INTRODUCTION
Since the 1980s, ischemia-reperfusion (I/R) injury of organs has been frequently encountered as a complex phenomenon in medicine and biology. Researches have been focused on I/R injury of organs such as the heart, brain, lungs, and the kidneys. In recent years, there has been an increase in the available information concerning the acute gastric mucosal injury induced by I/R, or gastric I/R injury[1-4]. The investigative key points have focused on the effects of individual factors on gastric mucosal injury induced by gastric I/R, including increase of oxygen free radicals and endothelin level, microvascular dysfunction, polymorphonuclear leukocyte infiltration, nitric oxide release, gastric acid secretion, prostaglandin decrease and so on[5-15]. Some researchers have quested the best methods to protect the gastric mucosa against gastric I/R injury, such as the endothelin converting enzyme inhibitor phosphoramidon[16], the anti-reactive oxygen species drug, tetrahydrabioptin[9] and the adhesion molecules of anti-leukocytes[17]. However, little is known about the systemic regulation of gastric I/R injury, particularly the role of related central nuclei.
It has been known that the gastric mucosal tissue integrity depends upon the interplay between cell renewal and death of damaged or aged cells. The gastric mucosa has a high renewal rate, and all gastric mucosal surface epithelial cells are replaced within 3-5 d as the exfoliated surface mucosal cells are replaced by cells migrating from the glandular foveolar and neck area[18]. The mucosa is maintained by the balance between proliferation and apoptosis of gastric mucosal cells. To study the imbalance between proliferation and apoptosis is of great significance in understanding the causes of gastric lesions.
Recently, literature reviews revealed that I/R could induce tissue injury and cellular apoptosis in many important organs[19-25]. Apoptosis of gastric mucosal cells has also been studied under varied conditions in rats, including ethanol, non-steroidal anti-inflammatory drugs, retinoic acids, H pylori infection[26-30]. Some reports of gastric mucosal cellular apoptosis induced by gastric I/R are contradictory. Fukuyama et al[31], for example, reported that apoptosis is not induced in the gastric mucosa after gastric I/R. Wada et al[32] demonstrated that gastric I/R induces significant apoptosis in the gastric mucosa. However, the central regulation of gastric mucosal cellular apoptosis and proliferation induced by gastric I/R in rats has not yet been fully elucidated.
In his early study[33], Salim pointed out that hypothalamus, especially the paraventricular nuclei (PVN), is extraordinarily complex in its structure and function. It participates in the regulation of various activities of the stomach, including secretion, electrical activation and gastric motor. Our previous study[34] showed that PVN and its associated nuclei could regulate the development of restraint and cold-water immersion stress-induced ulcer in stomach and that PVN is involved in the regulation of gastric I/R injury as one of the specific CNS areas attenuating gastric I/R injury[35]. The cellular mechanisms responsible for the attenuation of GI/R injury by electrical stimulation of PVN are still unknown.
The aim of our study is to investigate the molecular mechanisms of stimulating PVN in protecting against gastric I/R injury, to observe the characteristics of gastric mucosal cellular apoptosis and proliferation induced by gastric I/R at various times. It is believed that any progress in this field will help better understand the pathology of gastric I/R injury at cellular level.
MATERIALS AND METHODS
Reagents
M30 CytoDEATH monoclonal antibody was obtained from Roche (Basel, Switzerland). Mouse anti-rat proliferative nucleolus monoclonal antibody (proliferative cell nuclear antigen, PCNA), PowerVisionTM two-step immunohistochemistry detection kit, and 3.3′-diaminobenzidine (DAB; Maixin-Bio Co., China) substrate solution were obtained from Zhongshan Biotech Co. (Beijing, China).
Animals
Groups of six adult male Sprague-Dawley rats, weighing 180-220g,were supplied by the Experimental Animal Center of Xuzhou Medical College (usage certificate of experimental animals, No: SYXK (su) 2002-0038). All experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. The animals were housed under controlled temperature (22-24°C) and photoperiod (12 h light: 12 h dark) in wire-mesh-floor cages to prevent ingestion of hair and feces. The animals were fasted for 24 h before the experiment with free access to tap water. The animals were allocated to different groups to serve as gastric I/R injury models for different purposes in the experiments.
Electrical stimulation of PVN
Three days before the experiment, the rats were anesthetized with sodium pentobarbital (40 mg/kg i.p.) and mounted onto a stereotaxic apparatus. The scalp was incised, and a 0.5 mm hole was drilled in the cranium dorsal to the target site. The coordinates of PVN for the placement of electrodes were taken from the atlas of Paxinos and Watson[36] as follows: AP 1.5 mm, LR 0.4 mm, H 7.7-7.8 mm, and the incisor bar was positioned 3.3 mm below the center of the aural bars.
The concentric bipolar stimulating electrode is consisted of an insulated outer barrel formed from the needle of a medical syringe (0.4 mm in diameter) and an inner Ni-Cr wire, insulated except for the tip, which protrudes about 0.2 mm from the barrel. The stimulating electrode was inserted into PVN and fixed to the cranium with dental cement.
During the experiment, the rat was also anesthetized with sodium pentobarbital (40 mg/kg i.p.). PVN was stimulated before the induction of gastric I/R injury. The electrical stimulating parameters have been described previously[35]. Stimulation was performed 5 times with uniphasic, constant current square wave pulses (pulse width 0.2 ms, intensity 0.4 mA, frequency 50 Hz, duration 1 min), at 5 min intervals. In the sham PVN stimulation group, only the electrode was inserted, and no current was passed.
Electrical destruction of PVN
Three days before the experiment, the rat was anesthetized with sodium pentobarbital (40 mg/kg i.p.) and mounted onto a stereotaxic apparatus. The coordinates of PVN for the placement of destructing electrodes was similar to that of the stimulating electrodes. The destructing electrodes was in bilateral PVN. The destructing electrode were placed a single stainless steel wire, 0.3 mm in diameter, with a naked tip about 0.3 mm, and void of insulation. Bilateral lesions were then made by passing a positive DC of 1 mA for 10 s. The lesion electrode was immediately removed after destruction. Sham operations were performed with the same surgical procedures, and no current was passed.
Preparation of gastric I/R model
The animal, were kept fasting for 24 h with free access to water before experiment. All experiments were carried out under anesthesia with sodium pentobarbital (40 mg/kg i.p.), the supplemental dose of the anesthetic was given i.p. to maintain animal anesthesia throughout the experiment. GastricI/R injury was induced according to the method of Wada et al[37]. Briefly, the abdominal cavity was cut open, and the celiac artery and its adjacent tissues were carefully isolated. The celiac artery was first clamped with a small vascular clamp for 30 min to induce ischemia, and was then released to allow reperfusion for 30 min, 1 h, 3 h or 6 h respectively. Sham-operated animals underwent all of the surgical procedures except for the arterial clamping. At the end of each experiment, the rats with gastric I/R injury or sham-operated groups were killed by exsanguination via the abdominal aorta under anesthesia[38], while the rats with embedded electrode in PVN were killed by decapitation under deep anesthesia. The stomach was removed rapidly, opened along the great curvature, rinsed with ice-cold phosphate buffered saline (PBS, 0.1 mol/L), and spread onto a cold plate. The gastric mucosa was carefully examined grossly and microscopically.
Assessment of gastric mucosal injury index
Quantitative histological assessment of mucosal injury was performed with the method described by Guth et al[39] with slight modifications. The index is based on a cumulative-length scale. In which an individual lesion limited within the mucosal epithelium (including the pinpoint erosions, ulcers and hemorrhagic spots) is scored by giving points for its length. The score is 1 for a lesion ≤ 1 mm, 2 for a lesion > 1 mm and ≤ 2 mm, for a lesion > 2 mm ≤ 3 mm, and so on. For a lesion with a width > 1 mm, the lesion score is doubled. The sum of the scores represents the GMDI. To avoid the researcher's bias, the GMDI was determined by a researcher who was unfamiliar with the details of the experiment.
Immunohistochemical staining
Immunohistochemistry was performed with the streptavidin-peroxidase (SP) method[40]. The primary antibodies used were polyclonal antibody against proliferating cell nuclear antigen (PCNA), monoclonal antibody against M30 CytoDEATH. The gastric tissue samples were fixed in Bouin’s fixative for paraffin tissue slices. Five-μm thick sections were, deparaffinized, rehydrated, and boiled. The dewaxed sections were heated in a microwave oven (700 W) for 12 min to retrieve the antigens, and cooled to room temperature. The endogenous peroxide was blocked with 3% hydrogen peroxide (H2O2) for 15 min in methanol. The sections were washed with PBS (0.01 mol/L), blocked with 10% goat serum for 15 min to reduce the non-specific antibody binding, and then incubated with the primary antibody (M30CytoDEATh 1:10, PCNA 1:50) at 4°C overnight. The sections were washed twice for 5 min with PBS, incubated with the secondary anti-mouse immunoglobulin (Santa Cruz Inc., USA) conjugated with biotin at room temperature for 30 min, washed again with PBS (0.01 mol/L), and incubated with SP complex for l5 min. The reaction products of peroxidase were visualized by incubation with 0.05 mol/L Tris-HCl buffer (pH 7.6) containing 20 mg 3.3’-diaminobenzidine (DAB, Maixin-Bio Co. China) and 100 μL 5% H2O2 per 100 mL. The brown color signifying the presence of the antigen bound to the antibodies was detected by light microscopy. Finally, the sections were counterstained for nuclei with hematoxylin. The sections obtained from the control group were stained according to the same method except that PBS was substituted for the specific primary antibody. M30 CytoDEATH staining was quantified by calculating the average ratio of positive cells in 10 vision fields under a 400 × microscope. PCNA staining was quantified by calculating the average ratio of positive cells in 10 vision fields under a 100 × microscope.
Histological verification of stimulated or destructed PVN sites
At the end of each experiment, the PVN site was marked by passing a positive direct current of 1 mA for 10 s. The experimental rats were killed by decapitation under deep anesthesia, and the brains were removed and fixed in 10% formalin for 7 d. Frozen coronal serial sections (45 μm) were cut through the hypothalamus and mounted and stained with 1% neutral red to verify the sites of electrical stimulation under a light microscope. The data from rats whose target sites failed to meet the histological criteria were deleted from the statistical analysis.
Statistical analysis
All results are expressed as mean ± SE. Data were compared by an analysis of variance (ANOVA) followed by Student-Newman-keuls (SNK) test. All calculations were performed with SPSS 10.0 for Windows, P < 0.05 was considered statistically significant.
RESULTS
Histological verification of stimulated PVN sites
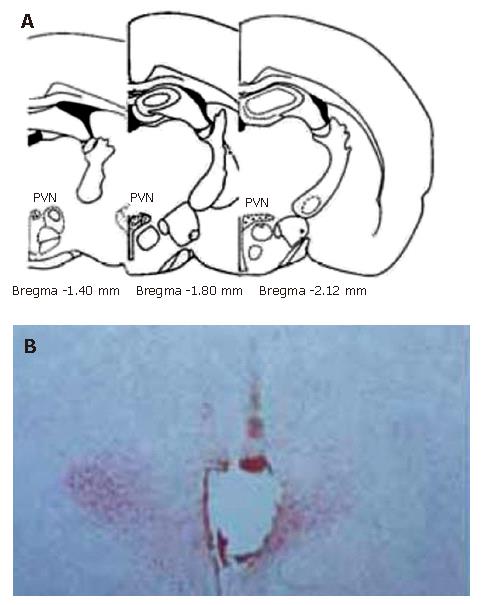
By referring to Paxinos and Watson’s Stereotaxic Atlas[36], histological verification was performed at all sites of electrical stimulation (Figure 1)
Figure 1 Sites of the stimulating electrode tip in PVN.
A: Standard atlas sections of the rat brain showing the distributions of stimulating sites of the experimental animals; B: Photomicrographs of stimulating sites in the rat brain. The section stained with neutral red, showing the position of stimulating electrode tip by passing a positive DC of 1 mA for 10 s, which indicates a placement within the PVN.
Effects of electrical stimulation and electrolytic destruction of PVN on gastric I/R injury in rats
Our previous experiments confirmed that the GMDI of the gastric I/R injury only group is similar to that of sham electrically stimulated PVN plus gastric I/R injury group (sham PVN + gastric I/R injury) at the different time points during reperfusion. Thus, we compared the differences between GI/R only and PVN + gastric I/R injury groups in subsequent experiments.
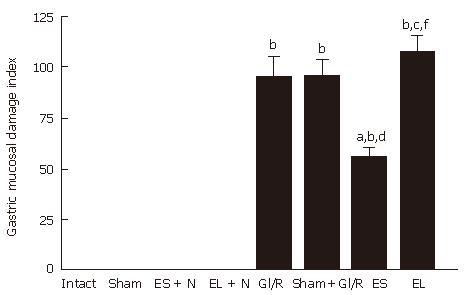
As shown in Figure 2, gastric mucosal injury was not found (the GMDI being zero) in the sham-operated group. The stimulated PVN + normal stomach group or the destructed PVN + normal stomach group was similar to that of normal rat group. In the gastric I/R injury group, the GMDI was 94.67 ± 25.57 at 1 h after reperfusion. The GMDI was significantly lower in the electrical stimulation of PVN + gastric I/R injury group than in the gastric I/R injury only group at 1 h after reperfusion (94.67 ± 25.57 vs 54.83 ± 12.64, P < 0.05). The GMDI was not significantly different in the bilateral PVN destruction + gastric I/R injury group compared with that in the gastric I/R injury injury only group or sham stimulating PVN plus gastric I/R injury group (106.94 ± 20.91 vs 94.67 ± 25.57, P > 0.05), but was markedly higher than that gastric I/R injury the electrical stimulation of PVN + GI/R group (106.94 ± 20.91 vs 54.83 ± 12.64, P < 0.05).
Figure 2 Effect of electrical stimulation and bilateral electrolytic destruction of PVN on gastric mucosal damage induced by I/R at 1 h after reperfusion in rats.
Intact: normal rat; sham: sham-operation (electrode was inserted, with no current passing and no artery clamped); ES + N: intact stomach after electrical stimulation of the PVN. EL + N: intact stomach after bilateral electrolytic destruction of the PVN; GI/R injury: gastric ischemia-reperfusion injury; Sham+GI/R injury: sham electrical stimulation of the PVN plus GI/R injury; ES: electrical stimulation of the PVN plus GI/R injury; EL: bilateral electrolytic destruction of the PVN plus GI/R injury. Values of GMDI are mean ± SE (n = 6). bP < 0.01 vs intact, dP < 0.01 ES vs ES + N, fP < 0.01 EL vs EL + N, aP < 0.05, ES vs GI-R, cP < 0.05, EL vs ES.
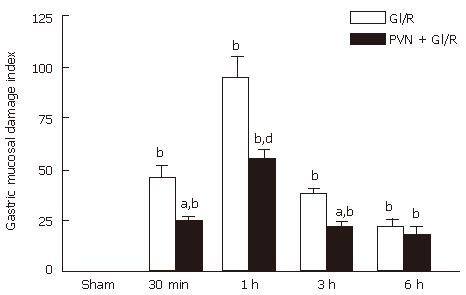
As shown in Figure 3, in the gastric I/R injury group, the gastric mucosal lesions appeared 30 min after reperfusion, the GMDI reached maximum at 1h after reperfusion (94.67 ± 25.57), but then declined. In the group of PVN + gastric I/R, the GMDI was significantly lower than that the gastric I/R group at 30 min, 1 h and 3 h after reperfusion (P < 0.01). At 6 h after reperfusion, there was no significant difference (P > 0.05) between the GI/R group and the stimulating PVN + gastric I/R group.
Figure 3 Effect of electrical stimulation of PVN on gastric mucosal damage induced by I/R at different times in rats.
Sham: sham-operation(electrode was inserted, with no current passing and no artery clamped); GI/R: gastric ischemia/reperfusion was maintained for 30 min, 1 h, 3 h and 6 h after 30 min of ischemia, respectively; PVN + GI/R: electrical stimulation of the PVN plus GI/R. Values: Each column represents mean ± SE (n = 6). bP < 0.01 vs sham; aP < 0.05, dP < 0.01, PVN + GI/R vs GI/R at different times.
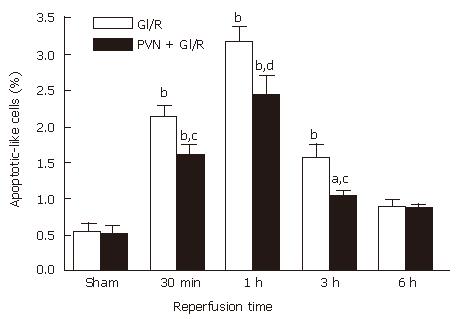
Effect of electrical simulation of PVN on apoptosis of gastric mucosal cells induced by GI/R in rats
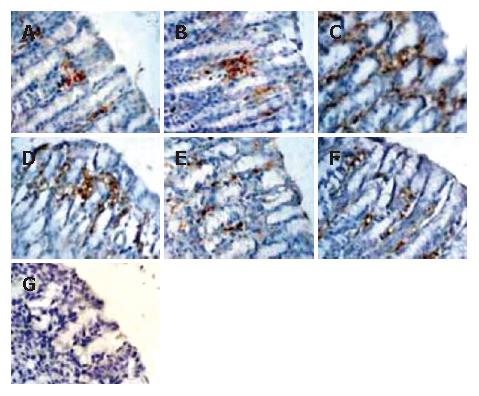
As shown in Figure 4, the M30 CytoDEATH positive apoptotic cells (i.e. apoptotic cells) were distributed predominantly in the upper part of the gastric glands and epithelial cells, where the cytoplasm was stained brown. In the normal gastric mucosa (Figure 4A and B), the few apoptotic cells were apparent. In the GI/R injury group, the percentage of apoptotic cells increased rapidly at the onset of reperfusion, and peaked at 1 h after reperfusion (6.32 times higher than that in the normal gastric mucosa, P < 0.01) (Figure 4C). Electrical stimulation of PVN markedly decreased the percentage of M30 CytoDEATH positive cells at 1 h after reperfusion (Figure 4 D), which declined to the basal level at 6 h after reperfusion (Figure 4E and F). Figure 4G indicates the negative control group.
Figure 4 Histological exhibition of electrical stimulation of PVN on gastric mucosal apoptosis induced by I/R at different times in rats.
The apoptotic-positive cells were probed with anti-M30 CytoDEATH antibody and counter-stained with hematoxylin in rat gastric mucosa (× 400). A: normal gastric mucosa; B: sham operation; C: GI/R at 1 h after reperfusion; D: PVN + GI/R at 1 h after reperfusion; E: GI/R at 6 h after reperfusion; F: PVN+GI/R at 6 h after reperfusion; G: negative control group.
As shown in Figure 5, the electrical stimulation of PVN significantly decreased the percentage of M30 CytoDEATH positive cells throughout the 6 h reperfusion (P < 0.05 or P < 0.01).
Figure 5 Effect of electrical stimulation of PVN on gastric mucosal cellular apoptosis induced by I/R at different time points in rats.
Sham: sham-operation; GI/R: reperfusion was maintained for 30 min, 1 h, 3 h and 6 h after 30 min of ischemia, respectively; PVN + GI/R: electrical stimulation of PVN plus GI/R. The percentage of apoptotic cells was taken by counting the cells in 10 microscopic fields (× 400). Each column represents mean ± SE (n = 6). aP < 0.05, bP < 0.01, GI/R vs sham-operation; cP < 0.05, dP < 0.01, PVN + GI/R vs GI/R at each time point.
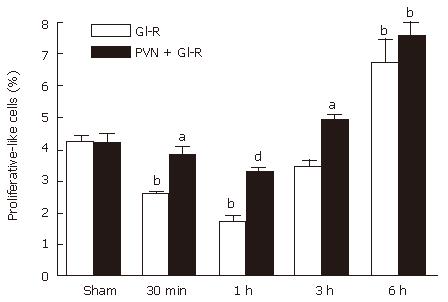
Effect of electrical stimulation of PVN on proliferation of gastric mucosal cells induced by GI/R in rats
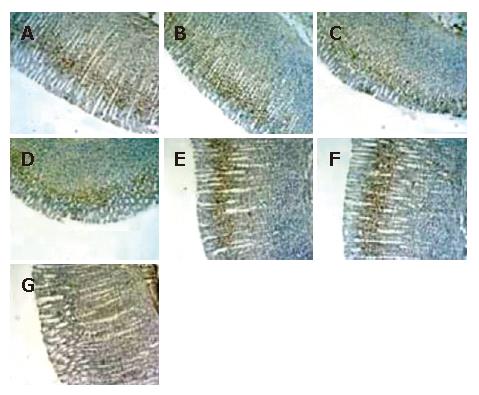
As shown in Figure 6, the proliferating-positive cells were found in the normal gastric mucosa, which were distributed predominantly in the glandular neck area of the gastric mucosa (Figure 6 A and B). The percentage of proliferating cells decreased rapidly at 1 h after reperfusion (Figure 6C). The percentage of proliferating positive cells were significantly higher in the stimulating PVN + gastric I/R injury group than in the gastric I/R injury group (Figure 6D). The proliferating-positive cells accumulated in both the gastric I/R injury only group and the stimulating PVN + gastric I/R injury group at 6 h after reperfusion, and the crowding was not significantly different at this time (Figure 6E and F). Figure 6G negative control group.
Figure 6 Histological exhibition of electrical stimulation of PVN on gastric mucosal cellular proliferation induced by I/R at different times in rats.
Proliferating-positive cells were probed with anti-mouse anti-proliferating cell nuclear antigen (PCNA) antibody and counter stained with hematoxylin in gastric mucosa (× 100). A: normal control gastric mucosa; B: gastric mucosa after sham operation; C: GI/R, 1 h after reperfusion; D: PVN + GI/R, 1 h after reperfusion; E: GI/R, 6 h after reperfusion; F: PVN + GI-R, 6 h after reperfusion; G: negative control group.
Figure 7 also showes that the electrical stimulation of PVN significantly increased the percentage of proliferating-positive cells at 30 min (1.47 times), 1 h (1.89 times) and 3 h (1.43 times) after reperfusion compared with the respective values in the gastric I/R injury group.
Figure 7 Effect of electrical stimulation of PVN on gastric mucosal proliferation induced by I/R at different times in rats.
Sham: sham-operation; GI/R: reperfusion was maintained for 30 min, 1 h, 3 h and 6 h after 30 min of ischemia, respectively; PVN+GI/R: electrical stimulation of PVN plus GI/R. The percentage of proliferating cells was taken by using cell count in 10 microscopic fields (× 100). Each column represents an average value expressed in mean ± SE (n = 6). bP < 0.01, GI/R vs sham operation; aP < 0.05 and dP < 0.01, PVN + GI/R vs GI/R.
DISCUSSION
In our present study, the gross gastric mucosa damage index was determined to evaluate the extent of gastric I/R injury. Immunohistochemistry was also used to assess the processes of gastric mucosal cellular apoptosis and proliferation in gastric I/R injury by detecting the apoptotic cells with the M30 CytoDEATH monoclonal antibody (which binds to a caspase-cleaved formalin-resistant epitope of the cytokeratin 18 cytoskeletal protein in the cytoplasm of apoptotic cells) and by labelling the proliferating cells with multi-clonal antibodies to PCNA (an assistant factor of DNA polymerase)[41,42]. We assessed these variables at different times from 30 min to 6 h after reperfusion. We reasoned that our histological analysis the process would help us better understand the cellular mechanisms responsible for the reperfusion injury.
In our analysis of the GMDI data, which was obtained with gastric I/R injury model of anesthetized (sodium pentobarbital, 40 mg. kg-1i.p.) rats, it was noticed that the gastric mucosal damage was localized within the mucosal epithelium, the lesions started to occur at 30 min after reperfusion, reached their peak at 1 h and then subsided. We dynamically observed the changes of apoptosis and proliferation of gastric mucosal cells at different times from 30 min to 6 h after gastric I/R injury, Histological study of the normal gastric mucosa revealed a few apoptotic cells in the superficial layer of gastric mucosa and proliferating cells only in the gastric glandular neck area. These patterns contrast with previous reports [43,44], supporting the notion that cell renewal and apoptosis are two essential processes maintaining the homeostasis of normal gastric mucosa. The pattern of simultaneous change in gastric mucosal cellular apoptosis during reperfusion is similar to that of gastric I/R injury; i.e., the level of apoptosis also reached its peak at 1 h after reperfusion when the gastric mucosal injury was most serious, and then declined. In contrast, the level of gastric mucosal cellular proliferation induced by ischemia/reperfusion also reached its minimum at 1 h after reperfusion, but the level of proliferation reached its maximum at 6 h after reperfusion, indicating that the proliferating process of gastric mucosal cells is slower than the apoptosis process. It is widely accepted that the gastric mucosal integrity depends on the balance between apoptosis and proliferation, which explains the equilibrium between cell renewal and death of damaged or aged cells, and that ischemia/reperfusion may induce mucosal injury by altering this balance.
In our present study, techniques of electrical stimulation and electrolytic destruction were adopted and the role of electrical stimulation of PVN in gastric mucosal injury, cellular apoptosis, and proliferation induced by gastric I/R injury were observed. It was found that PVN stimulation protected against gastric I/R injury. In the electrical stimulation of PVN plus gastric I/R group, the GMDI was significantly lower than that in the gastric I/R injury only group at 30 min, 1 h and 3 h after reperfusion. After bilateral PVN destruction, the GMDI was not significantly different from that in the gastric I/R only group, but it was significantly higher than that in electrical stimulation of PVN plus gastric I/R group (P < 0.05). Furthermore, our results also revealed that the electrical stimulation of PVN markedly decreased the expression of apoptotic-positive cells, and increased the expression of proliferating positive cells at 0.5, 1, 3 h after reperfusion, respectively. These results indicate that PVN participates in the regulation of gastric mucosal cellular apoptosis and proliferation after gastric I/R in rats. Inhibiting gastric mucosal cellular apoptosis and promoting gastric mucosal cellular proliferation might mediate the dramatic protective effect of PVN on gastric mucosal injury. Our data also support the notion that PVN is a specific center in the brain that maintains the integrity of gastric mucosa and modifies the equilibrium between gastric mucosal apoptosis and proliferation.
Our previous experimental results have testified that the protective effect against gastric I/R injury is related to the excitation of PVN neurons. We focused on the microinjection of L-glutamic acid (a neuronal stimulant) into PVN, and found that the chemical stimulation produces a similar effect to that of 0.4 mA electrical stimulation of PVN[35], and the histological evidence from present study illustrates that the destroyed locus is only restricted within PVN. These results imply that the protection against gastric I/R injury resulted from the excitation of PVN neurons rather than from the excitation of nerve fibers passing PVN or spreading the stimulating current. Therefore, PVN is closely related to the gastric mucosal cellular apoptosis and proliferation induced by gastric I/R, suggesting that PVN is one of the specific brain areas in regulating the gastric I/R injury.
The research on the neuro-endocrine mechanism of the protection of stimulating PVN on gastric I/R injury has been limited except for our previous reports. Our previous study indicates that PVN stimulation, electrical or chemical, activates its neurons to affect the activity of nucleus tractus solitarius (NTS)-dorsal vagal complex (DVC) through its descending fibers and then accomplish the protection against gastric I/R injury by the mediating vagus and sympathetic nerves[45]. Our another study also discovered that electrical stimulation of PVN significantly increases the stress gastric ulceration, while electrolytic lesion of PVN decreases it. PVN is an important brain site in regulating the development of stress-induced gastric ulcers, and is involved in classical neurotransmitters of ACh, NE and 5-HT, with the parasympathetic, sympathetic nervous systems and the three endocrine glands (thyroid, adrenal and gonad) taking part in the effect[34]. Nevertheless, when compared with our previous study on stress-induced gastric ulcer, in our present study the stimulating PVN attenuated gastric mucosal damage induced by gastric I/R, instead of aggravating it, as mentioned above, the contradictory results cannot be satisfactorily explained. It might be wise to regard the PVN as an extremely complicated regulatory mechanism integrating various stress information. Hence the mechanism of the protective effect of PVN against gastric I/R injury may not be a simple one. Recently, Tarnawski reviewed cellular and molecular mechanisms of gastrointestinal ulcer healing, and pointed out that the healing processes are controlled by many cytokines, growth factors (EGF, PDGF, KGF, HGF, TGFβ, VEGF, angiopoietins) and transcription factors activated by tissue injury in spatially and temporally coordinated manners[46]. It will be of interest to further examine this hypothesis in our experimental system.
In future, our further investigation will involve gathering more information and understand the mechanisms of PVN protecting against gastric reperfusion injury. At present, some efforts are being made in our laboratory to assess the effect of molecular mechanisms and signaling pathways of the protective effect of PVN.
COMMENTS
Background
Little is known about the systemic regulation of gastric I/R injury, particularly the role of related central nuclei. The present study found that the paraventricular nuclei (PVN) exerts protective effects against gastric I/R injury as a specific area in the brain, and the protection is associated with the inhibition of cellular apoptosis and promotion of gastric mucosal proliferation.
Research frontiers
The present study discussed the cellular mechanisms responsible for the gastric I/R injury and the possible cellular mechanisms of the attenuation of gastric I/R injury by electrical stimulation of PVN.
Innovations and breakthroughs
Our previous study showed that PVN is involved in the regulation of gastric I/R injury as one of the specific CNS areas attenuating gastric I/R injury. The cellular mechanisms responsible for the attenuation of gastric I/R injury by electrical stimulation of PVN are still unknown. It was the first time in this study to investigate the molecular mechanisms of stimulating PVN in protecting against gastric I/R injury, to observe the characteristics of gastric mucosal cellular apoptosis and proliferation induced by gastric I/R at various times.
Applications
The present study would help better understand the pathology of gastric I/R injury at cellular level and the regulation role of PVN in gastric function.
Terminology
M30 CytoDEATH is a caspase-cleaved formalin-resistant epitope of cytokeratin 18 cytoskeletal protein in the cytoplasm of apoptotic cells. PCNA is a proliferating cell nuclear antigen as an assistant factor of DNA polymerase.
Peer review
This is an interesting manuscript that links central neural mechanisms with protection of gastric mucosa following gastric I/R injury and found that stimulating PVN significantly inhibits gastric mucosal cellular apoptosis and promots gastric mucosal cellular proliferation, which is probably one of the protective mechanisms of electrical stimulation of PVN against gastric I/R injury. It was suggested that more methods should be performed to clarify the change patterns of gastric mucosal cellular apoptosis and proliferation.