depleted of erythrocytes and washed twice with PBS. The cells obtained were resuspended with complete RPMI 1640, and seeded in duplicate into flat-bottomed 24-well microtitration plates(Costar, Brumath, France) at 2 × 106 cells per well in 1 mL of culture medium containing 10 μg/mL HBsAg. The cell-free culture supernatants were harvested after 72 h and assayed for IFN-γ and IL-4 activity. The cytokine concentrations were determined by using a commercial ELISA kit (eBioscience Inc.) according to the manufacturer’s instructions, and the standard curves corresponding to known amounts of mouse recombinant IFN-γ, IL-4. The sensitivity limits for the assays are 15 pg/mL for IFN-γ, and 4 pg/mL for IL-4.
Erythrocytes were removed from the spleen cells of mice 14 d after immunization as above. The cells were resuspended with complete RPMI 1640, and then T cells were separated by a nylon wool column method[17]. In brief, 1 × 108 cells were drained through a nylon wool column (Polysciences. Inc., Warrington, PA) for 45 min at 37°C, and then nonadherent T cells were collected after two washes. T cells were labeled with 2.5 μmol/L CFSE and washed three times in the medium as described above, counted, and resuspended at a concentration of 1 × 106 cells/mL. The samples were seeded in triplicate into 24-well microtitration plates (Costar, Brumath, France) at 2 × 106 cells per well in 2 mL of culture medium containing 10 μg/mL HBsAg or PBS. T cells from the untreated mice were used as the negative control; and T cells from the untreated mice stimulated with 10 μg/mL concanavalin A (ConA) and 10 μg/mL HBsAg or PBS was served as the positive controls. The plates were incubated for 72 h in 5% CO2 at 37°C. Cell proliferation was estimated by flow cytometry[16]. Histogram was produced with the Motif 3.0 software package.
All data were presented as mean ± SD and analyzed using the Student-Newman-Keuls test and LSD multiple comparisons with SPSS11.5 software in the experiments. P < 0.05 was regarded as statistically significant.
We demonstrated an increase in CD83, IA/IE, and CD86 expression by DCs, stimulated with uric acid previously (Figure 1, Table 1). The effect of uric acid was dose-dependent and was still observed when the uric acid was administered at 100 μg/mL. After stimulation with uric acid (100-400 μg/mL), the percentage of various markers increased from 2.0- fold to 3.0-fold. The stimulatory effect elicited by uric acid at a concentration of 200-400 μg/mL was similar to that induced by LPS (1 μg/mL). After stimulation with uric acid or LPS, the CD11c expression in each group was high and similar, including the imDC group.
| Group | n | CD11C | CD83 | CD86 | MHC |
| RMPI-1640 | 5 | 72.85 ± 1.64 | 21.66 ± 5.34f | 37.77 ± 1.62f | 27.34 ± 1.81f |
| UA100 | 5 | 73.16 ± 1.05 | 47.71 ± 4.75bd | 78.48 ± 2.98bf | 75.83 ± 2.49bf |
| UA200 | 5 | 73.18 ± 0.95 | 52.23 ± 0.83b | 80.14 ± 1.01b | 79.47 ± 0.92b |
| UA400 | 5 | 73.36 ± 1.46 | 52.33 ± 0.94b | 81.08 ± 1.25b | 80.36 ± 1.22b |
| LPS | 5 | 73.44 ± 1.33 | 53.28 ± 1.12b | 82.50 ± 2.29bb | 81.42 ± 2.21b |
Using the ELISA technique, the IL-12 p70 secretion by DCs was detected 48h after stimulation with uric acid or LPS. IL-12p70 production was markedly increased in response to uric acid stimulation in a dose-dependent manner. The IL-12p70 production stimulated by 200-400 μg/mL uric acid was similar to LPS treatment (Table 2).
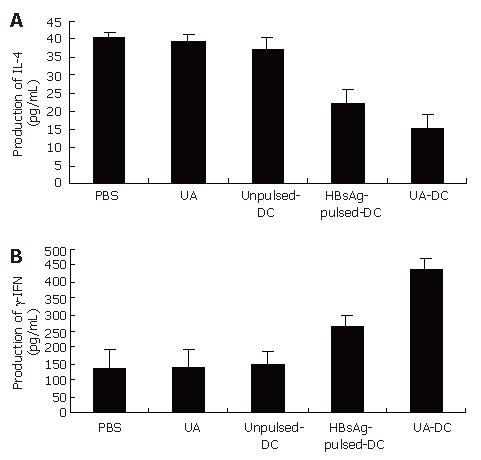
The supernatants of cultured immune spleen cells were evaluated for the production of IL-4 (Th2 cytokine) or IFN-γ (Th1 cytokine) in response to HBsAg re-stimulation on day 14 (Figure 2, Table 3).
The production of IL-4 in mice immunized with HBsAg-pulsed-DCs and uric acid was lower than that of mice immunized with HBsAg-pulsed-DCs alone or unpulsed-DCs alone or PBS alone(P < 0.001 for all) (Figure 2A).
The production of IFN-γ (Figure 2B) in mice immunized with HBsAg-pulsed-DCs and uric acid was significantly greater (P < 0.001 for all) than that in mice immunized with HBsAg-pulsed-DCs alone. Spleen cells from mice immunized with unpulsed-DCs alone or PBS alone produced a few IFN-γ.
Spleen cells from mice immunized with uric acid alone failed to enhance the secretion of IFN-γ or inhibit the secretion of the IL-4 (Figure 2).
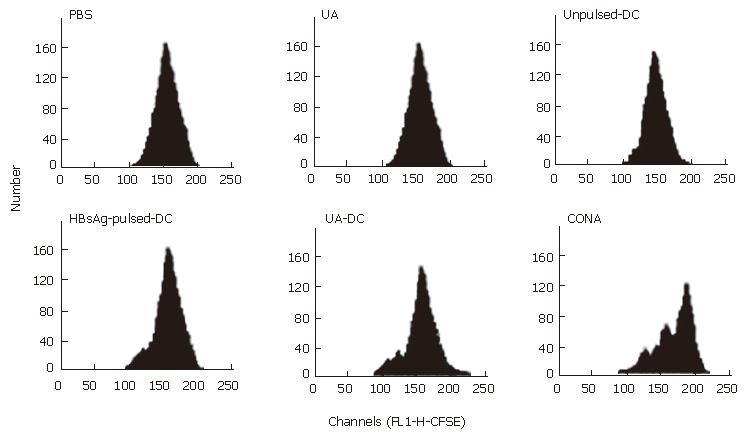
Fourteen days after immunization, CFSE-labeled-T cells from each mouse were re-stimulated with 10 μg/mL HBsAg or PBS for 72 h in vitro, and cellular proliferations were assayed by flow cytometry (Figure 3, Table 4).
A strong proliferative response to HBsAg re-stimulation was observed in T cells of mice immunized with HBsAg-pulsed-DCs and uric acid (Figure 3).
Proliferative response to HBsAg re-stimulation was also observed in T cells of mice treated with HBsAg-pulsed-DCs. Insignificant proliferation was observed in T cells of mice treated with unpulsed-DCs. No proliferation to HBsAg re-stimulation was observed in T cells of mice treated with uric acid alone or PBS (Figure 3).
PBS re-stimulation in vitro failed to stimulate T cell proliferation in each mouse (Table 4).
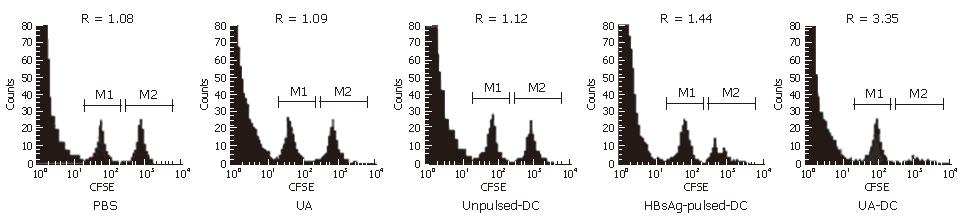
We directly determined the activity of HBsAg-specific-CTLs with an in vivo cytotoxicity assay. The extent of lysis of HBsAg-pulsed spleen cells was expressed as R-values (Figure 4) and the cytotoxicity activity of HBsAg-specific-CTLs were calculated (Table 5).
A significant strong cytotoxicity of HBsAg-specific CTLs was observed in the mice immunized with HBsAg-pulsed-DCs and uric acid; whereas immunization with HBsAg-pulsed-DCs alone or unpulsed-DCs induced low cytotoxicities of HBsAg-specific CTLs (Table 5).
In mice immunized with uric acid 200 μg alone or PBS, R values or CTL cytotoxicity were similar (P > 0.05) (Figure 4). It demonstrated that no significant specific lysis was observed and immunization with uric acid 200 μg alone failed to induce HBsAg-specific CTLs.
It has been shown that T-cell mediated immune responses are very important in overcoming HBV infection[1,2] and DCs can efficiently prime T-cell response, so the development of a vaccine of DCs has attracted considerable interest[18,19].
Several studies indicated that uric acid had excellent immune adjuvant activity, which could promote the specific immune response to vaccines efficiently[9,11]. Shi and colleagues have shown that the generation of responses from specific CTL activity was significantly enhanced, when uric acid was injected into mice along with the gp120 protein of the human immunodeficiency virus (HIV)[9]. Hu and colleagues showed that eliminating uric acid, by administration of allopurinol or uricase, delayed tumor immune rejection, whereas subcutaneous administration of uric acid enhanced the rejection process[11].
Uric acid crystals might be the biologically active form. It was shown that preformed crystals were highly stimulatory, whereas soluble uric acid was not[9-11]. The concentrations of uric acid that stimulated DCs corresponded to one at which uric acid crystals were precipitated. Injection of purified uric acid (> 70 μg /mL) was shown to boost CTL responses in spleen cells isolated from mice, which had been primed with particulate antigens, by triggering increased DC expression of the costimulatory molecules CD86 and CD80[9,11]. Allopurinol and uricase treatment, which substantially reduced plasma uric acid concentrations, was shown to markedly inhibit this T-cell priming. Uric acid crystals are known to stimulate monocytes to produce inflammatory mediators[20], and it seems likely that DCs are stimulated in a similar way.
As highly specialized antigen presenting cells (APC), DCs play a central role in antigen presentation to CD4+ or CD8+ T cells and allogeneic T cell proliferation[8]. It has been known that phenotypic and functional maturation was critical for DCs to activate immune responses effectively[21]. In a previous study, it was reported that uric acid could promote expression of co-stimulatory molecules (CD86, CD80) on DC surfaces[9]. Our data showed that uric acid promoted maturation of DCs (CD83high) and up-regulated the expression of co-stimulatory molecules CD86, and IA/IE (MHC-I molecule).
DCs have a crucial role in determining the type of T cell mediated response[22,23]. IL-12 is an important immune modulatory molecule, which specifically promotes Th1 cell differentiation and suppresses Th2 cell function, and induces a Th1 cell immune response[24]. In this study, uric acid could promote DCs to secrete IL-12p70 in vitro; after combination of immunization with uric acid, in spleen cells of mice, production of IFN-γ was significantly up-regulated,and IL-4 production was down-regulated. This indicated that uric acid might enhance Th1 cell immune responses by promoting DC to secrete IL-12. And then Th1 cells can induce the proliferation of CTLs and amplification of CD8+ T cell responses[25].
In addition, we showed that combination immunization of uric acid and HBsAg-pulsed-DCs could elicit a strong T cell-mediated immune response. Compared with HBsAg-pulsed-DCs vaccine alone, combination immunization elicited significantly greater T cell immune responses as evidenced by T cell proliferation to HBsAg re-stimulation, Th1 cytokine secretion and HBsAg-specific CTL responses. Uric acid may enhance the T cell immune responses by stimulating DC maturation and enhance its functions.
In hyperuricemia, it is well-known that uric acid can precipitate in the joints, where they cause gout, and/or in other tissues causing inflammation[26]. Therefore, the dose of uric acid administration is of crucial importance. In our study, the dose of uric acid was 200 μg. According to Shi and Hu et al[9,11], this dose of uric acid was safe and had an adjuvant effect.
We immunized both treated and control mice with 200 μg uric acid alone for two weeks. As expected, the T cell mediated immune responses were not enhanced. It demonstrated that uric acid has no adjuvant activity in the absence of exogenous antigens. It is important that no autoimmunity is induced, using uric acid as an adjuvant of vaccine.
In the murine model, combination of uric acid and HBsAg-pulsed-DCs seemed to be very effective. However, the anti-HBV effect of this vaccine strategy must be tested further in the HBV animal model.
In summary, we have demonstrated that uric acid can strongly enhance T cell immune responses to HBsAg-pulsed-DCs. We conclude that uric acid might serve as an effective adjuvant for DC vaccine against HBV infection. This strategy provides a model to develop therapeutic vaccines against HBV infection.
We are grateful to Professor Zuo-Ya Li, and Guan-Xin Shen for valuable advice on ELISA.
| 1. | Meyer zum Büschenfelde KH. Immunopathology of chronic liver diseases. Verh Dtsch Ges Pathol. 1995;79:186-197. [PubMed] |
| 2. | Bertoletti A, Gehring AJ. The immune response during hepatitis B virus infection. J Gen Virol. 2006;87:1439-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 295] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 3. | Akbar SM, Horiike N, Onji M. Immune therapy including dendritic cell based therapy in chronic hepatitis B virus infection. World J Gastroenterol. 2006;12:2876-2883. [PubMed] |
| 4. | Shimizu Y, Guidotti LG, Fowler P, Chisari FV. Dendritic cell immunization breaks cytotoxic T lymphocyte tolerance in hepatitis B virus transgenic mice. J Immunol. 1998;161:4520-4529. [PubMed] |
| 5. | Chen M, Li YG, Zhang DZ, Wang ZY, Zeng WQ, Shi XF, Guo Y, Guo SH, Ren H. Therapeutic effect of autologous dendritic cell vaccine on patients with chronic hepatitis B: a clinical study. World J Gastroenterol. 2005;11:1806-1808. [PubMed] |
| 6. | Santini SM, Belardelli F. Advances in the use of dendritic cells and new adjuvants for the development of therapeutic vaccines. Stem Cells. 2003;21:495-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109-1118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3910] [Cited by in RCA: 4000] [Article Influence: 125.0] [Reference Citation Analysis (0)] |
| 8. | Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10867] [Cited by in RCA: 10807] [Article Influence: 386.0] [Reference Citation Analysis (0)] |
| 9. | Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1206] [Cited by in RCA: 1248] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 10. | Jerome KR, Corey L. The danger within. N Engl J Med. 2004;350:411-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Hu DE, Moore AM, Thomsen LL, Brindle KM. Uric acid promotes tumor immune rejection. Cancer Res. 2004;64:5059-5062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693-1702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2890] [Cited by in RCA: 3100] [Article Influence: 91.2] [Reference Citation Analysis (0)] |
| 13. | Overwijk WW, Surman DR, Tsung K, Restifo NP. Identification of a Kb-restricted CTL epitope of beta-galactosidase: potential use in development of immunization protocols for "self" antigens. Methods. 1997;12:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Coles RM, Mueller SN, Heath WR, Carbone FR, Brooks AG. Progression of armed CTL from draining lymph node to spleen shortly after localized infection with herpes simplex virus 1. J Immunol. 2002;168:834-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 200] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Salio M, Palmowski MJ, Atzberger A, Hermans IF, Cerundolo V. CpG-matured murine plasmacytoid dendritic cells are capable of in vivo priming of functional CD8 T cell responses to endogenous but not exogenous antigens. J Exp Med. 2004;199:567-579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 155] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 16. | Lyons AB. Analysing cell division in vivo and in vitro using flow cytometric measurement of CFSE dye dilution. J Immunol Methods. 2000;243:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 520] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 17. | Dixon DM, Misfeldt ML. Proliferation of immature T cells within the splenocytes of athymic mice by Pseudomonas exotoxin A. Cell Immunol. 1994;158:71-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Böcher WO, Dekel B, Schwerin W, Geissler M, Hoffmann S, Rohwer A, Arditti F, Cooper A, Bernhard H, Berrebi A. Induction of strong hepatitis B virus (HBV) specific T helper cell and cytotoxic T lymphocyte responses by therapeutic vaccination in the trimera mouse model of chronic HBV infection. Eur J Immunol. 2001;31:2071-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Akbar SM, Furukawa S, Hasebe A, Horiike N, Michitaka K, Onji M. Production and efficacy of a dendritic cell-based therapeutic vaccine for murine chronic hepatitis B virus carrierer. Int J Mol Med. 2004;14:295-299. [PubMed] |
| 20. | Landis RC, Yagnik DR, Florey O, Philippidis P, Emons V, Mason JC, Haskard DO. Safe disposal of inflammatory monosodium urate monohydrate crystals by differentiated macrophages. Arthritis Rheum. 2002;46:3026-3033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 115] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Cella M, Sallusto F, Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol. 1997;9:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 992] [Cited by in RCA: 984] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 22. | Santana MA, Esquivel-Guadarrama F. Cell biology of T cell activation and differentiation. Int Rev Cytol. 2006;250:217-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Tan P, Anasetti C, Hansen JA, Melrose J, Brunvand M, Bradshaw J, Ledbetter JA, Linsley PS. Induction of alloantigen-specific hyporesponsiveness in human T lymphocytes by blocking interaction of CD28 with its natural ligand B7/BB1. J Exp Med. 1993;177:165-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 354] [Cited by in RCA: 386] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 24. | Kourilsky P, Truffa-Bachi P. Cytokine fields and the polarization of the immune response. Trends Immunol. 2001;22:502-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Gately MK, Wolitzky AG, Quinn PM, Chizzonite R. Regulation of human cytolytic lymphocyte responses by interleukin-12. Cell Immunol. 1992;143:127-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 230] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 26. | Gentili A. The advanced imaging of gouty tophi. Curr Rheumatol Rep. 2006;8:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
S- Editor Liu Y L- Editor Zhu LH E- Editor Ma WH