Published online Feb 21, 2007. doi: 10.3748/wjg.v13.i7.1003
Revised: December 25, 2006
Accepted: January 15, 2007
Published online: February 21, 2007
AIM: To evaluate the outcome predictors of percu-taneous ablation therapy in patients with unresectable hepatocellular carcinoma (HCC), especially to identify whether the initial treatment response contributes to the survival of the patients.
METHODS: The study cohort included 153 patients with single (102) and two or three (51) HCC nodules 5 cm or less in maximum diameter. As an initial treatment, 110 patients received radiofrequency ablation and 43 patients received percutaneous ethanol injection.
RESULTS: The Kaplan-Meier estimates of overall 3- and 5-year survival rates were 75% and 59%, respectively. The log-rank test revealed statistically significant differences in the overall survivals according to Child-Pugh class (P = 0.0275), tumor size (P = 0.0130), serum albumin level (P = 0.0060), serum protein induced by vitamin K absence or antagonist II level (P = 0.0486), and initial treatment response (P = 0.0130). The independent predictors of survival were serum albumin level (risk ratio, 3.216; 95% CI, 1.407-7.353; P = 0.0056) and initial treatment response (risk ratio, 2.474; 95% CI, 1.076-5.692; P = 0.0330) based on the Cox proportional hazards regression models. The patients had a serum albumin level 3.5 g/dL and the 3- and 5-year survival rates of 86% and 82%.
CONCLUSION: In HCC patients treated with percutaneous ablation therapy, serum albumin level and initial treatment response are the independent outcome predictors.
- Citation: Morimoto M, Numata K, Sugimori K, Shirato K, Kokawa A, Oka H, Hirasawa K, Koh R, Nihommatsu H, Tanaka K. Successful initial ablation therapy contributes to survival in patients with hepatocellular carcinoma. World J Gastroenterol 2007; 13(7): 1003-1009
- URL: https://www.wjgnet.com/1007-9327/full/v13/i7/1003.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i7.1003
Hepatocellular carcinoma (HCC) is one of the most prevalent human cancers with an increasing incidence worldwide[1], and about 70% of HCC is found in Asia[2]. Surveillance with ultrasonography (US) and alpha-fetoprotein in cirrhosis can detect small HCC at an early stage. For early stage patients (single HCC ≤ 5 cm or ≤ 3 nodules ≤ 3 cm), surgery is considered the first treatment option, however, because of accompanying chronic liver disease, many HCC patients can not undergo surgical resection[3-5]. As non-surgical treatment, various local ablation therapies such as percutaneous ethanol injection (PEI)[6,7] or percutaneous radiofrequency (RF) ablation have been proposed, and encouraging results of survival rates have been reported[8-10].
Although many reports of the prognostic predictors after surgical resection of early stage HCC have been reported[11-13], there have been few reports of the prognostic predictors after percutaneous ablation therapy. Sala et al in recent years reported that “Child-Pugh class” and “initial treatment response” as prognostic factors of the survival in those who received percutaneous ablation therapy[14]; however, most of the cases (83%) were treated with PEI. The current state of the main percutaneous ablation therapy changes from PEI to RF ablation; therefore, to establish an optimal therapeutic strategy based on the current state, we started this cohort study after 2000 when RF ablation was introduced in our institution.
In this study, we examined a group of HCC patients whose tumors were percutaneously treated using RF ablation or PEI and analyzed the factors pertinent to the prognosis. This approach permits (1) establishment of an optimal protocol in the percutaneous ablation therapy; and (2) assessment of the prognostic factors in RF ablation and PEI.
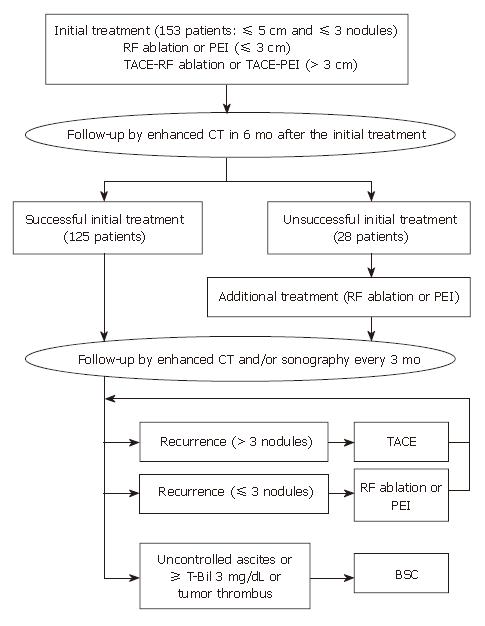
Between May 2000 and March 2005, 226 patients were diagnosed for the first time as having HCC lesions using US and contrast-enhanced CT and were hospitalized at Yokohama City University Medical Center. The criteria for entry in this study were (1) the presence of either a solitary lesion or up to three lesions, (2) a maximum tumor diameter of 5 cm or less, (3) patients who did not meet with the surgical criteria (resection or transplantation), (4) the lesion was detectable using US, and (5) no evidence existing of portal thrombosis, extrahepatic metastasis, or uncontrollable ascites. Seventy-three patients were excluded from the study and 153 patients were enrolled. The patients’ characteristics are depicted in Table 1. Ninety-three of the enrolled patients were men and 60 were women, ranging in age from 51 to 87 years (mean, 69 years). One hundred and thirty-three patients were Child-Pugh class A, and 20 patients were Child-Pugh class B. All had underlying cirrhosis due to hepatitis C virus (n = 134), hepatitis B virus (n = 8), alcohol use (n = 7), or other factors (n = 4). One hundred and two patients presented with solitary tumors and 51 patients had 2-3 lesions. The greatest tumor diameters ≤ 3 cm were seen in 120 patients, and > 3 cm in 33 patients. A confirmed diagnosis of HCC was made by the pathological examination of biopsied specimens obtained using a 21-gauge fine needle (Sonopsy, Hakko, Tokyo, Japan) and/or the radiological criteria[15] in all patients. Patients with a tumor more than 3 cm in maximum diameter were treated with transcatheter arterial chemoembolization (TACE) followed by RF ablation or PEI.
| Variables | All patients | PEI | RF ablation | P |
| (n = 153) | (n = 43) | (n = 110) | ||
| Age (yr) | ||||
| mean ± SD | 69 ± 7 | 69 ± 7 | 68 ± 7 | NS |
| Sex | ||||
| Male/Female | 93/60 | 22/21 | 71/39 | NS |
| Etiology | ||||
| HCV | 134 | 38 | 96 | |
| HBV | 8 | 1 | 7 | NS |
| Alcohol | 7 | 2 | 5 | |
| Others | 4 | 2 | 2 | |
| Child-Pugh class | ||||
| A/B | 133/20 | 37/6 | 96/14 | NS |
| Tumor size (cm) | ||||
| ≤ 3/> 3 | 120/33 | 33/10 | 87/23 | NS |
| Tumor number | ||||
| Single/Multiple | 102/51 | 19/24 | 83/27 | < 0.05 |
| Serum alpha-fetoprotein level (ng/mL) | ||||
| mean ± SD | 781 ± 8180 | 2501 ± 15289 | 109 ± 282 | NS |
| Serum PIVKA-II level (ng/mL) | ||||
| mean ± SD | 347 ± 1258 | 565 ± 1467 | 262 ± 1169 | NS |
| Serum albumin level (g/dL) | ||||
| mean ± SD | 3.8 ± 0.5 | 3.7 ± 0.5 | 3.8 ± 0.5 | NS |
| Initial treatment response | ||||
| Successful/Unsuccessful | 125/28 | 35/8 | 90/20 | NS |
The entire protocol was approved by the hospital ethics committee and was performed in compliance with the Helsinki Declaration. Written informed consent was obtained from all patients and relatives.
Percutaneous ethanol injection: Based on the reports of Llovet et al[16] and Livraghi et al[17], we selected PEI without selecting RF ablation when a tumor satisfied the following criteria: (1) existing in a subcapsular location, (2) existing in a location adjacent to a major vessel and another organ (heart, gallbladder, stomach and bowel), and (3) demonstrating poor differentiation. Patients with impaired clotting tests, or with a lower platelet count less than 5 × 1010/L were considered as being contraindicated for RF ablation and were treated by PEI. Ten patients (23% of patients treated by PEI) with tumors more than 3 cm in diameter were treated with TACE followed by PEI[18].
We used a real-time convex scanner or linear-array scanner with 3.5-MHz probes and a lateral attachable apparatus for needle guidance (Core-Vision 6000TM, Toshiba Medical Co., Tokyo, Japan). First, it was confirmed under US guidance that a 15- or 20-cm, 21-gauge puncture needle with a closed conical tip and three terminal side holes (PEIT needle; Hakko, Tokyo, Japan) was correctly positioned within the lesion, and 99.5% absolute ethyl alcohol was then slowly injected. Caution was taken to inject the deepest portions of the lesion first and then the more central and superficial portions, to prevent superficial spreading of the ethanol from masking the view for subsequent injections. We usually used one or more PEIT needles for each treatment session, and ethanol was injected into the tumor at one or more locations until the lesion was completely filled. Treatment was given twice a week. A treatment series usually consisted of six or more sessions, and the total dose of ethanol varied with the volume of the lesion, the texture, patient compliance, and distribution of the ethanol.
Radiofrequency ablation: We selected the RF ablation for the cases with low risks of tumor seeding and the hemorrhage based on the previous reports[16,17,19]. Twenty-three patients (21% of patients treated by RF ablation) with tumors more than 3 cm in diameter were treated with TACE followed by RF ablation[20].
At the beginning, grounding was achieved by attaching 2 or 4 pads to the patient’s thighs. A conscious sedation, consisting of a combination of intramuscular administration of pentazocine (PentazinTM 15 mg, Sankyo Pharmaceuticals, Tokyo, Japan) and hydroxyzine chloride (Atarax-PTM 25 mg, Pfizer Japan, Tokyo, Japan), was administered before treatment. Local anesthesia was achieved by injecting 1% lidocaine hydrochloride (XylocaineTM, Astra Japan, Tokyo, Japan). Sixty-five of 112 patients were treated with hooked, 15-gauge, 25-cm-long electrodes, which are expandable by 10 hooks to a maximum dimension of 3 or 3.5 cm (Le Veen Needle Electrode; Radiotherapeutics, Mountain View, CA), and RF ablation was applied using a generator (RTC 2000; Boston Scientific Japan, Tokyo, Japan). For the remaining 47 tumors, 17-gauge, cooled electrodes with a dimention of 2 or 3 cm (Cool-tip needle; Radionics, Burlington, MA) were used, attached to a 500-kHz RF generator (Radionics, Burlington, MA) capable of producing power of 200 W. A peristaltic pump (Watson-Marlow, Wilmington, MA) was used to infuse 0°C normal saline solution into the lumen of the electrodes to maintain the temperature below 25°C. For tumors more than 2 cm in diameter, several insertions were performed to obtain complete ablation of the entire tumors.
Transcatheter arterial chemoembolization: Thirty-three patients with tumors more than 3 cm in diameter were treated with TACE followed by RF ablation or PEI. We performed TACE by selectively introducing a microcatheter into the right or left hepatic artery or a segmental branch of the hepatic artery and injecting a mixture of iodized oil (Lipiodol; Andre Guerbet, Aulnay-sous-Bois, France) and epirubicin hydrochloride (30-50 mg per body surface) (Farmorubicin; Pharmacia and Upjohn, Tokyo, Japan) or styrene maleic acid neocarzinostatin (1.0-3.0 mg per body surface) (SMANCS; Yamanouchi Pharmaceutical, Tokyo, Japan). This was followed by introduction of a gelatin sponge (1 mm × 1 mm × 1 mm) (Spongel; Yamanouchi Pharmaceutical, Tokyo, Japan).
Initial treatment response was assessed by contrast-enhanced CT 1, 3, and 6 mo after initial treatment session. Successful initial treatment was defined as the absence of an enhanced area within the tumor assessed by contrast enhanced CT 6 mo after initial treatment session. If the presence of residual viable tumor within the treated area was defined by CT within 6 mo after initial treatment, the case was judged to be unsuccessful in the initial treatment. After the evaluation of initial treatment response, patients were followed up every 3 mo by contrast enhanced CT and/or US.
When the residual viable tumor and/or distant recurrence were detected by the follow-up CT, percutaneous ablation therapy was added if they were within 3 lesions, and TACE was selected for patients who were ineligible for percutaneous ablation therapy, with large and/or multifocal HCC who did not have vascular invasion or extrahepatic spread[15]. The above-mentioned treatment was repeated at once whenever the tumor recurrence was detected, until uncontrolled ascites or intravascular tumor thrombus appeared and serum bilirubin level reached 3 mg/dL or higher.
The end point of the study was the survival. The baseline characteristics of patients were expressed as mean ± standard deviation. Differences in proportions among the groups were analyzed by the Chi-square test. Mean quantitative values were compared by the Student’s t test. Follow-up data was dealt with from the beginning of the treatment and was maintained until the death or the last visit of the patients before April 30, 2006. The Kaplan-Meier method was used to calculate the survival rate and the log-rank test was used to analyze differences.
As pretreatment factors, fifteen variables were assessed in the univariate analysis: age (< 70 vs≥ 70 years); sex (male vs female); cause of underlying cirrhosis (HCV vs HBV vs alcohol vs others); Child-Pugh class (A vs B); tumor size (≤ 3 cm vs > 3 cm); tumor number (single vs multiple); serum alpha-fetoprotein levels (<400 vs≥ 400 ng/mL); serum protein induced by vitamin K absence or antagonist II (PIVKA-II) level (< 300 vs≥ 300 ng/mL); and serum albumin level (≤ 3.5 vs > 3.5g/dL); serum alanine aminotransferase (ALT) level (<80 vs≥ 80 U/L); serum bilirubin level (<2 vs≥ 2 mg/dL); serum platelet count (<9 vs≥ 9 × 109/L); prothrombin activity (≤ 70 vs > 70%); encephalopathy (none vs yes); and ascites (none vs yes). As treatment factors, two variables were assessed in the univariate analysis: type of treatment (PEI vs RF ablation); and initial treatment response (successful vs unsuccessful). Significant variables (P < 0.05) were included in a stepwise Cox regression analysis. Data analyses were performed with SPSS software (version 10.0J; SPSS, Tokyo, Japan). All P values were derived from two-tailed tests and P < 0.05 was accepted as statistically significant.
In general, the percutaneous ablation procedures were well tolerated in all the patients. Both cardiac and respiratory parameters remained stable throughout the treatment. The regimen of conscious sedation and local anesthesia used in this study was adequate for these ablation methods. None of the patients experienced bleeding as a result of these percutaneous ablation techniques.
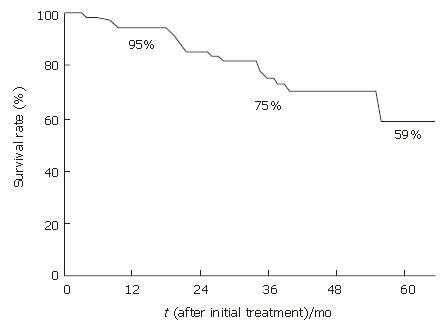
No local tumor residue was found in 125 of 153 (82%) patients as assessed by contrast enhanced CT 6 mo after the initial treatment (defined as successful initial treatment) (Figure 1). The remaining 28 (18%) patients judged as having residual viable tumor by contrast enhanced CT within 6 mo after the initial treatment (defined as unsuccessful initial treatment), were re-treated by RF ablation or PEI until no vascularities could be recognized within the tumor by contrast enhanced CT. With regard to the type of treatment, successful initial treatment was obtained in 90 (82%) of 110 patients treated with RF ablation, and in 35 (81%) of 43 patients treated with PEI, and no significant difference was observed between the two types of treatment. After a median follow-up of 34 mo (range, 1-66), the 1-, 3-, and 5-year survival rates were 95%, 75%, and 59%, respectively (Figure 2). At the time of the analysis, 83 (54%) patients had tumor recurrence (incomplete local treatment and/or distant recurrence), 24 patients died and 3 were l lost to follow-up. With regard to the type of treatment, tumor recurrences were observed in 58 (53%) of 110 patients treated with RF ablation, and in 25 (58%) of 43 patients treated with PEI. Death was due to tumor progression in 18 patients and due to liver failure in 6 patients.
In the univariate analysis, 5 variables were associated with survival (Table 2). Of the pre-treatment factors, Child-Pugh classification, tumor size, serum albumin level, and serum PIVKA-II level were found to be significant predictors of survival. And of the treatment factors, initial treatment response was found to be a significant predictor of survival while the type of ablation therapy (RF ablation vs PEI) was not (P = 0.9829). Multivariate analysis disclosed two independent predictors: serum albumin level ≤ 3.5g/dL vs >3.5g/dL (risk ratio 3.216, 95%CI: 1.407-7.353, P = 0.0056) and successful initial treatment vs unsuccessful initial treatment (risk ratio 2.474, 95%CI: 1.076-5.692, P = 0.0330) (Table 3). When only pre-treatment factors were included in the multivariate analysis, serum albumin level (risk ratio 3.199, 95%CI: 1.405-7.281, P = 0.0056) was proved to be an independent predictor.
| Variables | Patients | P |
| Pre-treatment factors | ||
| Age (yr) | ||
| < 70/≥ 70 | 74/79 | 0.0923 |
| Sex | ||
| Male/Female | 93/60 | 0.7284 |
| Child-Pugh class | ||
| A/B | 133/20 | 0.0275 |
| Tumor size (cm) | ||
| ≤ 3/> 3 | 120/33 | 0.0130 |
| Tumor number | ||
| Single/Multiple | 102/51 | 0.6298 |
| Serum alpha-fetoprotein level (ng/mL) | ||
| < 400/≥ 400 | 142/11 | 0.0722 |
| Serum PIVKA-II level (ng/mL) | ||
| < 300/≥ 300 | 133/20 | 0.0486 |
| Serum albumin level (g/dL) | ||
| ≤ 3.5/> 3.5 | 50/103 | 0.0060 |
| Treatment factors | ||
| Type of ablation therapy | ||
| RF ablation/PEI | 110/43 | 0.9829 |
| Initial treatment response | ||
| Successful/Unsuccessful | 125/28 | 0.0130 |
| Variables | Risk ratio (95% CI) | P |
| Tumor size (cm) | ||
| > 3 | 1.0 | |
| ≤ 3 | 0.459 (0.179-1.174) | 0.104 |
| Serum PIVKA-II level (ng/mL) | ||
| ≥ 300 | 1.0 | |
| < 300 | 0.542 (0.191-1.535) | 0.249 |
| Serum albumin level (g/dL) | ||
| > 3.5 | 1.0 | |
| ≤ 3.5 | 3.216 (1.407-7.353) | 0.006 |
| Initial treatment response | ||
| Successful | 1.0 | |
| Unsuccessful | 2.474 (1.076-5.692) | 0.033 |
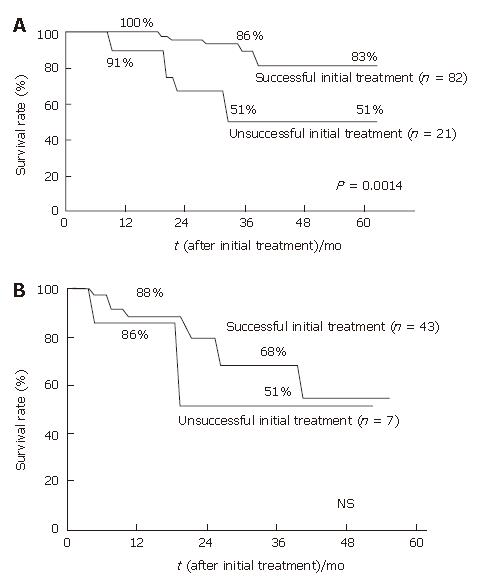
The sole predictive factor for survival in patients with a serum albumin level > 3.5g/dL proved to be the initial treatment response; therefore the survival was examined in case groups with serum albumin levels > 3.5g/dL (n = 103) and ≤ 3.5g/dL (n = 50) according to the initial treatment response. In patients with a serum albumin level > 3.5g/dL, the 1-, 3-, and 5-year survival rates in patients who were judged as having had a successful initial treatment were 100%, 86%, and 83%, respectively (n = 82), and the survival rates in patients who were judged as having had an unsuccessful initial treatment were 91%, 51%, and 51% at 1-, 3-, and 5-year, respectively (n = 21) (P = 0.0014) (Figure 3A). On the other hand, in patients with a serum albumin level ≤ 3.5g/dL, the multivariate analysis proved no independent predictors for survival. The survival rates in 43 patients with a successful initial treatment were 88% and 68% at 1 and 3 years, and in 7 patients with an unsuccessful initial treatment, the survival rates were 86% and 51% at 1 and 3 years, respectively (P = 0.3813) (Figure 3B).
Initial treatment response was significantly associated with the tumor number (P = 0.0031), tumor size (P = 0.0441), serum alpha-fetoprotein level (P = 0.0156), and serum PIVKA-II level (P = 0.0071), however it was not associated with the etiology of underlying cirrhosis, Child-Pugh class, and type of ablation therapy (Table 4).
| Initial treatment | ||
| Successful | Unsuccessful | |
| (n = 125) | (n = 28) | |
| Sex | ||
| Male/Female | 75/50 | 18/10 |
| Age (yr) | ||
| < 70/≥ 70 | 67/58 | 12/16 |
| Etiology | ||
| HCV/HBV/Alcohol/Others | 108/6/7/4 | 26/2/0/0 |
| Child-Pugh class | ||
| A/B | 110/15 | 23/5 |
| Serum albumin level (g/dL) | ||
| ≤ 3.5/> 3.5 | 43/82 | 7/21 |
| Tumor size (cm) | ||
| ≤ 3/> 3 | 102/23 | 18/10a |
| Tumor number | ||
| Single/Multiple | 90/35 | 12/16a |
| Type of ablation therapy | ||
| RF ablation/PEI | 90/35 | 20/8 |
| Serum alpha-fetoprotein level (ng/mL) | ||
| < 400/≥ 400 | 119/6 | 23/5a |
| Serum PIVKA-II level (ng/mL) | ||
| < 300/≥ 300 | 113/12 | 20/8a |
In the current study, we examined a group of HCC patients whose tumors were percutaneously treated using RF ablation or PEI and analyzed the predictors that contributed to the prognosis. Multivariate analysis disclosed two independent predictors: serum albumin level and initial treatment response. In the cases with a serum albumin level > 3.5 g/dL, our data consistently showed that a successful initial treatment was the most predictive factor for long-term survival.
Examinations of the factor that could contribute to the long-term survival of patients undergoing percutaneous ablation therapy, have been reported previously; however, most of them were evaluations using PEI. Pompili et al evaluated the therapeutic efficacy of PEI for patients with Child-Pugh class A and tumors > 5 cm in diameter, and reported that alpha-fetoprotein level and liver function were factors which contributed to survival[21]. Ebara et al evaluated the therapeutic efficacy of PEI for patients with ≤ 3 lesions of small HCC, and found that alpha-fetoprotein level and liver function were factors contributed to the suvival[22]. Recently, Sala et al[14] and Xu et al[23] reported the prognostic predictors after percutaneous ablation therapies. However, most of the cases were treated with PEI in the former study[14], and in the latter study[23], 63% of treated cases were recurrent HCC and the selection for ablation procedures (microwave vs RF ablation) was not fully clarified. To establish an optimal therapeutic protocol after the introduction of RF ablation, we started this cohort study after 2000 when RF ablation was introduced in our institution. In this study, 72% of the patients were treated with RF ablation, and this may reflect the current state of the available percutaneous ablation procedures.
In our study, the serum albumin level was shown as the most important pre-treatment predictor for survival. Hepatic functional reserve as indicated by the serum albumin level has generally been identified to be a good prognostic factor for survival. The serum albumin level has been shown to be one of the prognostic factors in HCC patients treated with hepatic resection[24] and PEI[25]. Ikeda et al reported that the serum albumin level was one of the independent factors associated with the carcinogens in HCV positive viral hepatitis[26]. In this study, 88% of patients had hepatitis C virus infection; therefore, the serum albumin level is selected as a significant factor that may provide for the recurrence of HCC after successful percutaneous local ablation therapy.
Furthermore, initial treatment response was shown as a second significant predictor for survival. Sala et al recently reported that initial treatment response was an outcome predictor of the survival after ablation therapy and initial complete tumor necrosis should be considered a relevant therapeutic target irrespective of tumor size and liver function[14]. In the present study, the impact of extensive tumor necrosis on survival has been observed in patients with good liver functional reserve (serum albumin level > 3.5 g/dL); however, it was not found in patients with poor liver functional reserve (serum albumin level ≤ 3.5 g/dL). Therefore, in patients with poor liver function reserve, optimal selection of patients as candidates for ablation therapies and of the appropriate therapeutic schedule for each single patient to control the tumor growth, avoiding a clinically significant worsening of liver function, is essential to obtain an improvement in survival.
Treatment response was generally judged by CT at one month after the treatment based on the WHO criteria[15], however in this study, the initial treatment response was judged by CT at 6 mo after the initial treatment. This difference of the surveillance after the initial treatment may be explained as follows. First, 22% of our patients underwent TACE prior to ablation therapy, so it was difficult to achieve a detailed evaluation of treatment response with CT because of dense lipiodol accumulation in the treated area[27]. Second, 72% of the patients received RF ablation therapy, resulting in an inflammatory increased vascular flow rim of the outline of the ablated zone for about one month following treatment, which can disturb the evaluation of the local treatment by contrast-enhanced CT[28].
In this study, alpha-fetoprotein level, PIVKA-II level, tumor size, and tumor number were significant factors for the initial treatment response by univariate analysis. Although there are few reports which have examined the factors for initial treatment response following percutaneous ablation therapy, there are several reports as to what contributes to local recurrence when RF ablation is selected. Lencioni et al showed that tumor size and type of ablation therapy were the factors for local recurrence-free survival in those cases treated with RF ablation and PEI[29]. Lin et al reported that tumor number, tumor size, histopathological (Edmondson’s) grade, and type of ablation therapy were significant factors of local recurrence[30].
In summary, we demonstrated that the best candidates for percutaneous ablation therapy are those patients who have good hepatic functional reserve and can be expected to achieve successful initial treatment. Attempts to fully ablate large tumors (3-5 cm in diameter) are achieved successfully by the combined use of TACE, however, further studies are needed to clarify whether or not the combined use of TACE in RF ablation will be a therapeutic option to achieve complete tumor necrosis for the treatment of large HCC tumors.
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2651] [Cited by in RCA: 2604] [Article Influence: 104.2] [Reference Citation Analysis (1)] |
| 2. | Bosch FX, Ribes J, Borràs J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 672] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 3. | Primary liver cancer in Japan. Clinicopathologic features and results of surgical treatment. Ann Surg. 1990;211:277-287. [PubMed] |
| 4. | Arii S, Yamaoka Y, Futagawa S, Inoue K, Kobayashi K, Kojiro M, Makuuchi M, Nakamura Y, Okita K, Yamada R. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology. 2000;32:1224-1229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 539] [Article Influence: 20.7] [Reference Citation Analysis (10)] |
| 5. | Fong Y, Sun RL, Jarnagin W, Blumgart LH. An analysis of 412 cases of hepatocellular carcinoma at a Western center. Ann Surg. 1999;229:790-799; discussion 799-800;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 591] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 6. | Ebara M, Ohto M, Sugiura N, Kita K, Yoshikawa M, Okuda K, Kondo F, Kondo Y. Percutaneous ethanol injection for the treatment of small hepatocellular carcinoma. Study of 95 patients. J Gastroenterol Hepatol. 1990;5:616-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 172] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Castells A, Bruix J, Bru C, Fuster J, Vilana R, Navasa M, Ayuso C, Boix L, Visa J, Rodés J. Treatment of small hepatocellular carcinoma in cirrhotic patients: a cohort study comparing surgical resection and percutaneous ethanol injection. Hepatology. 1993;18:1121-1126. [PubMed] |
| 8. | Rossi S, Di Stasi M, Buscarini E, Quaretti P, Garbagnati F, Squassante L, Paties CT, Silverman DE, Buscarini L. Percutaneous RF interstitial thermal ablation in the treatment of hepatic cancer. AJR Am J Roentgenol. 1996;167:759-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 507] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 9. | Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999;210:655-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 994] [Cited by in RCA: 878] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 10. | Curley SA, Izzo F, Delrio P, Ellis LM, Granchi J, Vallone P, Fiore F, Pignata S, Daniele B, Cremona F. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 805] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 11. | Suehiro T, Sugimachi K, Matsumata T, Itasaka H, Taketomi A, Maeda T. Protein induced by vitamin K absence or antagonist II as a prognostic marker in hepatocellular carcinoma. Comparison with alpha-fetoprotein. Cancer. 1994;73:2464-2471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Arii S, Tanaka J, Yamazoe Y, Minematsu S, Morino T, Fujita K, Maetani S, Tobe T. Predictive factors for intrahepatic recurrence of hepatocellular carcinoma after partial hepatectomy. Cancer. 1992;69:913-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Nagao T, Inoue S, Goto S, Mizuta T, Omori Y, Kawano N, Morioka Y. Hepatic resection for hepatocellular carcinoma. Clinical features and long-term prognosis. Ann Surg. 1987;205:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 230] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Sala M, Llovet JM, Vilana R, Bianchi L, Solé M, Ayuso C, Brú C, Bruix J. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology. 2004;40:1352-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 340] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 15. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3252] [Cited by in RCA: 3258] [Article Influence: 130.3] [Reference Citation Analysis (1)] |
| 16. | Llovet JM, Vilana R, Brú C, Bianchi L, Salmeron JM, Boix L, Ganau S, Sala M, Pagès M, Ayuso C. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 530] [Article Influence: 21.2] [Reference Citation Analysis (1)] |
| 17. | Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1051] [Cited by in RCA: 935] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 18. | Tanaka K, Okazaki H, Nakamura S, Endo O, Inoue S, Takamura Y, Sugiyama M, Ohaki Y. Hepatocellular carcinoma: treatment with a combination therapy of transcatheter arterial embolization and percutaneous ethanol injection. Radiology. 1991;179:713-717. [PubMed] |
| 19. | Morimoto M, Sugimori K, Shirato K, Kokawa A, Tomita N, Saito T, Tanaka N, Nozawa A, Hara M, Sekihara H. Treatment of hepatocellular carcinoma with radiofrequency ablation: radiologic-histologic correlation during follow-up periods. Hepatology. 2002;35:1467-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Yamakado K, Nakatsuka A, Ohmori S, Shiraki K, Nakano T, Ikoma J, Adachi Y, Takeda K. Radiofrequency ablation combined with chemoembolization in hepatocellular carcinoma: treatment response based on tumor size and morphology. J Vasc Interv Radiol. 2002;13:1225-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 151] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 21. | Pompili M, Rapaccini GL, Covino M, Pignataro G, Caturelli E, Siena DA, Villani MR, Cedrone A, Gasbarrini G. Prognostic factors for survival in patients with compensated cirrhosis and small hepatocellular carcinoma after percutaneous ethanol injection therapy. Cancer. 2001;92:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 22. | Ebara M, Okabe S, Kita K, Sugiura N, Fukuda H, Yoshikawa M, Kondo F, Saisho H. Percutaneous ethanol injection for small hepatocellular carcinoma: therapeutic efficacy based on 20-year observation. J Hepatol. 2005;43:458-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 127] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Xu HX, Lu MD, Xie XY, Yin XY, Kuang M, Chen JW, Xu ZF, Liu GJ. Prognostic factors for long-term outcome after percutaneous thermal ablation for hepatocellular carcinoma: a survival analysis of 137 consecutive patients. Clin Radiol. 2005;60:1018-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Chen MF, Tsai HP, Jeng LB, Lee WC, Yeh CN, Yu MC, Hung CM. Prognostic factors after resection for hepatocellular carcinoma in noncirrhotic livers: univariate and multivariate analysis. World J Surg. 2003;27:443-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Kuriyama H, Okada S, Okusaka T, Ueno H, Ikeda M. Prognostic factors in patients with small hepatocellular carcinoma treated by percutaneous ethanol injection. J Gastroenterol Hepatol. 2002;17:1205-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Ikeda K, Saitoh S, Suzuki Y, Kobayashi M, Tsubota A, Koida I, Arase Y, Fukuda M, Chayama K, Murashima N. Disease progression and hepatocellular carcinogenesis in patients with chronic viral hepatitis: a prospective observation of 2215 patients. J Hepatol. 1998;28:930-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 315] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 27. | Nakamura H, Hashimoto T, Oi H, Sawada S. Transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology. 1989;170:783-786. [PubMed] |
| 28. | Goldberg SN, Gazelle GS, Mueller PR. Thermal ablation therapy for focal malignancy: a unified approach to underlying principles, techniques, and diagnostic imaging guidance. AJR Am J Roentgenol. 2000;174:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 784] [Cited by in RCA: 708] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 29. | Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, Frings H, Laubenberger J, Zuber I, Blum HE. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 674] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 30. | Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm. Gastroenterology. 2004;127:1714-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 438] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
S- Editor Liu Y L- Editor Ma JY E- Editor Ma WH