Published online Feb 21, 2007. doi: 10.3748/wjg.v13.i7.1032
Revised: July 3, 2006
Accepted: September 20, 2006
Published online: February 21, 2007
AIM: To examine the factor(s) involved in differentiation of intestinal macrophages (IMACs) using a recently established in vitro model.
METHODS: To test whether soluble or membrane bound factors induce IMAC-differentiation, freshly elutriated monocytes (MO) were incubated with conditioned media or cell membranes of intestinal epithelial cells (IEC) or cultured with IEC in transwell systems. To determine the importance of an active migration of MO, three-dimensional aggregates from a 1:1-mixture of MO and IEC were examined by immunohistochemistry and flow cytometry. Apoptosis was examined by caspase-3 Western blots. Extracellular matrix production in differentiation models was compared by immunohistochemistry.
RESULTS: IMAC differentiation was observed in a complex three-dimensional co-culture model (multicellular spheroid, MCS) with IEC after migration of MO into the spheroids. By co-culture of MO with conditioned media or membrane preparations of IEC no IMAC differentiation was induced. Co-culture of MO with IEC in transwell-cultures, with the two cell populations separated by a membrane also did not result in intestinal-like differentiation of MO. In contrast to IEC-spheroids with immigrating MO in mixed MCS of IEC and MO only a small subpopulation of MO was able to survive the seven day culture period.
CONCLUSION: Intestinal-like differentiation of MO in vitro is only induced in the complex three-dimensional MCS model after immigration of MO indicating a role of cell-matrix and/or cell-cell interactions during the differentiation of IMACs.
-
Citation: Spoettl T, Hausmann M, Menzel K, Piberger H, Herfarth H, Schoelmerich J, Bataille F, Rogler G. Role of soluble factors and three-dimensional culture in
in vitro differentiation of intestinal macrophages. World J Gastroenterol 2007; 13(7): 1032-1041 - URL: https://www.wjgnet.com/1007-9327/full/v13/i7/1032.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i7.1032
Macrophages represent a component of the innate immune system which is of central importance. One of the largest populations of macrophages in the body is intestinal macrophages (IMACs)[1]. They are localized directly underneath the epithelial barrier at the sites of antigen entry, in particular in the sub-epithelial region of the small and large intestine and in the subepithelial domes of Peyer’s patches[2-4]. IMACs constitute 10%-20% of mononuclear cells in the human lamina propria[2,5-8]. They undergo a specific process of differentiation. This specific differentiation is believed to be essential for the specific functions of IMACs in the mucosal innate but also adaptive immune system. As IMACs are central players of both systems, a better understanding of the factors determining their differentiation may allow the definition of new targets for therapeutic interventions during acute and chronic mucosal inflammation. The importance of IMACs is supported by the finding that NOD2/CARD15, the first gene identified to increase susceptibility to Crohn’s disease, is mainly expressed in macrophages in the colonic mucosa[9,10].
The phenotype of IMACs is remarkable: Less than 10% of the macrophages (MACs) isolated from normal colonic mucosa express the typical MO/MAC-specific surface markers CD14, CD16, CD11b, CD11c[11-13]. Furthermore, the expression of co-stimulatory molecules B7-1 (CD80) and B7-2 (CD86) on IMACs is low[14,15]. In addition, the expression of pattern recognition receptor (PRR) toll like receptor (TLR) 2 and TLR 4 is also down-regulated in IMACs on transcriptional and translational levels[16]. The prototypic MO/MAC functions such as generation of superoxide radicals (oxidative burst reaction) are absent in normal mucosal IMACs due to a lack of NADPH-oxidase subunit expression[17], indicating that normal IMACs constitute a non-reactive cell population, which might be important for the induction of tolerance in the intestinal mucosa. A disturbance of the differentiation process followed by a reactive cell type retaining PRRs and activation functions could be followed by chronic inflammation.
Recently we used a three-dimensional co-culture model (multicellular spheroid model, MCS-model) of intestinal epithelial cells (IEC) and monocytes (MO) to induce the in vitro differentiation of intestinal-like macrophages[18]. We demonstrated that IEC clearly play an important role in the differentiation of IMACs. Freshly elutriated MO, which adhered and infiltrated IEC-MCS, changed their phenotype during a seven-day co-culture period[18]. Typical MO/MAC specific surface antigens such as CD14, CD16, CD11b and CD11c, which were detectable on invading cells after 24 h, were down-regulated after seven days. This differentiation was of functional relevance as seen by the loss of LPS-induced IL-1β transcription in IEC-MCS/MO co-cultures compared to control experiments[18]. As the gut specific differentiation of IMACs is of great functional importance and the MCS-model resembled the differentiation process in vitro, we addressed the question of which factor(s) induce the specific IMAC-differentiation.
Little is known about the direct interaction between IEC and MO/MAC. In normal intestinal mucosa tissue IMAC are separated from IEC by the basement membrane[2,4]. Doe and co-workers[19] have localized IMACs beneath the luminal epithelium[19]. It has been shown that IMACs or dendritic cells can transmigrate and return again across the basement membrane[20] and that the basement membrane is as easily permeable for large molecules as complement factors[21]. Martin and co-workers[22] found that murine MO/MAC and IEC are coupled by gap junctions and that gap junctional communication may provide a tool by which inflammatory cells regulate IEC function and vice versa.
In the present study, we aimed to study whether the induction of intestinal like differentiation of MAC is induced by soluble or secreted proteins, whether direct cell-cell interactions are necessary, whether cell-matrix interactions play a major role and whether all MO or just a subpopulation of MO is capable of differentiating into IMACs.
Primary blood MO were obtained by leukapheresis of healthy donors, followed by density gradient centrifugation over Ficoll/Hypaque as described previously[18]. Two intestinal epithelial cell lines (HT-29 and WiDr) and a control cell line of non-intestinal origin (urothelial carcinoma, J82) were used. All cell lines were cultured under standard tissue-culture conditions[23,24]. The isolation of monocytes was approved by the local institutional review board.
Confluent monolayers of IEC lines or the control cell line were incubated with cell culture medium with or without fetal calf serum (FCS). After 48 h the medium was removed, centrifuged and stored at -20°C. Freshly elutriated MO were incubated in conditioned medium for up to seven days.
Immunohistochemical staining was carried out according to the standard alkaline phosphatase anti-alkaline phosphatase (APAAP) or diaminobenzidine (DAB) technique[25]. The following monoclonal antibodies against MO/MAC-antigens were used: anti-CD68 (clone: KP1, Dako, Hamburg, Germany), anti-CD11b (clone: BEAR1, Immunotech, Hamburg, Germany), anti-CD11c (clone: BU15, Immunotech, Hamburg, Germany), anti-CD14 (clone: RMO52, Immunotech, Hamburg, Germany) and anti-CD16 (clone: 3G8, Immunotech, Hamburg, Germany). For detection of extracellular matrix antibodies against fibronectin (clone: 568, Progen, Heidelberg, Germany), laminin (clone: 4C7, Dako, Hamburg, Germany) and collagen IV (clone: CIV 22.(1), Dako, Hamburg, Germany) were used.
Fixed MO were washed with NKH buffer (0.14 mol/L NaCl, 5 mmol/L KCl and 2 mmol/L HEPES, all Merck, Darmstadt, Germany) and unspecific binding was blocked with 10% FCS in NAG buffer (0.1% NaN3, 2 mmol/L HEPES, 0.2% gelatine, all Merck, Darmstadt, Germany and 0.2% BSA, Sigma-Aldrich, Deisenhofen, Germany). Antibodies against CD68, CD14, CD16 and β2-microglobuline (anti-β2M, Dianova, Hamburg, Germany) as a positive control were applied. After rinsing, rabbit anti-mouse IgG (Dako, Hamburg, Germany) was added and incubated with peroxidase-conjugated goat-anti-rabbit antibody (Immunotech, Hamburg, Germany). Subsequent incubation with 1.2-phenylendiamine-dihydrochloride substrate (OPD, Fluka, Deisenhofen, Germany) for exact 12 min resulted in a yellow color. The reaction was stopped with 1 mol/L H2SO4 (Merck, Darmstadt, Germany) and the extinction was determined photometrically. As a reference value the extinction of ß2M was set 1. All measured values were standardized on the β2M-extinction.
A total of 100 000 MO per well were grown either in conditioned media of IEC or in control media in 96-well plates for seven days. A colorimetric assay (MTS, Endogen, Woburn, Germany) for quantification of cell proliferation and cell viability was performed according to the manufacturer’s protocol. The absorption was measured 8 h after addition of the MTS-labeling mixture.
IEC or control cells were washed with PBS, resuspended in homogenization buffer (Tris 50 mmol/L, EDTA 1 mmol/L, PMSF 1 mmol/L, benzamidine 1 mmol/L, saccharose 0.25 mmol/L) and lyzed by sonification. Cell membranes were isolated in three subsequent centrifugation steps (400 ×g for 10 min, 8500 ×g for 10 min, 25 000 ×g for 30 min). During the third centrifugation step, the cell membranes were sedimented, re-suspended in PBS and incubated in 96-well plates to allow adherence to the plastic surface. After 30 min the supernatants were removed and replaced by a suspension of freshly elutriated MO.
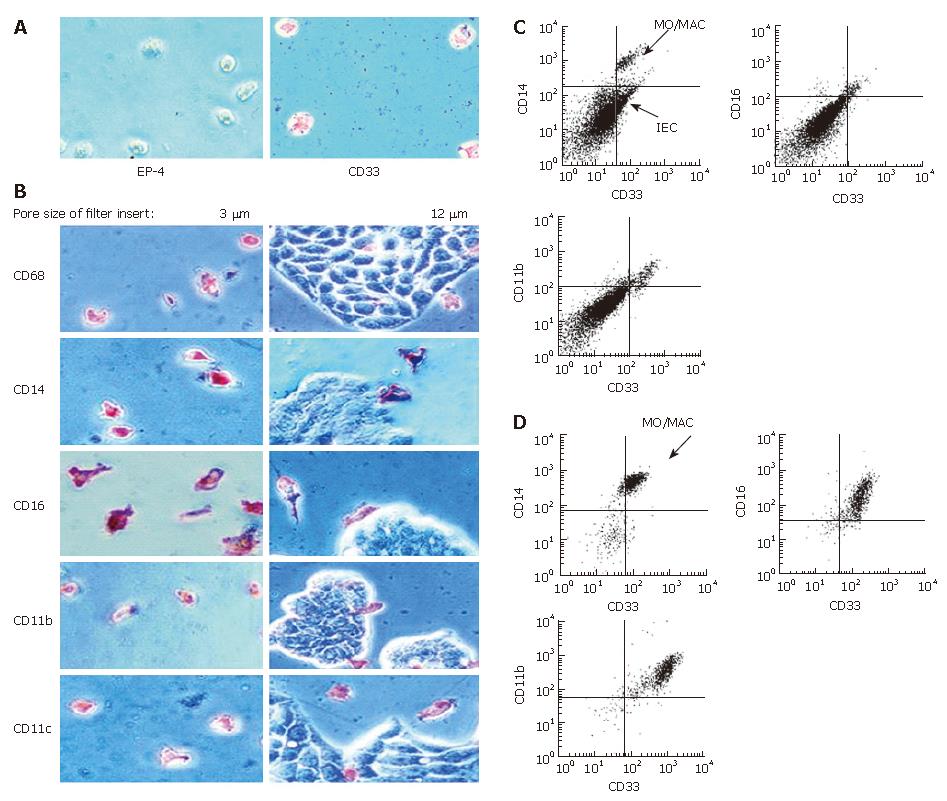
IEC were seeded onto filter inserts with a pore size of 12 μm or 3 μm (preventing IEC from transmigration through the membranes). After formation of an IEC-monolayer the supernatant was removed and freshly elutriated MO in medium supplemented with 2% of human AB-serum were added to each filter insert. After seven days of incubation migrated cells were fixed for immunohistochemistry. Cells in suspension were collected separately and subjected to flow cytometrical analysis.
MCS from only IEC or from a 1:1 mixture of IEC and MO were generated according to the liquid overlay culture technique[18]. Mixed spheroids were also generated with addition of a blocking anti-Fas antibody (Upstate Biotechnology, Lake Placid, USA) to the cell suspension 30 min before seeding.
Flow cytometry was performed using a Coulter EPICS® XL-MCL (Coulter, Krefeld, Germany). Cells were double stained with a FITC-conjugated anti-CD14 antibody (clone Tük4, Coulter, Krefeld, Germany) and a PE-conjugated anti-CD33 antibody (clone MY9, Coulter, Krefeld, Germany) as described previously.
Data acquisition and analysis were performed using WIN-MDI software (http://facs.scripps.edu/help/html/).
Cells were resuspended in RIPA buffer (1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 mmol/L Na3VO4, 50 mmol/L NaF and 1 tablet of complete proteinase inhibitor cocktail [Boehringer, Mannheim, Germany] per 50 mL PBS) for 10 min on ice and centrifuged (12 000 ×g for 15 min at 4°C). The protein concentration of the supernatant (protein fraction) was determined by BCA protein assay (Sigma-Aldrich Chemie, Deisenhofen, Germany). Thirty μg of protein was mixed with an equivalent volume of 2 × protein loading buffer containing 2-β-mercaptoethanol and boiled for 5 min before it was loaded onto SDS polyacrylamide gels. After electrophoresis, proteins were transferred onto nitro-cellulose membranes using the Xcell blot module (Invitrogen BV/NOVEX; Gronigen, Netherlands) and blocked in TBST (50 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 0.05% Tween 20) containing 5% non-fat dry milk powder. Protein immunoblots were performed using specific antibodies to caspase-3 (clone 19, Transduction Laboratories, Lexington, USA) and β-actin (clone JLA20, Calbiochem, Cambridge, USA). The membranes were further incubated with peroxidase-conjugated secondary antibodies and protein bands were visualized using a chemoluminescence kit (ECL Plus™, Amersham, Buckinghamshire, UK) according to the manufacturer’s protocol.
Recently we demonstrated in vitro differentiation of MO into IMACs in complex three- dimensional co-culture models (MCS model) with IEC after migration of MO into the IEC complexes. Here we further studied whether soluble factors or cell-cell interactions might be more relevant to this differentiation.
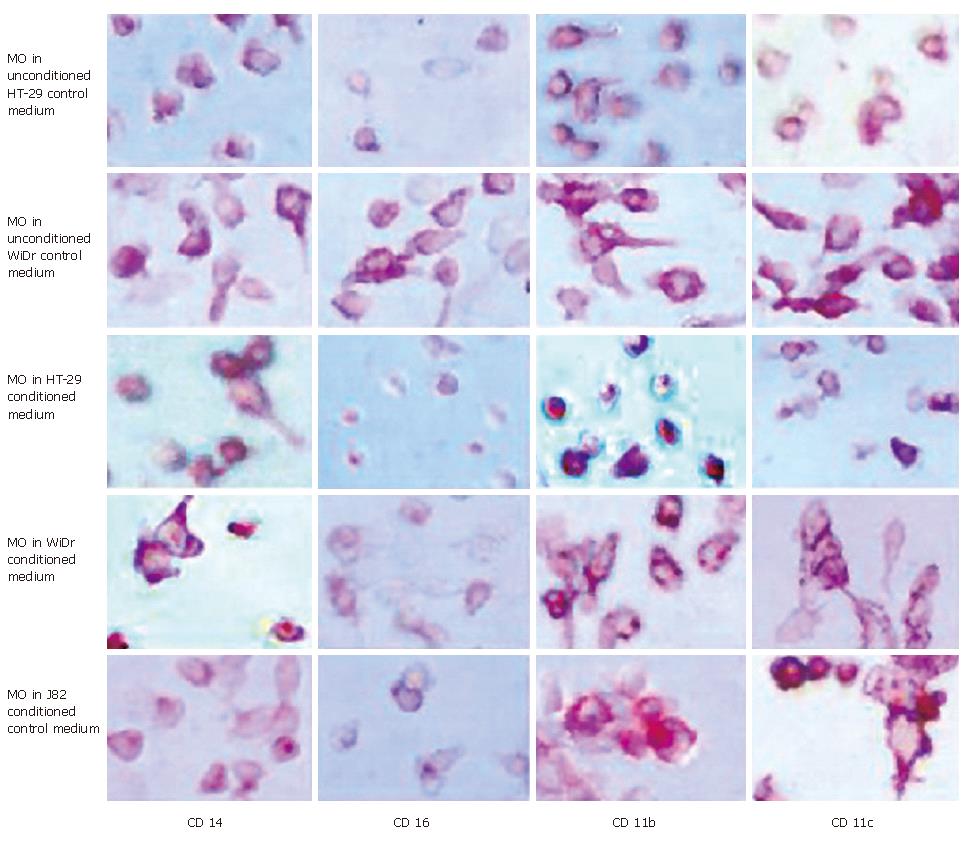
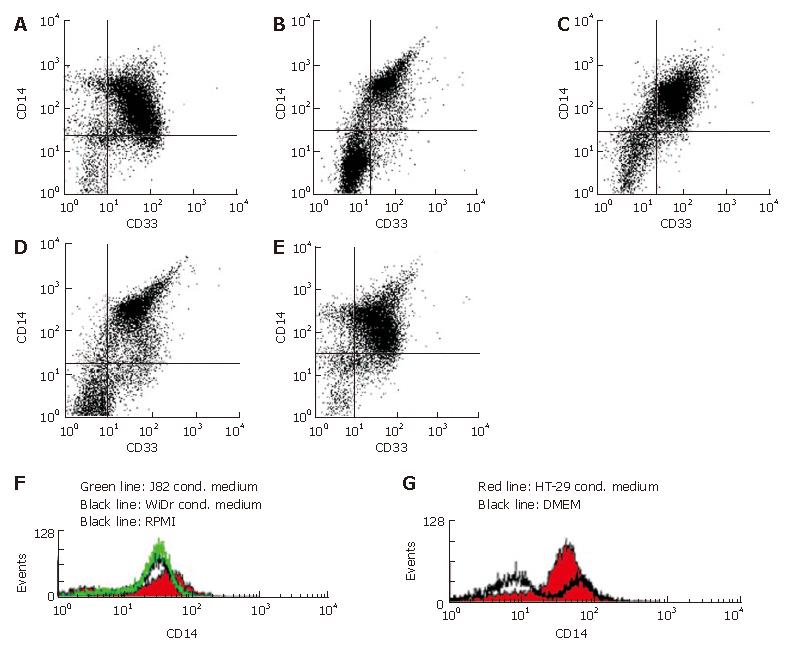
To test whether soluble factors secreted by IEC induce the intestinal-like differentiation of MO, freshly elutriated MO were incubated with IEC-conditioned medium for seven days. Immunohistochemical analysis of MO showed no intestinal-like differentiation. CD14, CD16, CD11b and CD11c, which are down-regulated during differentiation of IMACs and therefore absent on MAC from normal non-inflamed mucosa, were all detectable. CD14 was expressed by 70%-80%, CD16 by 50%-60%, CD11b by 80%-90% and CD11c by 90%-100% of the MO/MAC incubated in HT-29-conditioned medium for seven days. Same results obtained with conditioned medium of the second tested IEC line WiDr were not significantly different from the values obtained with the MO/MAC incubated in control media (Figure 1). When cells were analysed by flow cytometry these findings were confirmed as no down-regulation of CD14, CD16, CD11b and CD80 expression could be observed in MO after seven-day co-culture with IEC-conditioned media (CD14 expression shown in Figure 2).
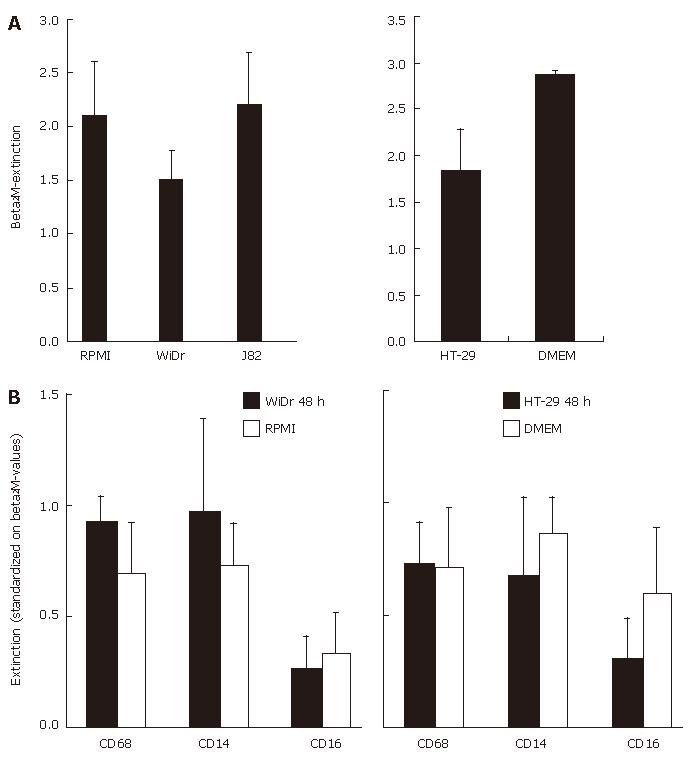
The results were further confirmed and quantified using the cell-ELISA technique able to detect minor changes in antigen expression. As a positive control and for reference values we determined the expression of the MAC housekeeping gene β2M (Figure 3A). All other values were standardized in relation to ß2M-expression. The antigen expression of MO/MAC, incubated in IEC-conditioned medium did not differ significantly from that of cells, incubated in control medium. The expression of CD16 was always slightly decreased, but could also be observed in control experiments and was not specific for IEC-conditioned media (Figure 3B).
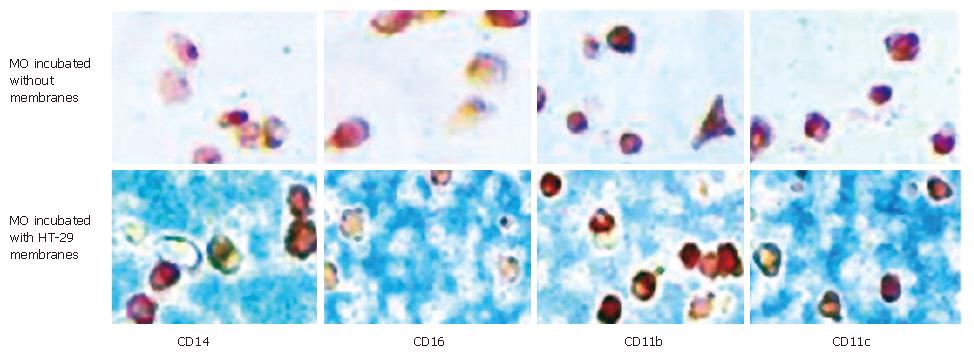
To determine whether membrane-bound factors of IEC induce the intestinal-like differentiation of MO, freshly elutriated blood MO were co-cultured with membrane preparations of IEC as described in Materials and Methods. After an incubation period of seven days MO co-cultured with IEC-membranes showed no differentiation into intestinal-like MAC. The tested antigens CD14, CD16, CD11b and CD11c were still detectable after seven days of culture of MO/MAC together with membrane preparations of the IEC line HT-29. MO/MAC incubated for seven days without membrane preparations showed the same pattern of antigen expression (Figure 4).
Same results were obtained with membranes of a second intestinal epithelial cell line (WiDr) and the control cell line J82 (data not shown).
To test whether a short direct contact between MO and IEC is able to induce intestinal-like differentiation of MO, we incubated MO and IEC in so called “transwell-cultures”. MO were added to IEC grown in filter inserts. When filters with a pore size of three μm were used, only MO were able to migrate through the membrane as confirmed by negative staining for the epithelial cell marker EP-4 and positive staining for the MO/MAC-marker CD33 (Figure 5A). Twelve-μm long filters allowed also IEC to migrate through the membranes. IEC could be easily distinguished from MO/MAC by morphology and showed no expression of the tested MO/MAC-specific antigens. MO either migrated through the IEC layer or stayed in the upper compartment of transwell-culture. Antigen expression of cells adherent to the plastic dishes after transmigration was examined by immunohistochemistry. MO/MAC were all positive for the intracellular MO/MAC marker CD68 and showed a high expression of CD14, CD16, CD11b and CD11c after the seven day culture period (Figure 5B). Non adherent cells from the upper or lower compartment of the filter insert were analysed by flow cytometry and showed a similar antigen pattern with high CD14, CD16 and CD11b expressions (Figure 5C and D).
To test whether the process of invading the three-dime-nsional IEC-spheroids is necessary for the differentiation of MO into IMACs, we generated “mixed spheroids” from a 1:1-mixture of IEC and MO. In these experiments MO were added during the generation of MCS and did not invade the three-dimensional aggregates.
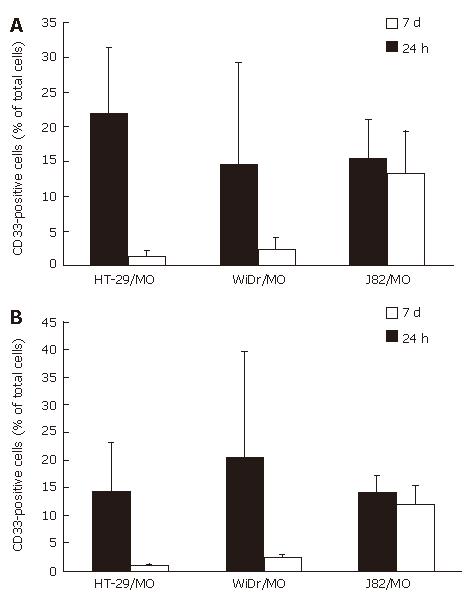
Flow cytometrical analysis showed 15.2% MO/MAC inside spheroids of the control cell line J82 after 24 h of co-culture. This percentage was nearly constant during a seven-day culture period (13.2%, d 7, n = 5). In spheroids with the IEC line HT-29 a different effect could be observed. After 24 h 21.7% MO/MAC inside the spheroids could be detected. On day seven of co-culture the percentage of MO/MAC inside the spheroids decreased to 1.4% (n = 4). Similar results could be obtained with spheroids of the IEC line WiDr. The percentage of MO/MAC inside the spheroids decreased from 14.6% (24 h) to 2.4% (d 7, n = 4) (Figure 6A). Remaining MO/MAC inside the aggregates showed no differentiation into IMACs. The results showed that active invasion of the aggregates by MO was an essential step in the process of IMAC differentiation.
To test whether the observed decrease in the relative amount of MO in “mixed spheroids” is due to Fas-induced apoptosis, we added a blocking anti-Fas antibody to the cell suspensions before generating “mixed spheroids”. Addition of the anti-Fas antibody did not change the results significantly. The number of MO/MAC decreased from 14.2% (24 h) to 0.8% (7d) in HT-29 MCS and from 20% (24 h) to 2.3% (7d) in WiDr spheroids. In control experiments with J82 spheroids the number of MO/MAC was almost constant with 13.9% at 24 h and 12% on day seven (n = 3) (Figure 6B).
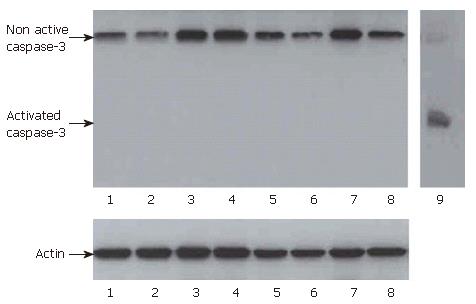
In addition, Western-blots for caspase-3 were performed. After 24 h and three days no activated caspase-3 was detected in ”mixed MCS” of IEC and MO or in control cells and MO (Figure 7).
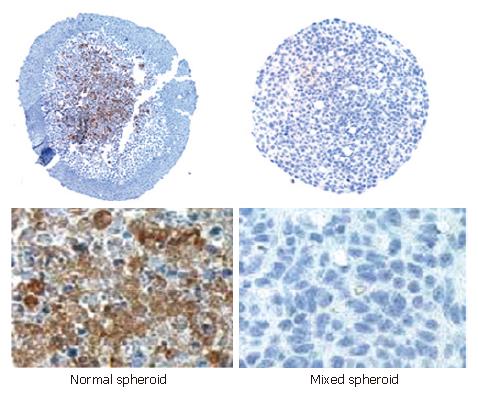
The expression of extracellular matrix (ECM) proteins (fibronectin, laminin and collagen IV) in the “normal” and mixed spheroids was determined by immunohistochemistry. Laminin and collagen IV were not detectable in both mixed spheroids and spheroids invaded by MO. In contrast, a strong expression of fibronectin could be detected in spheroids invaded by MO, which was up-regulated from three to seven days of culture and was absent in mixed spheroids incubated for the same time. The expression of fibronectin was localized in the inner region of aggregates (Figure 8).
As IMACs are essential players in local immune responses and the innate immune system of intestinal barrier, their specific phenotype must be of importance. Compared to IMACs from inflammatory bowel disease (IBD) patients, IMACs from normal intestinal mucosa show a down-regulation of several surface markers, co-stimulatory molecules and proteins necessary for LPS-induced signal transduction[11-13,16], which may be responsible for the induction of tolerance.
Recently we have shown that MO differentiate into IMACs after immigration into the MCS co-culture with IEC in vitro[18]. In this study, factor(s) inducing IMAC differentiation in vitro were analysed. We showed that the specific IMAC differentiation was not mediated by soluble or membrane bound factors of IEC alone, as no intestinal-like differentiation was observed in freshly elutriated MO cultured together with IEC-conditioned medium or IEC-membranes. Also “weak interactions” between MO and IEC in trans-well cultures, in which MO and IEC (monolayers) were only separated by filter membranes, were not sufficient for differentiation. IMAC differentiation could only be induced in the complex three-dimensional MCS model with close contact between MO, IEC and ECM[18]. Loss of MO observed in 1:1 “mixed spheroids” of the IEC lines HT-29 and WiDr was obviously not due to apoptosis, as we could neither block this effect by adding an anti-Fas antibody nor detect activated caspase-3 during the incubation period.
Although MCS do not resemble the in vivo situation where IMACs are separated from the IEC by the basement membrane, many conditions such as O2-gradient, pH or ECM production are similar to those in the body[26-28]. In MCS, cells are also able to form cell-cell and cell-matrix contacts which are found in vivo[29-31] and may therefore be regarded as a useful model to study the important aspects of IMAC differentiation.
In general, interactions between different cell types, special cytokine milieus and contact with components of the ECM can trigger cells to develop a special phenotype. Hohn and co-workers[32] showed that components of the basement membrane and ECM are involved in choriocarcinoma cell differentiation. Hanspal et al[33] have demonstrated the importance of cell-cell interactions during the regulation of erythropoiesis. The ECM protein, vitronectin, controls the differentiation of cerebellar granular cells[34]. It was reported that ECM also plays a role in the differentiation of embryonic stem cells[35] and skeletal muscle cells[36]. Armstrong et al[37] have shown that ECM proteins have effects on MAC differentiation, growth and function.
Jacob et al[38] induced differentiation of MO to MAC in vitro by culturing MO on different ECM protein substrates, and found that specific markers associated with differentiation are changes in the expression of cell surface antigens. FACS analysis showed a down-regulation of CD14 occurring in a substrate dependent manner, which was the highest in MO maintained on fibronectin in their study[38].
In our experiments, MCS invaded by MO and cultured for seven days contained a large amount of ECM protein fibronectin. In addition, the expression of fibronectin was up-regulated during the culture period, which is correlated with the previously described down-regulation of CD14 and other MO-specific surface markers[18]. In mixed spheroids no fibronectin expression could be detected, which could be due to a stronger degradation of ECM proteins by the larger amount of MO added or a lack of synthesis induction. Fibronectin present in the described co-culture model seems to be an important factor contributing to the in vitro differentiation of IMACs as well as the active invasion of MO into the three-dimensional aggregates, indicating that only a subpopulation of blood MO is able to differentiate into IMACs.
The loss of MO in the “mixed spheroids” could have several reasons. First, differentiation into the intestinal phenotype is necessary for survival in the epithelial cell environment. Second, the higher number of mononuclear cells in the mixed spheroids compared to the model with MO invasion induces factors that lead to cell death or prevent the synthesis of survival factors. Third, we cannot exclude that this is a self protection effect of the tumor cell lines used and that MO only enter the MCS in the MCS model.
We cannot exclude that there is a pre-primed subpopulation of MO in the peripheral blood that is especially suited to get in contact with IEC. According to such a hypothesis only this pre-primed subpopulation would enter the MCS model or the mucosa and further differentiate into IMACs. This natural selection would not be observed in co-cultures of all elutriated peripheral MO with conditioned media, membrane preparations or in mixed spheroids. The number of differentiating cells would be too low to reach a significant difference. In the present study, we investigated this possibility of a pre-primed subpopulation of MO suited for IMAC differentiation.
To understand the differentiation process of IMACs in healthy individuals may further help to find an approach for the therapy of IBD. Local induction of a tolerogenic and anergic IMAC cell type could down-regulate or stop mucosal inflammation. A therapeutic approach inducing differentiation of this cell type would be one step up the inflammatory cascade not aimed at T-cells but at the site of the first contact with antigen entry in the mucosa.
| 1. | Lee SH, Starkey PM, Gordon S. Quantitative analysis of total macrophage content in adult mouse tissues. Immunochemical studies with monoclonal antibody F4/80. J Exp Med. 1985;161:475-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 335] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 2. | Pavli P, Doe WF. Intestinal macrophages. Inflammatory bowel disease. New York: Elsevier 1992; 177-188. |
| 3. | LeFevre ME, Hammer R, Joel DD. Macrophages of the mammalian small intestine: a review. J Reticuloendothel Soc. 1979;26:553-573. [PubMed] |
| 4. | Bockman DE, Boydston WR, Beezhold DH. The role of epithelial cells in gut-associated immune reactivity. Ann N Y Acad Sci. 1983;409:129-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 47] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Bull DM, Bookman MA. Isolation and functional characterization of human intestinal mucosal lymphoid cells. J Clin Invest. 1977;59:966-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 317] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Donnellan WL. The structure of the colonic mucosa. The epithelium and subepithelial reticulohistiocytic complex. Gastroenterology. 1965;49:496-514. [PubMed] |
| 7. | Golder JP, Doe WF. Isolation and preliminary characterization of human intestinal macrophages. Gastroenterology. 1983;84:795-802. [PubMed] |
| 8. | Pavli P, Woodhams CE, Doe WF, Hume DA. Isolation and characterization of antigen-presenting dendritic cells from the mouse intestinal lamina propria. Immunology. 1990;70:40-47. [PubMed] |
| 9. | Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4223] [Cited by in RCA: 3932] [Article Influence: 157.3] [Reference Citation Analysis (0)] |
| 10. | Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3555] [Cited by in RCA: 3498] [Article Influence: 139.9] [Reference Citation Analysis (1)] |
| 11. | Andus T, Rogler G, Daig R, Falk W, Schölmerich J, Gross V. The role of macrophages. Inflammatory bowel disease. The Netherlands: Kluwer Dordrecht 1995; 281-297. |
| 12. | Rogler G, Hausmann M, Vogl D, Aschenbrenner E, Andus T, Falk W, Andreesen R, Schölmerich J, Gross V. Isolation and phenotypic characterization of colonic macrophages. Clin Exp Immunol. 1998;112:205-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 143] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Rogler G, Andus T, Aschenbrenner E, Vogl D, Falk W, Schölmerich J, Gross V. Alterations of the phenotype of colonic macrophages in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1997;9:893-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Rugtveit J, Bakka A, Brandtzaeg P. Differential distribution of B7.1 (CD80) and B7.2 (CD86) costimulatory molecules on mucosal macrophage subsets in human inflammatory bowel disease (IBD). Clin Exp Immunol. 1997;110:104-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 89] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Barbosa IL, Gant VA, Hamblin AS. Alveolar macrophages from patients with bronchogenic carcinoma and sarcoidosis similarly express monocyte antigens. Clin Exp Immunol. 1991;86:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Hausmann M, Kiessling S, Mestermann S, Webb G, Spöttl T, Andus T, Schölmerich J, Herfarth H, Ray K, Falk W. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122:1987-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 375] [Article Influence: 15.6] [Reference Citation Analysis (2)] |
| 17. | Hausmann M, Spöttl T, Andus T, Rothe G, Falk W, Schölmerich J, Herfarth H, Rogler G. Subtractive screening reveals up-regulation of NADPH oxidase expression in Crohn's disease intestinal macrophages. Clin Exp Immunol. 2001;125:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Spöttl T, Hausmann M, Kreutz M, Peuker A, Vogl D, Schölmerich J, Falk W, Andreesen R, Andus T, Herfarth H. Monocyte differentiation in intestine-like macrophage phenotype induced by epithelial cells. J Leukoc Biol. 2001;70:241-251. [PubMed] |
| 19. | Pavli P, Maxwell L, Van de Pol E, Doe F. Distribution of human colonic dendritic cells and macrophages. Clin Exp Immunol. 1996;104:124-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | D'Amico G, Bianchi G, Bernasconi S, Bersani L, Piemonti L, Sozzani S, Mantovani A, Allavena P. Adhesion, transendothelial migration, and reverse transmigration of in vitro cultured dendritic cells. Blood. 1998;92:207-214. [PubMed] |
| 21. | Fujigaki Y, Nagase M, Kojima K, Yamamoto T, Hishida A. Glomerular handling of immune complex in the acute phase of active in situ immune complex glomerulonephritis employing cationized ferritin in rats. Ultrastructural localization of immune complex, complements and inflammatory cells. Virchows Arch. 1997;431:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Martin CA, Homaidan FR, Palaia T, Burakoff R, el-Sabban ME. Gap junctional communication between murine macrophages and intestinal epithelial cell lines. Cell Adhes Commun. 1998;5:437-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Marshall CJ, Franks LM, Carbonell AW. Markers of neoplastic transformation in epithelial cell lines derived from human carcinomas. J Natl Cancer Inst. 1977;58:1743-1751. [PubMed] |
| 24. | Noguchi P, Wallace R, Johnson J, Earley EM, O'Brien S, Ferrone S, Pellegrino MA, Milstien J, Needy C, Browne W. Characterization of the WIDR: a human colon carcinoma cell line. In Vitro. 1979;15:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 108] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Aigner A, Neumann S. Immunchemie: Grundlagen, Anwendungen, Perspektiven. Jena: Gustav Fischer 1997; . |
| 26. | Inch WR, McCredie JA, Sutherland RM. Growth of nodular carcinomas in rodents compared with multi-cell spheroids in tissue culture. Growth. 1970;34:271-282. [PubMed] |
| 27. | Mueller-Klieser W. Multicellular spheroids. A review on cellular aggregates in cancer research. J Cancer Res Clin Oncol. 1987;113:101-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 290] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 28. | Konur A, Kreutz M, Knüchel R, Krause SW, Andreesen R. Cytokine repertoire during maturation of monocytes to macrophages within spheroids of malignant and non-malignant urothelial cell lines. Int J Cancer. 1998;78:648-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Sutherland RM. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science. 1988;240:177-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1301] [Cited by in RCA: 1230] [Article Influence: 32.4] [Reference Citation Analysis (14)] |
| 30. | Olive PL, Durand RE. Drug and radiation resistance in spheroids: cell contact and kinetics. Cancer Metastasis Rev. 1994;13:121-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 182] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 31. | Davies CD, Müller H, Hagen I, Gårseth M, Hjelstuen MH. Comparison of extracellular matrix in human osteosarcomas and melanomas growing as xenografts, multicellular spheroids, and monolayer cultures. Anticancer Res. 1997;17:4317-4326. [PubMed] |
| 32. | Hohn HP, Grümmer R, Bosserhoff S, Graf-Lingnau S, Reuss B, Bäcker C, Denker HW. The role of matrix contact and of cell-cell interactions in choriocarcinoma cell differentiation. Eur J Cell Biol. 1996;69:76-85. [PubMed] |
| 33. | Hanspal M. Importance of cell-cell interactions in regulation of erythropoiesis. Curr Opin Hematol. 1997;4:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Wechsler-Reya RJ. Caught in the matrix: how vitronectin controls neuronal differentiation. Trends Neurosci. 2001;24:680-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Czyz J, Wobus A. Embryonic stem cell differentiation: the role of extracellular factors. Differentiation. 2001;68:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 171] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 36. | Osses N, Brandan E. ECM is required for skeletal muscle differentiation independently of muscle regulatory factor expression. Am J Physiol Cell Physiol. 2002;282:C383-C394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 134] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 37. | Armstrong JW, Chapes SK. Effects of extracellular matrix proteins on macrophage differentiation, growth, and function: comparison of liquid and agar culture systems. J Exp Zool. 1994;269:178-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | Jacob SS, Shastry P, Sudhakaran PR. Monocyte-macrophage differentiation in vitro: modulation by extracellular matrix protein substratum. Mol Cell Biochem. 2002;233:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
S- Editor Liu Y L- Editor Wang XL E- Editor Ma WH