Published online Feb 14, 2007. doi: 10.3748/wjg.v13.i6.970
Revised: December 15, 2006
Accepted: January 17, 2007
Published online: February 14, 2007
To treat postoperative bleeding after hepato-pancreato-biliary surgery, interventional radiology has become essential. We report a case of coincidental pseudoaneurysm and jejunal varices that were both successfully treated by stent-grafts. After a pancreaticoduodenectomy, the patient developed a pseudoaneurysm in the hepatic artery and a stenosis in its periphery. After establishing hepatic arterial flow by placing stent-grafts over both the pseudoaneurysm and the stenosis, the pseudoaneurysm was embolized with microcoils. Nine months later, the patient developed jejunal varices caused by a severe stricture in the main trunk of the portal vein. Percutaneous transhepatic portography was performed and stent-grafts were placed over the stenotic segment. A venoplasty using stent-grafts normalized the portal blood flow and the jejunal varices vanished. Although stenosis occurred due to scarred tissues from leakage after pancreaticoduodenectomy, stent-grafts were useful for managing jejunal bleeding post-operatively.
- Citation: Ichihara T, Sato T, Miyazawa H, Shibata S, Hashimoto M, Ishiyama K, Yamamoto Y. Stent placement is effective on both postoperative hepatic arterial pseudoaneurysm and subsequent portal vein stricture: A case report. World J Gastroenterol 2007; 13(6): 970-972
- URL: https://www.wjgnet.com/1007-9327/full/v13/i6/970.htm
- DOI: https://dx.doi.org/10.3748/wjg.v13.i6.970
Postoperative bleeding is one of the leading causes of death after pancreaticoduodenectomy[1]. In particular, delayed hemorrhage occurring five or more days post-operatively[2] is difficult to manage because the hepatic artery and/or the portal vein are frequently affected. That is, not only hemostasis, but also the maintenance of hepatic blood flow has to be considered in treating these patients. Above all other methods, embolization using an interventional radiology (IVR) technique has been used for surgical treatment because of its efficacy and minimal invasiveness[3]. In this paper we report jejunal varices due to a portal venous stricture and a coincidental pseudoaneurysm in the hepatic artery after a pancreaticoduodenectomy. These complications occurred metachronously and each was successfully treated with an IVR technique using stent-grafts. Although treatment for this condition has not yet been standardized, we give a detailed account of our approach.
In July 2004, a 64-year-old woman underwent a pancreaticoduodenectomy with a Child’s reconstruction for carcinoma of the distal bile duct[4]. During the postoperative course, a minor leak from the hepaticojejunostomy occurred, which was resolved within ten days. However, on the 26th postoperative day, 250 mL of blood through an intraabdominal drain was collected.
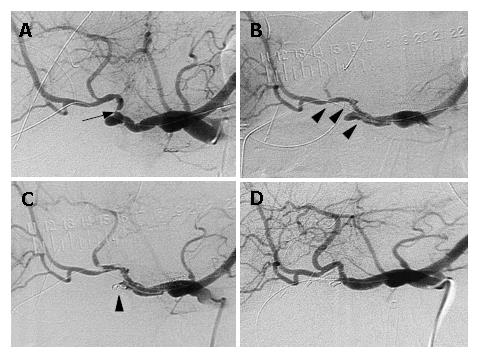
Angiography was carried out immediately. As shown in Figure 1A, there was no extravasation, but a severe stricture in the proper hepatic artery and pseudoaneurysm at the cutting stump of the gastroduodenal artery were noted. To secure the hepatopetal arterial flow, an angioplasty was performed. Two pieces of stent-grafts, each 2.5 mm in diameter and 13 mm or 23 mm in length (MULTI LINK Penta®, GUIDANT Indianapolis, IN, USA) were introduced sequentially through the stenotic segment and the pseudoaneurysm in the hepatic artery (Figure 1B). After stenting, embolization of the pseudoaneurysm was attempted using microcoils (GDC Detachable coil®: Boston scientific; Natick, MA, USA) to stop the inflow into the pseudoaneurysmal lumen (Figure 1C). The patency of the hepatic artery and the disappearance of the pseudoaneurysm were confirmed by arteriography 8 d after treatment (Figure 1D). The patient was discharged from the hospital 4 wk after treatment.
This patient did well for 9 mo after hospital discharge. In April 2005, however, she was readmitted with gastrointestinal bleeding. Laboratory data upon admission showed severe anemia and hyperammoninemia. Gastrointestinal fiberscopy showed bleeding from a varix in the lifted limb of the jejunum near the hepaticojejunostomy.
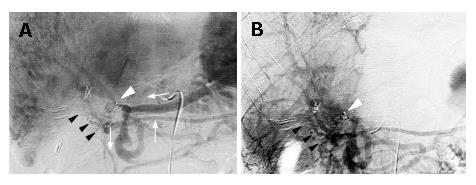
Angiography was performed. Hepatic arterial flow was normal as seen in Figure 1D. However, celiac and superior mesenteric arteriography during the portal phase (Figure 2A and B) revealed a severe stricture at the main trunk of the portal vein, adjacent to the microcoils that had been inserted 10 mo earlier. Hepatofugal collaterals had also developed via the left gastric vein and the inferior mesenteric vein. Moreover, collateral vessels were observed around the hepaticojejunostomy creating jejunal varices in the lifted limb toward the hepatic hilum. These jejunal varices were identified as the source of the intestinal bleeding. Since an abdominal computed tomography showed no recurrent tumor, the portal vein stenosis was most likely caused by inflammatory changes that occurred after a leak at the hepaticojejunostomy.
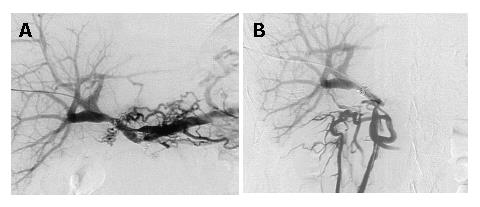
Because the main flow of the portal vein was not completely interrupted, percutaneous transhepatic portography (PTP) into the mesenteric side of the portal vein beyond the stenosis was performed. As seen in Figure 2A and B, PTP findings were consistent with those obtained by conventional angiography. To maintain the main stream of the portal vein, two pieces of uncovered-type, self-expandable metallic stent-grafts, 8 mm in diameter and 20 or 30 mm in length (LUMINEXX®, BARD, Covington, GA, USA) were placed in succession over the stenotic segment. The stent-grafts were then mechanically dilated using a balloon dilator (Power Flex®; Cordis, Miami, FL, USA), resulting in full expansion of the grafts. However, portal blood flow was not corrected immediately. We speculated that possible causes for this were vasospasm of the portal system and/or thrombi generated during stenting and dilation. Therefore, a heparin-coated catheter was left in the mesenteric side of the portal trunk, and urokinase (Nihon Pharmaceutical Co., Tokyo, Japan) 240 000 U a day (Nihon Pharmaceutical Co., Tokyo, Japan) was infused for 7 d. As shown in Figure 3A and B, portography after the urokinase infusion revealed good portal blood flow through the stent-grafts and the jejunal varices had disappeared. Before placement of the stent-grafts, portal venous pressure was 32 cmH2O, which dropped to 25 cmH2O after correcting portal blood flow. Gastrointestinal fiberscopy confirmed the absence of jejunal varices around the hepaticojejunostomy. Currently, the patient is doing well without repetitive episodes of gastrointestinal bleeding for 9 mo after the procedure.
Rupture of a pseudoaneurysm of the hepatic artery is the main cause of postoperative death after pancreaticoduodenectomy[5]. In the majority of cases, there is concurrent leakage at the site of the pancreatico-intestinal or choledocho-jejunal anastomosis. Therefore, reentering the previously scarred operative field for repair is not easy and hemostasis by suturing the bleeding point often fails because of a re-encroachment on the affected vessels[6]. On the other hand, using a remote approach like IVR via blood vessels is minimally invasive, thus preferable in these situations. However, when hepatic arterial flow is interrupted by IVR to stop hemorrhage, consecutive events such as liver cell necrosis, a breakdown of the choledochojejunostomy, and multiple liver abscesses could occur. Therefore, when using IVR after embolizing pseudoaneurysms, patient outcomes depend a great deal on the maintenance of hepatic arterial flow. In this case, stent-grafts in the hepatic artery prior to the embolization of a pseudoaneurysm were used. However, using stent-grafts in these cases is controversial. For example, several radiologists have suggested that placing a stent-graft in blood vessels with inflammatory changes is inappropriate[7]. Conversely, Paci et al[8] reported the efficacy of stent-grafts in the treatment of pseudoaneurysms in hepatic arteries. We used stent-grafts to secure hepatic arterial flow by dilating the stenotic part of the artery and guiding the blood flow into the proper direction out of the pseudoaneurysmal lumen, avoiding the possibility of migrating microcoils interfering with arterial flow to the liver.
In cases of varices generated in the intestines, extrahepatic portal obstruction often plays a causative role[9]. Jejunal varices caused by portal venous obliteration could be treated with either a shunt for portal decompression[10,11], an angioplasty by an IVR technique such as balloon dilation, stenting to correct the portal blood flow[12] or embolizing varices by an IVR technique to stop inflow[13,14]. Making a treatment choice is complex, involving various factors such as portal hemodynamics, the extent of liver fibrosis, and the cause of portal venous obliteration. However, a priority is establishing a permanent correction of portal blood flow assuring long-lasting effectiveness of hemostasis. That is, re-bleeding from jejunal varices or newly-developed gastroesophageal varices could occur if hepatopetal flow through the main portal trunk is impaired.
Since in this case, there was a possibility for normal portal venous flow, an angioplasty using either stent-grafts or balloons was used. If the portal vein had been completely obstructed by inflammatory changes and the obstructed segments were wider than an inch in length as occurred in our previous cases[15,16], treatments other than angioplasty would have been chosen.
In addition, it was possible to dilate the stricture and secure a lumen of 8 mm in diameter using a balloon dilator. This mechanically-forced dilation could inadvertently interfere with an immediate correction of portal blood flow, however, a urokinase infusion through the portal vein to resolve thrombi attached was valuable[15,16].
Another unsolved issue using this intervention is the duration of patency of the stent-grafts. Funaki et al [17]reported that patency was maintained with their stents in portal veins for lengths of time ranging from 4 to 29 mo ,and in most cases stents were obstructed by malignancies. Since we have only followed our patient for 14 mo, continued surveillance to determine patency of the stent is essential.
| 1. | Trede M, Schwall G. The complications of pancreatectomy. Ann Surg. 1988;207:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 269] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 2. | Kim J, Kim JK, Yoon W, Heo SH, Lee EJ, Park JG, Kang HK, Cho CK, Chung SY. Transarterial embolization for postoperative hemorrhage after abdominal surgery. J Gastrointest Surg. 2005;9:393-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Sato N, Yamaguchi K, Shimizu S, Morisaki T, Yokohata K, Chijiiwa K, Tanaka M. Coil embolization of bleeding visceral pseudoaneurysms following pancreatectomy: the importance of early angiography. Arch Surg. 1998;133:1099-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 107] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Child CG. Carcinoma of the duodenum. Ann Surg. 1943;118:838-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Brodsky JT, Turnbull AD. Arterial hemorrhage after pancreatoduodenectomy. The 'sentinel bleed'. Arch Surg. 1991;126:1037-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Otah E, Cushin BJ, Rozenblit GN, Neff R, Otah KE, Cooperman AM. Visceral artery pseudoaneurysms following pancreatoduodenectomy. Arch Surg. 2002;137:55-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 95] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Bürger T, Halloul Z, Meyer F, Grote R, Lippert H. Emergency stent-graft repair of a ruptured hepatic artery secondary to local postoperative peritonitis. J Endovasc Ther. 2000;7:324-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Paci E, Antico E, Candelari R, Alborino S, Marmorale C, Landi E. Pseudoaneurysm of the common hepatic artery: treatment with a stent-graft. Cardiovasc Intervent Radiol. 2000;23:472-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Moncure AC, Waltman AC, Vandersalm TJ, Linton RR, Levine FH, Abbott WM. Gastrointestinal hemorrhage from adhesion-related mesenteric varices. Ann Surg. 1976;183:24-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Chen VT, Wei J, Liu YC. A new procedure for management of extrahepatic portal obstruction. Proximal splenic-left intrahepatic portal shunt. Arch Surg. 1992;127:1358-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Katoh H, Shimozawa E, Kojima T, Tanabe T. Modified splenorenal shunt with splenopancreatic disconnection. Surgery. 1989;106:920-924. [PubMed] |
| 12. | Hiraoka K, Kondo S, Ambo Y, Hirano S, Omi M, Okushiba S, Katoh H. Portal venous dilatation and stenting for bleeding jejunal varices: report of two cases. Surg Today. 2001;31:1008-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 13. | Sato T, Asanuma Y, Ishida H, Hashimoto M, Tanaka J, Andoh H, Yasui O, Kurokawa T, Komatsuda T, Konno K. A case of extrahepatic portosystemic shunt without portal hypertension treated by laparoscopically assisted embolization. Surgery. 1999;126:984-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Sato T, Yasui O, Kurokawa T, Hashimoto M, Asanuma Y, Koyama K. Jejunal varix with extrahepatic portal obstruction treated by embolization using interventional radiology: report of a case. Surg Today. 2003;33:131-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Smith SC, Winters KJ, Lasala JM. Stent thrombosis in a patient receiving chemotherapy. Cathet Cardiovasc Diagn. 1997;40:383-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Bilodeau L, Hearn JA, Dean LS, Roubin GS. Prolonged intracoronary urokinase infusion for acute stent thrombosis. Cathet Cardiovasc Diagn. 1993;30:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Funaki B, Rosenblum JD, Leef JA, Hackworth CA, Szymski GX, Alonso EM. Angioplasty treatment of portal vein stenosis in children with segmental liver transplants: mid-term results. AJR Am J Roentgenol. 1997;169:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
S- Editor Liu Y L- Editor Zhu LH E- Editor Lu W